Introduction
In the weaning process, nursery pigs face several stressful events. This process involves separation from the mother, change of pens, new social interaction, as well as new nutritional regimens, which generate physiological alterations (Campbell et al., 2013; Wijtten et al., 2011). These changes affect the use of nutrients (Boudry et al., 2004) and allow non-beneficial bacteria to disturb the balance of the commensal microbiota (Gresse et al., 2017; Wei et al., 2020). The non-beneficial bacteria, such as E. coli or Clostridium generate the appearance of post-weaning diarrhea in pigs, causing a low use of nutrients and even the death (Wensley et al., 2021).
The nutritional industry has been generating additives or antimicrobials as feed supplements to control pathogenic bacteria in nursery pigs (de Lange et al., 2010). Among some additives, antibiotics have been one of the most used in feed formulation for nursery pigs, generating a positive effect in the control of pathogenic bacteria growth (Shin et al., 2005; Yun et al., 2017). However, scientific findings have shown that the use of antibiotics as growth promoters generates bacterial resistance, causing detrimental effects on human health (He et al., 2020; Verstegen & Williams, 2002).
Pharmacological levels of zinc oxide in diets for pigs have been shown to stimulate the immune system and improve antioxidant capacity (Wang et al., 2020). Additionally, high zinc oxide levels in pig diets have been demonstrated to modulate the microbiota in pigs during the nursery stage (Yu et al., 2017). The National Research Council (2012) establishes, approximately, 100 ppm as the nutritional requirement of zinc in pigs in the beginning stage. However, the best responses in relieving post-weaning stress were obtained when diets were formulated with a zinc level over 1500 ppm (Hill et al., 2001).
In the European Union, zinc has been banned from pigs diet formulation due to its environmental impact as a heavy metal, its use is still accepted in other countries to counteract gastro intestinal tract (GIT) disorders. Due to the high human health concern about the resistance to antibiotics and the continued use of antibiotic based treatments, the objective of this research to evaluate the effect of a high level of zinc as a substitute to antibiotics in nursery pigs.
Material and methods
General conditions
The study was carried out from June to August 2022 in the Swine Research Center of the University of Panama, in the province of Chiriqui. It is located at 8° 23’ 15.12” north latitude and 82° 19’ 47.48” West longitude, with an elevation of 26 meters above sea level. The reported environmental temperature of the zone was between 24 to 32 ºC, and a relative humidity of 71 % to 86 %.
Animals and experimental diets
A total of 54 nursery pigs (Sus scrofa domestica) (23±2 day old; 6.95±0.06 kg) were assigned to three dietary treatments. Each treatment had six replicates (pen) with three pigs per replicate. The treatments were: 1) control diet, formulated to meet the nutritional requirements for pigs (National Research Council, 2012); 2) similar to control diet plus 200 mg of amoxicillin/kg feed (Amoxin 200, DQSA, Panama) during phase 1 (P1; d 0-14), and 40 mg of florfenicol/kg feed (Pecflor Premix, PL-Pecuarius, Mexico) during phase 2 (P2; d 15-28); and 3) similar to control diet plus 2000 ppm and 1600 ppm of zinc (National Zinc, Panama) during P1 and P2, respectively. In phase 3 (P3; d 29-42), all pigs were fed a common diet (Table 1).
Table 1 Composition and calculated nutritional profile of the experimental feeding diets of pigs. Swine research center of the Universidad de Panama, Chiriquí, Panama. 2022.
