Introduction
The adhesive system called universal adhesives are known as “multi-mode” because this latest generation of adhesives can be applied by either self-etching or an etch-and-rinse mode (1,2,3). This new multi-mode generation of adhesives has already revealed favorable immediate clinical performance, comparable with that of gold-standard etch-and-rinse and self-etch adhesives (4). Some manufacturers have recently introduced universal adhesives with a “no-wait” or “quick bonding” concept. These universal adhesives provide less technical sensitivity and simplified procedures for clinicians and require no time to wait after adhesive application.
Although a shorter application time may be clinically appealing, this procedure may have negative consequences to adhesive infiltration and solvent evaporation (5). Dentin has a heterogeneous structure, consisting of collagen and hydroxyapatite (HAp), and the water content is higher than that of enamel. The bonding effectiveness of self-etch adhesives depend on the chemical reaction between functional monomers of the adhesive and HAp. The high water and solvent levels in some universal adhesives allow ionization of the included acidic functional monomers and induce resin monomer infiltration (6,7). However, residual water inhibits resin monomer polymerization (8,9); therefore, a specific length of application time should allow the residual water and solvents to evaporate (10,11).
Universal adhesives are increasing in popularity in clinical practice, but the manufacturer’s instructions for universal adhesives are unclear; for example, “is the application procedure active or passive?” or “what is the required time for application process?”. The method of adhesive application is based on the clinician’s preference because the procedures are not described in detail by the manufacturer’s instructions.
In this study, two light-cured universal adhesives and one self-cured universal adhesive were used. The aim of this study was to evaluate the effects of alternative self-etch application modes on the resin-dentin microtensile bond strength (µTBS) of three “no wait concept” universal adhesives. The null hypothesis tested was that alternative self-etch application methods do not affect the µTBS to dentin.
Materials and methods
Preparation od dentin specimens
This study protocol was approved by the Ethical Research Committee (2019/363) of the Karadeniz Technical University. In this study extracted impacted non-carious human third molars were used. After extraction, all teeth were stored in an aqueous solution of 0.1% thymol for a maximum of one month. Teeth were embedded in self-curing acrylic resin (Imıcryl, SC, Konya, Turkey) in cylindrical silicone molds. The occlusal third was removed using a low-speed diamond saw (Micracut 125, Low Speed Precision Cutter, Metkon, Bursa, Turkey) under running water, and flat surfaces were prepared in mid-coronal dentin. The dentin surfaces were prepared with 600-grit SiC paper to create a standardized smear layer.
Bonding procedures
Twenty-seven teeth were randomly divided into three experimental groups, as follows, according to the adhesives that were used: 1) Clearfil Universal Bond Quick (CUQ); 2) G-Premio Bond (GPB); and 3) Tokuyama Universal Bond (TUB). Material compositions and details are provided in Table 1. The teeth assigned for each adhesive were further randomly divided into three subgroups (n=3). The following three different application procedures were used for the dentin surfaces: the adhesives were applied and immediately subjected to air-dry (IA); the adhesives were applied followed by a 10-second wait (PA); or the adhesives were rubbed for 10 seconds (AA). The adhesives were air-dried as stated in each manufacturer’s instructions (Table 2). Two layers of 2-mm thick composite resin (Filtek Z250 universal, 3M ESPE, St Paul, MN, USA) were applied to the dentin surface. Each layer was cured using a LED light-curing unit (Elipar S10, 3M ESPE, St Paul, MN, USA).
Table 1 Universal adhesives used in this study.
| Materials (Lot.) | - | Compositions | pH | Manufacturers |
|---|---|---|---|---|
| Clearfil Universal Bond Quick (5H0033) | CUQ | Bis-GMA, 10-MDP, HEMA, hydrophilic amide monomer, ethanol, water, NaF, accelerator, silane coupling agent, Colloidal silica, dl-Camphorquinone | 2.3 | Kuraray Dental, Tokyo, Japan |
| G-Premio Bond (1903252) | GPB | MDP, 4-MET, MEPS, BHT (butylated hydroxytoluene), acetone, dimethacrylate resins, photoinitiator, aluminium oxide, water, phosphoric acid ester monomer | 1.5 | GC Corp, Tokyo, Japan |
| Tokuyama Universal Bond (024E18) | TUB | Liquid A: phosphoric acid monomer (3D-SR monomer), Bis-GMA, TEGDMA, HEMA, MTU-6 Liquid B: acetone, isopropyl alcohol., water, borate catalyst, c-MPTES, peroxide | 2.2 | Tokuyama Dental, Tokyo, Japan |
Table 2 Alternative self-etch application modes.
