Introduction
Damage to bone tissue due to trauma or the presence of pathology represents a challenge at the time of treatment. Certain conditions, such as diabetes, osteoporosis, development of neoplasms, or very extensive lesions, hinder the healing process (1). The regeneration strategy -the autograft- is considered the gold standard; however, it has the great disadvantage of presenting high resorption rates and sometimes developing low-quality bone tissue and morbidities that may occur at the donor site (2).
To avoid these limitations the concept of tissue engineering was developed, which focuses on the use of biomaterials that act as scaffolds that mimic the extracellular matrix of the tissue to be regenerated, together with cells with high regenerative potential (3). Three-dimensional (3D) cell culture is a strategy that has been studied alone or in combination with scaffolds for tissue regeneration. The main characteristic of this type of cell culture is the interaction of the cells with the environment in its three dimensions, which allows them to maintain a similar morphology that resembles an in vivo physiological microenvironment, favoring survival, gene expression, and differentiation potential, among others (4).
In the field of tissue regeneration, the application given to 3D cell culture has been very broad. In a recent study, the co-culture behavior of cell spheroids of periodontal ligament stem cells and human umbilical vein endothelial cells was analyzed, showing enhanced new cementum formation (5). In contrast, umbilical cord stem cell spheroids have shown potential in the differentiation of Schwann cells, demonstrating possible future application in the regeneration of peripheral nerves (6).
However, the number of projects that focus on the potential of this type of 3D cell spheroid culture in the treatment of bone defects has been significantly increasing in recent years. A variety of methodologies have been used for developing 3D spheroids culture formation making the comparison of results complex; thus, this systematic review aims to (i) perform a critical analysis focused on the current role of 3D cell spheroid culture in bone regeneration strategies; and (ii), address the main challenges in the experimental phase in order to optimize the clinical application of the 3D spheroid strategy of culture. The review was based on the PRISMA criteria by consulting the PubMed, Scopus, and ScienceDirect databases. Studies whose main objective was the behavior of 3D spheroid culture in bone tissue regeneration were selected; in vitro and in vivo studies were considered.
Materials and methods
Selection criteria
Search strategy
The search was carried out in the electronic databases PubMed, Scopus, and ScienceDirect, using the following keywords: "3D cell culture", "spheroid", and " bone regeneration", employing independent search strategies with the Boolean operators "OR" and "AND". An additional search of the references of the selected studies was carried out to detect potential studies that met the selection criteria. The investigation was limited to the years 2010-2020.
Sslection of studies
For the selection of the studies, initial filtering was performed for each title and abstract that mentioned the analysis of 3D cell culture spheroid in bone regeneration. The selected studies were placed in a bibliographic database in which second full-text filtering was performed to identify specific bibliographies that met the established criteria. The selection of the studies was carried out independently by two reviewers; in case of discrepancy, a third reviewer participated.
Data extraction and analysis
The information collected was the type of study (in vitro/in vivo); the type of cells used for the formation of cell spheroid; the methodology used for 3D cell culture of developing spheroids; the number of cells; and a brief description of the objectives and conclusions of the studies. Due to the review's objective, only descriptive statistics of the data collected were performed, reporting measures of central tendency for quantitative variables and frequencies for qualitative variables. The data were entered into the SPSS V.22 program.
Risk of bias
In order to guarantee the transparency of the results, the evaluation of risk of bias was carried out. The risk-of-bias tool to address in vitro studies developed by the United States National Toxicology Program was applied (7). The evaluation was performed independently by two evaluators.