| Ingredients, % | Phase 1 | Phase 2 | Phase 3 | ||||
| Treatments1 | Common diet | ||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | |
| Corn | 50.85 | 50.85 | 50.85 | 56.00 | 56.00 | 56.00 | 56.00 |
| Soybean meal | 35.96 | 36.00 | 36.14 | 33.66 | 33.71 | 33.76 | 33.66 |
| Rice polish | 2.44 | 2.19 | 1.49 | 1.48 | 1.21 | 0.80 | 1.48 |
| molasse | 2.45 | 2.45 | 2.45 | 2.40 | 2.40 | 2.40 | 2.40 |
| Palm oil | 4.83 | 4.93 | 5.2 | 3.34 | 3.45 | 3.62 | 3.34 |
| Salt | 0.20 | 0.20 | 0.20 | 0.30 | 0.30 | 0.30 | 0.30 |
| M-dicalcium | |||||||
| phosphate | 1.49 | 1.49 | 1.49 | 1.28 | 1.28 | 1.28 | 1.28 |
| Calcium carbonate | 0.98 | 0.98 | 0.98 | 0.85 | 0.85 | 0.85 | 0.85 |
| Premix Vit-min2 | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 |
| L- Lysine | 0.27 | 0.27 | 0.27 | 0.19 | 0.19 | 0.19 | 0.19 |
| DL-Methionine | 0.09 | 0.09 | 0.09 | 0.07 | 0.07 | 0.07 | 0.07 |
| L-Threonine | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| L-Tryptophan | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Myco-AD A-Z | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| ZnO | 0.00 | 0.00 | 0.40 | 0.00 | 0.00 | 0.30 | 0.00 |
| Amoxin 2003 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pecflor premix4 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 |
| Nutritional content | Phase 1 | Phase 2 | Pase 3 | Phase 1 | Phase 2 | Phase 3 | Phase 1 |
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | |
| Dry matter, % | 87.00 | 87.00 | 87.00 | 86.90 | 86.90 | 86.90 | 86.90 |
| ME,(Kcal/kg) | 3400.4 | 3400.1 | 3400.0 | 3350.8 | 3350.8 | 3350.0 | 3350.8 |
| Crude protein, % | 21.00 | 21.00 | 21.01 | 20.10 | 20.10 | 20.10 | 20.10 |
| Ca, % | 0.79 | 0.79 | 0.79 | 0.70 | 0.70 | 0.70 | 0.70 |
| Available P, % | 0.65 | 0.65 | 0.65 | 0.60 | 0.60 | 0.60 | 0.60 |
| Zn, ppm | 138.000 | 138.0 | 2010.0 | 138.6 | 138.6 | 1600.0 | 138.6 |
| Lysine, % | 1.35 | 1.35 | 1.35 | 1.23 | 1.23 | 1.23 | 1.23 |
| Methionine, % | 0.39 | 0.39 | 0.39 | 0.36 | 0.36 | 0.36 | 0.36 |
| Threonine, % | 0.79 | 0.79 | 0.79 | 0.75 | 0.75 | 0.75 | 0.75 |
| NDF, % | 8.76 | 8.73 | 8.66 | 8.84 | 8.81 | 8.77 | 8.84 |
| ADF, % | 3.15 | 3.14 | 3.13 | 3.09 | 3.09 | 3.08 | 3.09 |
1 Treatments: 1) control diet; 2) diet with antibiotics; and 3) diet with zinc oxide. / Tratamientos: 1) dieta de control; 2) dieta con antibióticos; y 3) dieta con óxido de zinc.
2 The vitamin premix provided per kilogram of diet contains: 11375 IU of vitamin A, 3500 IU of vitamin D3, 26.3 IU of vitamin E, 3.5 mg of vitamin K3, 3.5 mg of vitamin B1, 8.8 mg of riboflavin, 5.4 mg of vitamin B6, 0.03 mg of vitamin B12, 17.5 mg of pantothenic acid, 35.0 mg of niacin, 1.75 mg of folate, and 0.14 mg of biotin. The mineral premix provided per kilogram of diet includes: 64.4 mg of Cu (cupric glycinate), 165.4 mg of Fe (iron glycine), 47.8 mg of Mn (manganese glycinate), 47.8 mg of Zn (zinc glycinate), 0.54 mg of Se (yeast selenium), 0.68 mg of Ca (calcium iodate), and 0.1 mg of Co (cobaltous sulfate). / El premix de vitaminas proporcionado por kilogramo de dieta contiene: 11375 UI de vitamina A, 3500 UI de vitamina D3, 26,3 UI de vitamina E, 3,5 mg de vitamina K3, 3,5 mg de vitamina B1, 8,8 mg de riboflavina, 5,4 mg de vitamina B6, 0,03 mg de vitamina B12, 17,5 mg de ácido pantoténico, 35,0 mg de niacina, 1.75 mg de folato y 0,14 mg de biotina. El premix mineral proporcionado por kilogramo de dieta incluye: 64,4 mg de Cu (cobre glicinato), 165,4 mg de Fe (hierro glicina), 47,8 mg de Mn (manganeso glicinato), 47,8 mg de Zn (zinc glicinato), 0,54 mg de Se (selenio de levadura), 0,68 mg de Ca (yodato de calcio) y 0,1 mg de Co (sulfato cobiloso).