| Universal Adhesives | Manufacturers’ Instructions/ immediate application | Prolonged application time | Active application/rubbing |
|---|---|---|---|
| - | IA | PA | AA |
| Clearfil Universal Bond Quick | Apply adhesive. Immediately medium air-dry for 5s. Light cure for 10s. | Apply adhesive and wait for 10s. Medium air-dry for 5s. Light cure for 10s. | Apply adhesive and rub it for 10s. Medium air-dry for 5s. Light cure for 10s. |
| G-Premio Bond | Apply adhesive. Immediately maximum air-dry for 5s. Light cure for 10s. | Apply adhesive and wait for 10s. Maximum air-dry for 5s. Light cure for 10s. | Apply adhesive and rub it for 10s. Maximum air-dry for 5s. Light cure for 10s. |
| Tokuyama Universal | Apply adhesive. Immediately weak air-dry for 5s. No light cure. | Apply adhesive and wait for 10s. Weak air-dry for 5s. No light cure. | Apply adhesive and rub it for 10s. Weak air-dry for 5. No light cure. |
Microtnesile bond strength test (µTBS)
After storage in 37°C distilled water for 24 h, all the bonded teeth were cut into 1 mm² sections using a low-speed diamond saw (Micracut 125 Low Speed Precision Cutter, Metkon, Bursa, Turkey) under running water. Then, five samples (sections) per tooth from the central region were randomly selected and 15 sections from three teeth were tested immediately after cutting (n=15). The sections were fixed onto a tensile testing jaw using cyanoacrylate adhesive and subjected to a tensile force at a crosshead speed of 1 mm/min in a testing apparatus (Microtensile Tester, Bisco, IL, USA). µTBS values were expressed in MPa, and the data were analyzed.
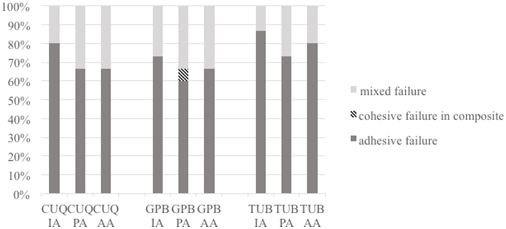
To determine the type of failure that resulted from the microtensile bond strength test, fracture surfaces were examined under 40× magnification using a stereomicroscope (Leica MZ16, Wetzlar, Germany). Failure modes were classified as adhesive, cohesive in the composite, or mixed.
Statistical analysis
Differences between adhesives groups were analyzed using a Kruskal-Wallis test. The Mann-Whitney U test was used for making comparisons within application modes. The statistical analysis was performed by SSPS Windows for 17.0 (SSPS Inc., Chicago, IL, USA) and p<0.05 was considered to be significant.
Results
µTBS values were significantly influenced by the application mode factors (Table 3). No significant differences were found µTBS values in IA between all adhesive (p>0.05). For all adhesives, PA increased µTBS values compared with IA (p<0.05), and AA increased µTBS values except for TUB (p=0.225). When µTBS values of CUQ and GPB were compared, there were no significant difference in PA (p=0.850), but a significant difference were found in AA (p=0.024). TUB had statistically lower values in PA and AA compared to CUQ and GPB (PA: p(CUQ-TUB)=0.007, p(GPB-TUB)=0.008; AA: p(CUQ-TUB)<0.001, p(GPB-TUB) <0.001). There were no significant differences between PA and AA in µTBS values of CUQ (p=0.657).
The different failure modes are shown in Figure 1. Adhesive failure was the most commonly observed type of failure in all specimens, irrespective of the type of adhesive, application time, or application method used. Cohesive failure in the composite was observed only for GPB adhesives in the prolonged application time mode.
Table 3 Mean microtensile bond strength values (µTBS) ± standart deviation in MPa (n=15).
| - | CUQ | GPB | TUB | KW p |
|---|---|---|---|---|
| IA | 13.83±2.19 ᴬᵃ | 14.63±3.55 ᴬᵃ | 13.23±2.90 ᴬᵃ | 0.496 |
| PA | 19.20±3.37 ᴬᵇ | 18.97±3.13 ᴬᵇ | 15.82±2.91 ᴮᵇ | 0.009 |
| AA | 18.62±3.70 ᴬᵇ | 23.11±6.30 ᴮᶜ | 11.97±2.69 Ca | 0.001 |
| KW p | 0.001> | 0.001> | 0.004 | - |
Different uppercase superscript letters indicate a statistically significant difference among adhesives, Different lowercase superscript letters indicate a statistically significant difference between application methods (p <0.05). KW,Kruskal-Wallis IA: immediate application PA: Prolonged application time AA: Active application /rubbing
Discussion
In the present study, the effect of alternative self-etch application methods on the dentin bond strength of three “no wait concept” universal adhesive systems were evaluated. This study showed that the application time and method had a significant effect on the bond strength to dentin (Table 3). Therefore, the null hypothesis that the alternative self-etch application methods do not affect the µTBS of universal adhesive systems was rejected.