Results
Search strategy
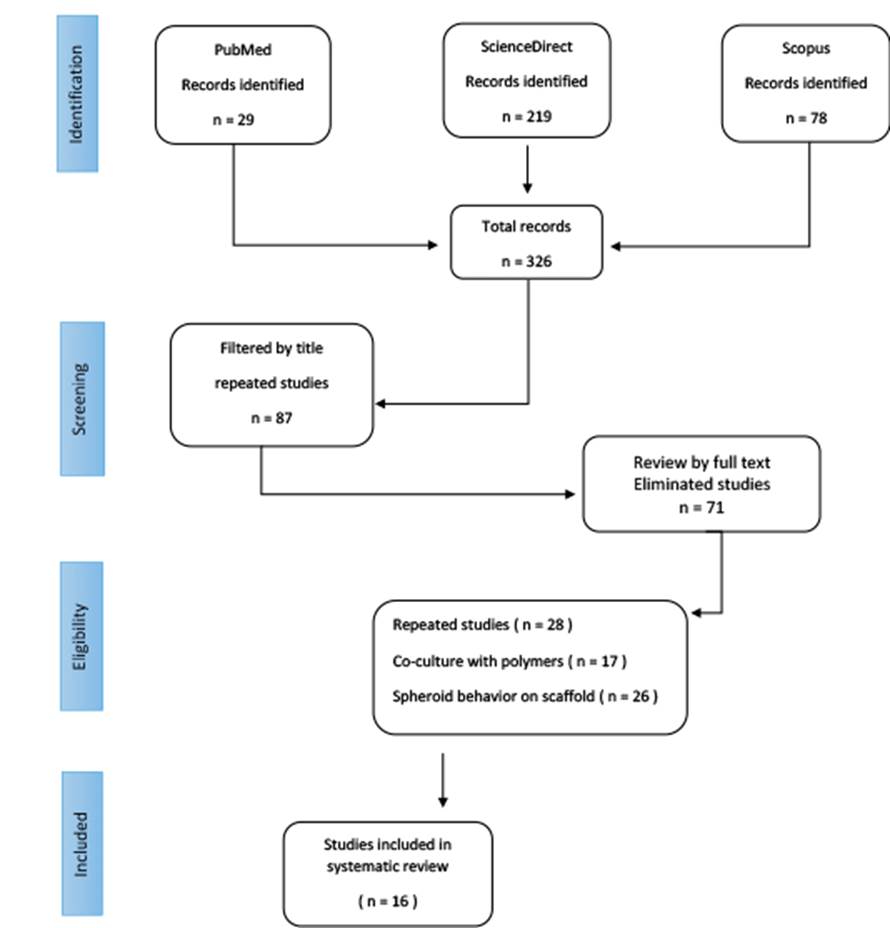
In the first search performed in the different databases, 326 studies were identified; when the first filtering was performed by title and abstract, 87 articles were selected. The studies were placed in a bibliographic database to identify repeated studies and those that did not meet the selection criteria. A total of 71 studies were eliminated, and only 16 studies were identified that focused on analyzing the behavior of cell spheroid alone without the presence of scaffolds (Figure 1).
Description data
Of the 16 studies selected, 50% corresponded to in vitro projects (8 studies) and the remaining 50% to in vitro/in vivo studies (n=14; 87.5%) of the articles reviewed used stem cells for the formation of cell spheroid from different origins: adipose tissue, bone marrow, gingival, rat, or human. The number of cells used to fabricate cell aggregates ranged from 1000 or 2000 to 1×10⁷ cells. The most common methodology for 3D cell culture development was low adhesion surfaces in 8 of the studies evaluated (Table 1).
Risk of bais
For the risk of bias analysis, a modification of the tool used was made using only questions related to experimental conditions, blinded studies, exclusion of cases or samples, and analysis of the results. The evaluation showed that the total number of included studies had a low level of risk of bias as shown in Table 2.
Table 1 Information from the selected studies related to the type of study, in vitro or in vivo, the cells used for the development of 3D spheroid cell culture, technique, number of cells, objective, and conclusion.