3 Amoxicillin trihydrate: 20 g of amoxicillin per 100 grams of product. /Amoxicilina trihidratada: 20 g de amoxicilina por 100 gramos de producto.
4 Florfenicol: 40,0 g of florfenicol per 1000 grams of product. /Florfenicol: 40,0 g de florfenicol por 1000 gramos de producto.
Pigs were treated with the same sanitary protocol including an injection of 200 mg of iron dextran intramuscularly on the second day of life, tail docking and ear notching two and three days after birth, respectively.
Chemical analysis of diets
Sample diets were pre-dried in a mechanical convection oven at 60 °C for 72 hours (Yamato DKN810, New York, USA) and ground to a particle size of 1 mm (Restsch GmbH & Co., Germany) for further analysis of the nutritional content. The feed samples were placed in an oven at 105 °C for 24 hours (40GC Lab. Oven; Quincy Lab. Inc.; IL, USA) to determine the percentage of dry matter according to method 930.15 (Association of Analytical Chemists (AOAC), 2016), and then at 600 °C for 3 hours (Thermolyne, Thermo Scientific, NC, USA) for the determination of the ash content according to method 942.05 (AOAC, 2016). Concentration of Ca, P, and Zinc were determined by atomic absorption spectrophotometry (Analytik Jena-nov 400P, Germany) based on the methods 927.02, 964.06 986.15, respectively (AOAC, 2016).
Nitrogen content was analyzed using the Kjeldahl methodology (Velp Scientifica, Germany) according to method 976.05 (AOAC, 2016). The neutral detergent fiber (NDF) and acid detergent fiber (ADF) analysis were carried out in the ANKOM Tech. equipment (Ankom Technology, NY, USA), based on the method of Goering and Van Soest (1970). The digestibility of dry matter (DDM) was determined based on the equation: % DDM = 88.9 - (%ADF x 0.779), for its subsequent use in the determination of metabolizable energy through the equation: EM = 3.61 x DDM (Table 2).
Table 2 Nutritional content determined from experimental diets used for feeding pigs (Sus scrofa domestica). Animal Nutrition Laboratory of the Instituto de Innovación Agropecuaria. Chiriquí, Panama. 2022.
| Phase 1 | Phase 2 | Pase 3 | |||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | |
| Dry matter, % | 88.20 | 88.50 | 87.90 | 87.70 | 88.10 | 87.10 | 87.40 |
| *ME (Kcal/kg) | 3070.90 | 3112.80 | 3106.60 | 3099.90 | 3110.60 | 3117.60 | 3099.90 |
| Crude protein, % | 20.84 | 20.94 | 21.03 | 20.78 | 21.2 | 20.98 | 20.78 |
| Ca, % | 0.73 | 0.74 | 0.75 | 0.73 | 0.71 | 0.73 | 0.72 |
| P, % | 0.62 | 0.61 | 0.62 | 0.62 | 0.58 | 0.61 | 0.62 |
| +NDF,% | 14.40 | 10.80 | 11.80 | 14.40 | 13.30 | 10.80 | 14.40 |
| ≠ADF,% | 4.92 | 3.43 | 3.65 | 3.89 | 3.51 | 3.26 | 3.89 |
*ME: Metabolizable energy, +NDF: Neutral detergent fiber, ≠ADF: Acid detergent fiber, Ca: Calcium, P: Phosphorus. / *ME: energía metabolizable, +NDF: fibra en detergente neutro, ≠ADF: fibra en detergente ácido, Ca: calcio, P: fósforo.
Data collection
The body weight of each pig was measured at the beginning of the experiment (d0) and at the end of each phase (day 14, 28, and 42) to determine the average daily gain (ADG). Similarly, feed left in feeder was weighed at the end of each phase to determine average daily feed intake (ADFI). The ADFI was divided by ADG to determine the feed: gain ratio of each treatment by phase.