In the present study, PA increased µTBS values in all adhesives compared with IA. The GPB was categorized as an intermediately strong self-etching adhesive that provides higher etching ability to the smear layer (12), while CUQ and TUB adhesives are mild, self-etching adhesives (6). Additionally, longer application time might compensate for the lower etching capability of mild self-etch adhesives (10). Saikaew et al. (5) evaluated the effect of a shortened application time of universal adhesive on long-term bond strength, and they reported that a shortened application time can compromise bonding performance. Huang et al.(13) compared two alternative application modes of GBP and found that the prolonged application time improved the bonding performance. Higher µTBS values can be explained by the prolonged application time, which provides increased monomer infiltration (11).
Increased dentin bond strength with active application of universal adhesives has been reported as a result of increased resin monomer infiltration and solvent evaporation (14,15,16). The pressure that occurs during the active application causes compression of the collagen network. When the pressure is released, the compressed collagen expands and this process provides the infiltration of the adhesive into collagen network while the solvents evaporates (7,17). The AA mode with GPB adhesive showed the highest µTBS values. This might be explained by AA and also different solvent ingredient of GPB. The adhesives are generally formulated with acetone, ethanol, and water or solvent combinations (18,19). GPB contains acetone as a solvent whereas CUQ is ethanol-water-based and TUB is isopropyl alcohol- based (Table 1). The vapor pressure of ethanol is lower than that of acetone (12,20). Itoh et al. (21) reported that vapor pressure (at 25°C) is 44mm Hg for isopropyl alcohol, 200 mm Hg for acetone, and 54.1mm Hg for ethanol. Thus, evaporation of isopropyl alcohol by air-drying is more difficult than acetone and ethanol. In addition, CUQ and TUB includes 2-Hidroksietil methacrylate (HEMA), which is an adhesion-promoting monomer that decreases the vapor pressure of water and alcohol due to its hydrophilicity. Therefore, it can prevent insufficient solvent evaporation from adhesive (20). For the ingredients in the adhesives that were used (Table 1), CUQ and GPB contains 10-methacryloyloxydecyl dihydrogen phosphate (MDP) monomer. The MDP monomers provide an ionic bond with the calcium in hydroxyapatite of enamel and solvent type and the application modes can explain the higher µTBS values for CUQ and GPB compared to TUB.
TUB is a self-curing adhesive that requires no light-irradiation, and the borate catalyst content as the polymerization initiator (Bo SE Technology) promotes polymerization from the adhesive interface (Contact Cure). A thin “bonding layer” that is formed after air drying due to the rapid progression of the self-curing technology also provides bonding to the composite resin. It was developed based on three-dimensional (3D) self-reinforcing (SR) technology as an adhesive monomer and. 3D-SR has the potential for chemical bonding to the tooth structure by forming multiple bonding sites with calcium (23,24). The “gel effect” that is provided with borate-based SR adhesive content is beneficial for the penetration of adhesive monomers into the dentin tubules (25). TUB with AA exhibited a significantly lower µTBS value compared with the other adhesives. This finding might be related to the negative effect of the rubbing action on the self-curing chemical polymerization process, and it probably results from degradation of the interface bonding layer.
The fracture modes were mainly categorized as adhesive failure and mixed failure (Figure 1). Saikaew et al. (5) reported that pores present in adhesive interface may cause failures and these pores represent solvent and water that could not evaporate due to shortened application time in universal adhesives. The high percentage of adhesive failure in IA may be due to shortened application time.
The limitation of this in vitro study was the lack of aging procedures which should be taken into consideration for clinical success. Further in-vitro and clinical studies are needed to evaluate effect of application modes on the long-term performance of universal adhesives. The 'no-wait concept' of adhesives may make the bonding procedure less technique sensitive in clinical practice. However, this study showed that the immediate application procedure did not provide the highest bonding strength. Because of the varying adhesive ingredients, application procedures might positively or negatively affect the bond strength to the dentin. Thus, instructions for the materials should be specified more clearly and details should be provided.
Conclusions
Within the limitations of this study, the application time and application method affected µTBS. CUQ, GPB, and TUB exhibited significantly higher µTBS values to dentin with prolonged application time compared to immediate application. However, active application increased the µTBS values of CUQ and GPB, but decreased the µTBS values of self-curing universal adhesive TUB.