| - | Spheroid formation methodology | - | - | - | - | - |
|---|---|---|---|---|---|---|
| Study | Type of study | Cells | Technique | # Cells/mL | Objetive | Conclusions |
| He et al. (2017) (8) | in vitro/in vivo | Mouse cranial stem cell | Low attachment culture | 2×10⁵ | Spheroid culture might favor the expansion of cranial stem cells and promote maintenance of their stemness. | The development of a reliable protocol to isolate stem cells from mouse calvaria and their regulation by specific chemical inhibitors of PI3K/AKT signaling pathways may be effective agents to modulate cranial bone repair by targeting specific stem cells in local tissues. |
| Moritani et al. (2018) (9) | in vitro/in vivo | Human periodontal ligament mesenchymal stem cells | Microwell chips | 2×10³ | Examine the stemness of spheroid hPDL-MSCs isolated from hPDLs and their osteogenic potential of hPDL-MSC spheroids. | Spheroid form of PDL-MSCs enhances osteogenic potential by increasing the expression of osteogenic genes. |
| Suenaga et al. (2015) (10) | in vitro/in vivo | Human bone-marrow- derived MSC | Rotational culture system | 1×10⁷ | Assess the effectiveness of the MSC-spheroids generated by the rotation culture system in the repair of cranial bone defects in a well-established rat model. | MSC-spheroids may simply induce an osteogenic response, enhancing osteoblast recruitment to the implantation site. |
| Yamaguchi et al. (2014) (11) | in vitro/in vivo | Bone marrow stem cells | Low attachment culture | 1×10⁴ | Examine the antigenerative potential of the spheroids of rMSCs isolated from bone marrow compared with monolayer rMSC. | In vitro and in vivo results reveal that MSCs in the spheroid culture exhibit enhanced osteogenic efficiency compared with those in the monolayer culture. |
| Imamura et al. (2020) (12) | in vitro/in vivo | Mouse bone marrow stromal precursor | Low attachment culture | 4×10⁵ | Examine the effects of transplanting wild-type or knockout spheroid MSCs on new bone formation in mice calvarial defect model. | 3D spheroid MSCs exhibited increased stemness molecules, and the expression of osteogenesis-related molecules was upregulated in 3D spheroid MSCs together with the activation of Wnt/β-catenin signaling. |
| Walser et al. (2013) (13) | in vitro/in vivo | Primary human osteoblasts, human dermal microvascular endothelial cells and normal human dermal fibroblasts | Low attachment culture | 5×10⁴ | Generate spheroid- based individual vascularization units for bone tissue engineering by aggregating different combinations of primary HOB, HDMEC and normal human dermal fibroblasts in the form of co-culture spheroids. | HOB-HDMEC spheroids exhibit a high viability and rapidly develop a functional vascularization after transplantation. |
| Tiaden et al. | in vitro/in vivo | Human bone-marrow- derived MSC | Hanging drop technique | 2.5×10³ | Examine the effects of recombinant mammalian HTRA1 on the osteogenesis of human BMSCs and matrix mineralization. | HTRA1 is a positive regulator of both osteogenesis of MSCs and mineralization of differentiating bone-forming cells, possibly at the expense of adipogenic differentiation. |
| Rumiński et al. (2020) (15) | in vitro | Adipose-derived mesenchymal stem cells | Low attachment culture | 1 × 104 | Study the effect of cAMP regulation on the osteogenic differentiation of ADSCs by employing a 3D spheroid culture to provide improved osteogenic stimulation and analyze the possible role of protein kinase A activity in 3D-induced osteogenesis. | cAMP level manipulation might be used to steer the differentiation of ADSCs into osteoblasts prior to implantation on 3D scaffolds in prospective bone-regeneration clinical studies. |
| Yamamoto (2014) (16) | in vitro | Mouse dental papilla cells | Low attachment culture | 3 × 104 | Evaluate the effects of 3D spheroid culture on the phenotype immortalized MDPs that have the ability to differentiate into odonto/osteoblasts. | 3D spheroid culture promotes odonto/osteoblastic differentiation of MDPs and is probably mediated by integrin signaling. |