At the beginning of the experiment and on day 14, 28 and 42, a pig with a body weight closer to the average weight of the pigs in each pen was used to collect blood samples via the jugular vein and stored in a 2 mL K2 EDTA tube. Blood samples were processed from two to four hours after collection to determine the blood cell count using a hematology analysis system (Mindray, BC-2800Vet, China). Additionally, sterile swabs were used to collect fresh fecal samples from two pigs per pen. The samples were weighed and dried in an oven (Yamato DKN810 mechanical convection oven, New York, USA) for 72 hours to determine the percentage of fecal dry matter (FDM).
Statistical analysis
The performance data were analyzed using the PROC ANOVA of SAS (SAS Institute Inc., Cary, NC, USA) as a completely randomized block design, with diets as main factor, initial body weight as the block effect and pens as the experimental unit for ANOVA. For fecal consistency and blood data, the PROC ANOVA of SAS (Cary NC, USA) was used, as a repeated measurement analysis. Diets and days of sampling were the factors of the experiment, and their interaction was evaluated. The Tukey method was used to contrast the mean differences. Statistically, p values <0.05 were considered as differences, while p values < 0.10 as a tendency to differ. The graphs were made using Graph Pad Prism (V.8.0.2, San Diego, CA, USA).
Results
Pigs supplemented with high level of zinc had a higher ADG compared to pigs fed the control diet or antibiotic diet during P1 and P2 (p < 0.05). In the P3; pigs fed control diet and antibiotic diet had similar ADG and both were higher than those pigs fed with high level of zinc (p < 0.05). In the overall phase (P1-3), pigs fed with high level of zinc had higher ADG than the antibiotic group (p < 0.05), but similar response to the control group (p > 0.05). These results support the response obtained on the final body weight of day 14 and 28, where pigs fed with high level of zinc were heavier than pigs fed antibiotic diet or control diet (p < 0.05; Table 3).
Table 3 Effects of high levels of zinc, as an alternative to antibiotics, on productive performance in weaned pigs. Swine Research Center of The Universidad de Panama. Chiriquí, Panama. 2023.
| Treatment (Trt) | SEM | Pr > F | |||
| 1 | 2 | 3 | Trt | ||
| BW*, lb | |||||
| d 0 | 6.94 | 6.91 | 6.98 | 0.066 | 0.906 |
| d 14 | 7.74a | 7.85a | 8.18b | 0.069 | 0.002 |
| d 28 | 12.76ª | 12.08ª | 14.25b | 0.212 | <0.0001 |
| d 42 | 21.18ab | 20.44a | 21.92b | 0.252 | <0.0001 |
| ADG+, lb | |||||
| P1 (d 0-14) | 0.06ª | 0.06ª | 0.09b | 0.006 | 0.014 |
| P2 (d 15-28) | 0.36b | 0.30a | 0.43c | 0.015 | <0.0001 |
| P3 (d 29-42) | 0.60a | 0.59a | 0.55b | 0.015 | 0.034 |
| P1-3 (d 0-42) | 0.33ab | 0.32a | 0.35b | 0.006 | 0.009 |
| ADFI≠, lb | |||||
| P1 (d 0-14) | 0.18 | 0.21 | 0.22 | 0.027 | 0.564 |
| P2 (d 15-28) | 0.61 | 0.57 | 0.63 | 0.024 | 0.192 |
| P3 (d 29-42) | 1.06 | 1.08 | 1.04 | 0.027 | 0.569 |
| P1-3 (d 0-42) | 0.61 | 0.62 | 0.63 | 0.019 | 0.800 |
| F:G± | |||||
| P1 (d 0-14) | 3.19 | 3.28 | 2.67 | 0.248 | 0.213 |
| P2 (d 15-28) | 1.70ab | 1.88b | 1.46a | 0.070 | 0.008 |
| P3 (d 29-42) | 1.76 | 1.82 | 1.90 | 0.078 | 0.708 |
| P1-3 (d 0-42) | 1.81 | 1.93 | 1.77 | 0.058 | 0.165 |
*BW: Body weight, +ADG: average daily gain, ≠ADFI: average daily feed intake, ±F:G: feed: gain ratio, d: day, P: phase, SEM: standar error of mean. / *BW: peso vivo, +ADG: ganancia diaria de peso, ≠ADFI: consumo promedio de alimento diario, ±F:G: relación consumo: ganancia, d: día, p: fase, SEM: error estándar de la media.
Pigs fed with high level of zinc was one of the groups with the greatest weight at the end of the study, since it showed significant differences with respect to treatment with antibiotics (p < 0.05), but similar to the control group. Regarding ADFI, no significant differences (p > 0.05) were found among treatments in any phase. Also, no statistical differences were found in the F: G ratio in P1; while in P2, pigs fed with high level of zinc had lower F: G ratio than pigs fed with antibiotic (p < 0.05), with the control group as intermediate. In the p3 and the overall P1-3, there were not statistical differences among treatments regarding the F: G ratio (p > 0.05; Table 3).
Significant differences were found between treatments in the percentage of FDM, with the highest percentage in those pigs fed with high level of zinc (Figure 1A; p < 0.05). Similarly, there were significant differences between sampling days regarding FDM (p < 0.05), with the highest percentage, numerically, in day 42 (Figure 1B). Additionally, a tendency for the treatment per day interaction was found on FDM (p < 0.10), with high values for those supplemented with control diet at the end of the study (Figure 1C).
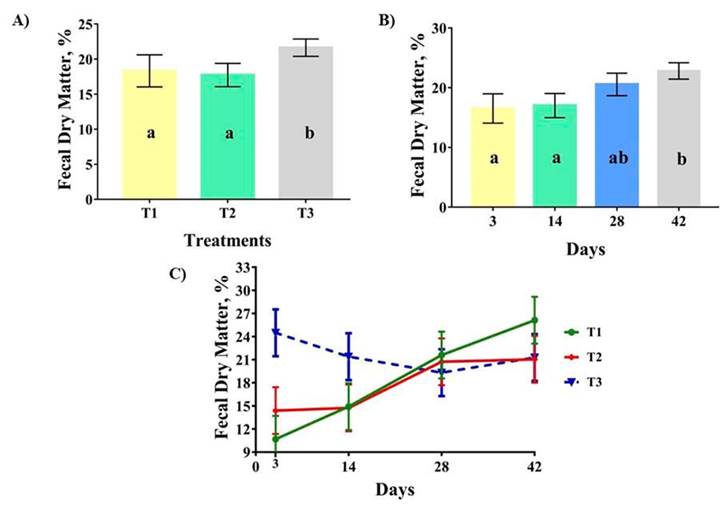
Figure 1 Effects of zinc level on fecal dry matter in nursery pigs. A). Fecal dry matter content in different treatments; B). Fecal dry matter content on different sampling days; and C). Interaction treatment per day on dry matter content of feces. Swine Research Center of the Universidad de Panama. Chiriquí, Panama. 2022.
Regarding the hematological profile, there were significant differences between treatments in the concentration of hemoglobin (HGB), with the highest concentration in pigs fed with high level of zinc (p < 0.05). In addition, significant differences were found in the mean corpuscular volume (MCV), where pigs fed with high level of zinc had higher concentration than those pigs fed antibiotic (p < 0.05), but similar to the control group. Regarding the white blood cells, there was a significant difference in the percentage of monocyte among treatments (p < 0.05), with the highest percentage in pigs fed with high level of zinc (Table 4). In addition, there were statistically significant differences between days of sampling for all the hematological variables evaluated (p < 0.05), except for the percentage of eosinophiles.