| Kamoya et al. (2016) (17) | in vitro | Mouse bone-marrow-derived mesenchymal stem cells | Oxy-chip | 100 × 104 | Compare the osteoblastic differentiation of the mouse bone-marrow-derived mesenchymal stem cells grown on the Oxy chip to those grown on a conventional non-oxygen permeable chip as well as a monolayer culture. | The oxygenated spheroid culture system improved the cell viability and osteoblastic differentiation, and inhibited alternate differentiation pathways. |
| Saiz et al. (2019) (18) | in vitro/in vivo | Rat bone marrow stromal cells | Rotational culture system | 5 × 106 | Bone formation would be enhanced by local myokine presentation, and the myoblast secretome would be augmented by exposure to factors secreted by MSC spheroids. | The synergistic presentation of SPH-MYO with rBMSCs resulted in significant increases in bone volume and near complete bridging in 12 weeks. |
| Fu et al. (2018) (19) | in vitro/in vivo | Rat bone marrow mesenchymal stem cells | Low attachment culture | 5 × 103 | Report mineralized ECM/stem cell microspheroids, in which self-assembly of the stem cell spheroids and mineralization of the self-produced ECM occurred simultaneously. | The mineralized ECM/stem cell microspheroids exhibited enhanced stiffness and provided a physiological microenvironment conducive to cell proliferation, intracellular communication, collagen secretion, and osteogenic differentiation. |
| Murphy et al. (2016) (20) | in vitro | Human bone-marrow-derived MSCs | Hanging drop technique | 15 × 103 | Osteogenically induced MSCs formed into 3D spheroids would exhibit a more sustained osteoblastic phenotype than dissociated MSCs when encapsulated in a collagen hydrogel. | MSCs formed as spheroids sustain their osteoblastic phenotype better than dissociated MSCs when encapsulated within a collagen hydrogel. |
| Baraniak & McDevitt (2012) (21) | in vitro | Murine bone-marrow-derived MSCs | Rotational culture system | 1.8-6 × 106 | Form mesenchymal stem cell spheroids of different sizes using a forced aggregation technique and maintain in suspension culture for extended periods of time thereafter; compare cell proliferation and differentiation potential spheroids to conventional adherent monolayer cultures. | The study demonstrates the successful development of a 3D scaffold-free culture system for MSCs that maintains and enhances the in vitro multipotent differentiation of these cells. |
| Gurumurthy et al. (2017) (22) | in vitro | Human adipose derived stem cells | Low attachment culture | 50 × 104 | Study the in vitro osteogenic activity of hASCs, by culturing them as spheroids in tissue culture polystyrene plates coated with polymer elastin-like polypeptide. | The use of the ELP-PEI coating method for in vitro 3D spheroid culture shows that hASCs cultured in this way have improved differentiation properties compared to the 2D monolayer culture. |
| Rumiński et al. (2019) (23) | in vitro | Human adipose derived stem cells | Rotational culture system | 1 × 103 | Study spheroids generated on non-adhesive surfaces without providing any external attachment substrate throughout the investigated culture period, to elucidate the role of a cell-derived spheroid microenvironment on ADSC osteogenic commitment. | Scaffold-free 3D spheroid culture increased the commitment of ADSCs into pre-osteoblasts compared to that of 3D scaffold and monolayer conditions, but efficient osteoblast maturation and production of the mineralized extracellular matrix required an attachment substrate for the cells. |
3D, 3 dimensional; ADSC, adipose-derived stem cell; BMSCs, bone mesenchymal stromal cells; cAMP, cyclic adenosine monophosphate; ECM, extracellular matrix; hASCs, human adipose-derived stem cells; HDMEC, human dermal microvascular endothelial cells; HOB, human osteoblasts; hPDL-MSC, human periodontal ligament-mesenchymal stem cells; HTRA1, high-temperature requirement serine protease A1; MDPs, mouse dental papilla cells; PDL, periodontal ligament; rBMSCs, rat bone mesenchymal stem cells; rMSC, rat mesenchymal stem cells.