Table 4 Effects of high levels of zinc, as an alternative to antibiotics, on the hematological profile in nursery pigs. Swine research center of Universidad de Panama. Chiriquí, Panama. 2022.
| Treatment (Trt) | SEM | Pr > F | |||||
| 1 | 2 | 3 | Day | Trt | Day x Trt | ||
| RBC, x106/µ | 6.64 | 6.13 | 7.83 | 0.9679 | <.0001 | 0.4782 | 0.9652 |
| HGB, g/dL | 9.63a | 9.93a | 11.03b | 1.0999 | <.0001 | 0.0115 | 0.0535 |
| HCT, % | 30.46 | 29.91 | 33.08 | 3.7087 | 0.0003 | 0.1022 | 0.0541 |
| MCV, fL | 49.68b | 42.28a | 53.10b | 8.0210 | 0.0001 | 0.0094 | 0.8667 |
| MCH, pg | 15.58 | 15.11 | 17.00 | 3.1240 | <.0001 | 0.3949 | 0.8714 |
| MCHC, g/dL | 31.18 | 31.42 | 31.53 | 1.4889 | <.0001 | 0.7384 | 0.9978 |
| WBC, x103/µL | 16.01 | 16.18 | 14.48 | 4.1804 | 0.0005 | 0.5552 | 0.9775 |
| Limphocyte, % | 56.35 | 51.00 | 56.67 | 12.7519 | 0.0017 | 0.4895 | 0.8938 |
| Monocyte, % | 3.25a | 3.17a | 5.50b | 1.5811 | 0.0028 | 0.0017 | 0.4823 |
| Neutrophil, % | 39.67 | 44.83 | 36.82 | 12.7465 | 0.0041 | 0.3206 | 0.9053 |
| Eosinophil, % | 0.83 | 1.00 | 1.08 | 1.0672 | 0.9947 | 0.8441 | 0.9822 |
RBC: red blood cells; HGB: hemoglobin; HCT: hematocrit; MCV: mean corpuscular volume; MCH: corpuscular volume of hemoglobin; MCHC: mean corpuscular hemoglobin concentration; WBC: White blood cells, SEM: Standar error of mean. / RBC: glóbulos rojos; HGB: hemoglobina; HCT: hematocrito; VCM: volumen corpuscular medio; MCH: volumen corpuscular de hemoglobina; MCHC: concentración media de hemoglobina corpuscular; GB: glóbulos blancos, SEM: error estándar de la media.
Additionally, a tendency for treatment per day interaction was found in the concentration of HGB and the percentage of hematocrit (p < 0.10), with the higher level along the study in those pigs fed with high level of zinc (Table 4; Figure 2).
Discussion
The supplementation with high level of zinc improved the ADG by 0.03 kg and 0.13 kg compared to the control diet or antibiotic diet, respectively, during the first month after weaning. Similar results were reported by Kim et al. (2015), where pigs supplemented with high level of zinc (2500 ppm) showed better weight gain compared to those supplemented with antibiotic (120 ppm apramycin) or the basal diet. Similar weight gains for pigs on a zinc fortified diet versus pigs fed antibiotic, and both the zinc and antibiotic groups performed better than pigs supplemented with the basal diet (Han et al., 2011).
There was a reduction in ADG once feed was switched to a common diet without zinc inclusion during P3. Similar results were found by Mudarra et al. (2022) who reported a reduction in ADG when pigs were fed a diet devoid of high level of zinc during the last nursery phase (day 29 to 42) after a constant supply of high zinc in feed. The promoting effect of zinc on ADG appears to be dependent on its constant inclusion and not exerting an effect after its withdrawal, as could be evidenced by the antibiotic group in this study.
Even though the inclusion of antibiotic or high level of zinc did not exert any modulatory effect on feed consumption along the study, pigs under the high zinc diet, in P2, showed a higher utilization of nutrients than the antibiotic group, provided by the reduction in F: G ratio. This higher efficiency in nutrient utilization in pigs fed with high level of zinc could be due to the modulatory effect that zinc has been evidenced on digestive enzyme activity (Pieper et al., 2012) such as amylase, lipase, carboxypeptidase, trypsin, and chymotrypsin (Hedemann et al., 2006) as well as changes in the intestinal morphology, including increments in the duodenal villus height and villus height to crypt depth ratio (Lei & Kim, 2018).
The percentage of FDM is widely used as an indicator of digestive disorders in post-weaning pigs (Kim et al., 2008). Scientific evidence indicates that pigs fed with high level of zinc have better fecal consistency than pigs fed diet devoid of it (Oh et al., 2021). As previously mentioned by Kim et al. (2008), zinc has shown to generally improve the health status of small intestine through the thickness of the mucus layer. Hedemann et al. (2006) mentioned that the supplementation of high level of zinc, approximately 2500 ppm, stimulate the synthesis of mucins.