Table 2 The risk of bias of the analysis.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Risk |
|---|---|---|---|---|---|---|---|---|
| He et al. (2017) | + | - | + | + | + | + | + | Low |
| Moritani et al. (2018) | + | - | + | + | + | + | + | Low |
| Suenaga et al. (2015) | + | - | + | + | + | + | + | Low |
| Yamaguchi et al. (2014) | + | - | + | + | + | + | + | Low |
| Imamura et al. (2020) | + | - | + | + | + | + | + | Low |
| Walser et al. (2013) | + | - | + | + | + | + | + | Low |
| Tiaden et al. (2012) | + | - | + | + | + | + | + | Low |
| Rumiński et al. (2020) | + | - | + | + | + | + | + | Low |
| Yamamoto et al. (2014) | + | - | + | + | + | + | + | Low |
| Kamoya et al. (2016) | + | - | + | + | + | + | + | Low |
| Saiz et al. (2019) | + | - | + | + | + | + | + | Low |
| Fu et al. (2018) | + | - | + | + | + | + | + | Low |
| Murphy et al. (2016) | + | - | + | + | + | + | + | Low |
| Baraniak & McDevitt (2012) | + | - | + | + | + | + | + | Low |
| Gurumurthy et al. (2017) | + | - | + | + | + | + | + | Low |
| Rumiński et al. (2019) | + | - | + | + | + | + | + | Low |
Discussion
With the introduction of 3D cell culture, a much clearer and more accurate ap-proximation of cell behavior within an organism was achieved (24). Thus, its analysis in the field of bone regeneration has been widely addressed to optimize mineral tissue neoformation.
There are many methodological questions in the study of 3D cell culture, such as defining the culture method and determining the number of cells needed for the stability of the 3D structure after being seeded. Since 3D spheroid was discovered, different methodologies have been developed, including seeding cells on surfaces with low adhesion plates, rotational systems cultures that force the proximity between cells for the interaction of cell-cell contact, and suspension techniques that use the force of gravity for an initial approach to the cells. According to the information obtained, in most studies, the 3D spheroid formation is carried out on low adherence surfaces (n=8). The main characteristic of this methodology is that the surface of the plate is not functionalized for improved the adherence of the cells to the bottom, thus the cells are floating, and the system does not exert forces that stimulate the proximity between the cells. Thus, as the cells are not being able to adhere to the surface of the plate, the cells begin to adhere to each other and starting the cell-cell signaling process allow to obtain a rounded morphology that is not modified, and the amount of nutrients that are received by the spheroid structure in the long-term culture is not limited (2).
The 3D cell spheroid culture, by its own structure, is characterized by a central region with an inadequate nutritional and oxygen supply, which allows a necrotic nucleus to be formed, and the high-density of the cell aggregates have a lower expression of markers in their nucleus related to cell proliferation (25). Therefore, it has been recommended that in 3D aspects, spheroids with smaller morphology of approximately 150 µm in diameter decrease the hypoxic areas and improve the survival (26). For this reason, one of the technical challenges of 3D cell spheroid culture is to establish the optimal number of cells in such a way that the 3D spheroid structure remains vital and stable for long periods of time. The number of cells recommended for the formation of stable spheroids is diverse throughout the literature; however, according to the results obtained, the cell density could be optimized from 1000 or 2000 cell/mL to millions of cells/mL depends on the application of the spheroid.
To limit the variability in the size of 3D cell spheroid, different methodologies have been developed, such as the use of micropatterns based on the use of biomaterials that favor cell adhesion in a determined area, so the cell spheroid will have a con-trolled size (27), or on the use of devices that allow a constant supply of oxygen, pre-venting hypoxia and necrosis of the nucleus by the forming spheroids with a cell density of 2.5 x 10⁵ (17).
Furthermore, the formation of 3D cell spheroid could be carried out with a wide range of adults or tumor cell types where the applications of cell spheroid been used for oral carcinogenesis (28), brain and nerve tumors (29), drug and radiation testing (30,31), cardiac tissue (32), and cartilage tissue (33), to understand cancer, cell function, tissue formation, and bioprinting applications.