Mucin is a glycoprotein that composes the mucus layer of the GIT, and plays a key role in cell signaling pathway and barrier defense of the host epithelium from pathogenic microorganisms and their toxins (Grondin et al., 2020). The supplementation of high level of zinc might improve the health status of intestine through the synthesis of mucins, reducing the capacity of pathogens to adhere to the epithelium and the release their toxins, thus avoiding the onset of post-weaning diarrhea, and maintaining better fecal consistency, as reported in the percentage of FDM of pigs fed with high level of zinc in this study.
The pattern of FDM response was not similar among treatments along the experimental period. Mudarra et al. (2022) reported an increment of the percentage of FDM in pigs supplemented with 1600 ppm of zinc as pigs get older. Our results are not consistent with those reported by Mudarra et al. (2022) where pigs fed with high level of zinc had higher FDM on day 3, decreasing until day 28 and finalizing with similar percentage of FDM to the antibiotic group on day 42, while the control group maintain a constant increment along the study. Further studies have to be done to evaluate the modulatory effect of zinc oxide in FDM through the first month after weaning.
The supplementation of pigs with diets containing high level of zinc increased the hemoglobin concentration by 1.1 g/dL and 1.4 g/dL compared to the control group or those fed with antibiotic diet, respectively. The hemoglobin level in pigs is reduced after weaning due to low iron synthesis in the sow’s milk as well as low feed intake after weaning (Lee et al., 2008), having as consequence the presence of anemia during first month postweaning (Seip et al., 2020). The hemoglobin levels with concentrations close to 8 g/dL can be considered anemia (Lee et al., 2008), and it is related to reductions in productive performance in pigs (Knight & Dilger, 2018).
Anemia is mainly described as the reduction in the percentage of hematocrit based on a low concentration of red blood cells, as well as in a decrease in the concentration of hemoglobin in the blood (Jeng & Chen, 2022). The hemoglobin level is highly correlated with the percentage of hematocrit (Knight & Dilger, 2018). Our results agree with that reported by Knight and Dilger (2018), where the hemoglobin concentration throughout the study had a similar pattern to the percentage of hematocrit in all the evaluated treatments. Interestingly, the highest percentage of hematocrit and hemoglobin throughout the study was found in pigs supplemented with high level of zinc.
In mammals, erythropoiesis is regulated by the hormone erythropoietin (EPO), a humoral cytokine that targets erythroid cells and their progenitors (Broxmeyer, 2013). Konomi and Yokoi (2005) indicate that hematopoiesis is influenced by zinc level, where it has direct effect in the synthesis of EPO, as well as for the expansion of erythrocytic compartment (Huber & Cousins, 1993). In previous studies, zinc supplementation stimulate erythropoiesis (Feng et al., 2019) and red blood cell formation in the bone marrow in rats (Chen et al., 2018). In this study, the inclusion of pharmacological levels of zinc might increase the total percentage of hematocrits, and with this an increment in the concentration of hemoglobin.
Zinc is involved in more than 300 enzymatic reactions, and a constant flow of zinc is important for the proliferation and differentiation of immune system cells (Haase & Rink, 2009). As mentioned before, zinc plays a key role in hematopoiesis (Konomi & Yokoi, 2005), with several evidence of its effect on proliferation of myeloid cells (Haase & Rink, 2009; Martin et al., 1991; Sapkota & Knoell, 2018). In addition to erythrocytes and mast cells, the myeloid lineage cells give rise to polymorphonuclear neutrophils, monocytes, and macrophages which are the first line of defense to recognize and eliminate pathogens. In short, zinc supplementation stimulate the proliferation of myeloid stem cells, thus increasing the presence of monocytes in blood.
Conclusion
The supplementation between 1600 ppm and 2000 ppm as zinc oxide during the first month after weaning increases the hemoglobin concentration, reduces the incidence of diarrhea, and improve growth performance in nursery pigs. The use of pharmacological level of zinc oxide might replace the promoting effect of antibiotic during the nursery period.
