However, for bone tissue applications, the most commonly used cells are primary osteoblast and stem cells, either from bone marrow, adipose tissue, or dental tissues (8,23,34). These cells are characterized by clonality, self-renewal capacity, and the ability to differentiate into different lineages with the specific growth factor cocktail of induction; the 3D culture spheroid of stem cells maintains and potentiates these characteristics (35). According to the review carried out, this type of 3D spheroid has shown the capacity to support the expression of markers such as Nanog, Oct-4, and Sox-2, which potentiate the expression of alkaline phosphatase (ALP) -a marker of early expression in the mineralization process-(8,9, 23), and enhance the expression of genes related to the formation of the mineral extracellular matrix (9,11,23), or increase the recruitment of cells to the implant site (8,10). One of the molecular mechanisms involved in these behaviors is the activation of the Wnt/β-catenin pathway and SMADs that potentiate bone formation in vivo (12), and the mechanisms mediated by α2β1 integrins (16,20). The above demonstrates that 3D spheroid culture simultaneously activates multiple signaling pathways, which directly impacts the maintenance of stemness or the upregulation of stem cells to guide osteoblast differentiation and bone matrix formation.
With the above, we can elucidate that the study of 3D spheroids in bone regeneration is an issue with particular technical challenges. The main objectives are (i) the development of culture techniques that optimize the morphology of the cellular spheroid focusing on the uniform size, in combination with (ii) the development of devices that optimize the permeability of nutrients and oxygen inside the structure as an Oxy-chip (17). On the other hand, the study of the behavior of the 3D spheroid through the expression of markers related to the differentiation process towards the osteoblastic lineage should be approached with future implications for in vivo clinical applications. Undoubtedly, the cell source is a variable to be considered; the cells used most often are mesenchymal stem cells because of the characteristics previously described; bone-marrow- derived stem cells are the ones with the highest level of characterization (10,12,14,17,18,19,20,21,34). However, the challenge for obtaining these particular stem cells is a major limitation when considering the possible clinical dental application, so the search for other sources -such as adipose tissue or different types of oral tissues- has become the object of in vitro and in vivo analysis (8,9,15,16, 22,23).
In addition, 3D spheroid culture has great advantages in bone tissue regeneration. However, the strategies most common that have been developed in bone application is in combination with the use of organic and inorganic materials in order to optimize the functionality of 3D spheroid cultures: (i) the use of fumarate-vinyl acetate copolymer and chitosan microgels has been evaluated and they create a 3D environment that enables the spheroid to have an extracellular matrix, which allows it to reorganize and acquire mobility (36); and (ii) gelatin methacrylate (GelMA)/ Na2CO3-based microspheres have been developed to allow the formation of CaCo3 crystals, which increase cell adhesion as well as the expression of ALP, suggesting that this model can be applied in the engineering of tissues focused on the regeneration of bone tissue using 3D cultures (37).
Although the advances in research on the potential use of 3D cell spheroids culture in bone regeneration have progressed quickly, as the bioprinting strategy as promising technology for developing construct with specific morphology, there is still a long way to go for clinical application. This is because the constant innovation in spheroid formation methodologies and new technologies for designing functional 3D biomaterials are needed to continue researching the optimal interaction of 3D cell spheroid cultures focused on bone tissue engineering.
Conclusions
The constant affectations suffered by bone tissue caused by multiple etiologies have led to the search for new strategies to optimally regenerate damaged structures. One of these is 3D cell spheroid cultures from which the strategy for the developing formation and the analysis of the biological behavior of the spheroid in terms of proliferation, survival, and differentiation need continuously be optimized. This confirms that 3D cell spheroid culture has characteristics far superior to monolayer 2D culture. The methods most often used for the formation of 3D spheroid are low adherence surfaces followed by rotational methods, which are simple and easily accessible methodologies. Without a doubt, mesenchymal stem cells are the most widely used cell models due to their regenerative potential; therefore, part of their study involves the identification and analysis of accessible sources. Hence, this study shows a clear and concise update of the different methodologies used for the analysis of 3D spheroid and the future of the use in the application for bone tissue engineering.
Author contribution statement
Conceptualization: M.V.C.G. and M.A.A.P. Investigation: M.V.C.G. and F.S.O.
Methodology: M.V.G.C. and J.C.C.G.
Validation: M.V.G.C., J.C.C.G. and M.A.A.P.
Data curation: M.V.G.C., J.C.C.G. and F.S.O. Writing-original draft preparation: M.V.C.G. and F.S.O. Writing-review and editing: J.C.C.G. and M.A.A.P. Funding acquisition: M.A.A.P.