Introduction
The Amazon viper or the common lancehead, Bothrops atrox (L., 1758) (Squamata: Viperidae), is medium size and slightly robust snake (Fraga et al., 2013; Monteiro et al., 2020). This species can be differentiated from other species of snakes by having a spear-shaped head, with an olive-green color, uniform or slightly darker comparing with its body, generally without spots (Da Silva et al., 2017). Besides that, B. atrox presents a dark-brown post-orbital line starting located on the eyes to the sides of the oral cavity, covering the last three supralabial scales, showing a golden bronze iris color (Fraga et al., 2013; Monteiro et al., 2020).
This snake has a wide distribution in the Neotropical region, inhabiting the Andes in Colombia, and other South American countries, such as Ecuador, Peru, Bolivia, Guyana, Surinam, and Brazil, in an altitudinal range from the sea level to 1 200 meters (Fraga et al., 2013; Da Silva et al., 2017). Also, B. atrox is predominantly nocturnal, but daytime activities are not uncommon (Egler et al., 1996). The diet includes invertebrates, fish, amphibians, reptiles, and small mammals (Bisneto & Kaefer, 2019). Recent studies regarding the biology of this species, reveal that their juveniles are arboreal and feed on ectodermal prey, while the terrestrial adults feed on endothermic prey, being possible that these preys may be intermediate hosts for endoparasites, such as nematodes and trematodes (Fraga et al., 2013; Rodrigues et al., 2016).
Currently, there are 150 valid species of parasitic trematodes of reptiles in South America, with 112 species recorded to the Brazilian territory (Kohn & Fernandes, 2014). These parasites can be found in various sites of infection in reptiles, and specifically, into the Plagiorchiidae, they have been recorded from the intestine, lungs, tongue, trachea, esophagus, stomach, ureters, kidney, rectum, cloaca, oral cavity, oviduct, liver, heart, spleen, and mesentery (Kohn & Fernandes, 2014).
Sticholecitha serpentisPrudhoe, 1949 (Trematoda: Plagiorchiidae), the only species of the genus, described in Surinam in the esophagus of the snake Chironius carinatus L., 1758 (Squamata: Colubridae), is a poorly known parasite with an unclear taxonomy. This species has also been reported in Bothrops moojeni Hoge, 1966 (Squamata: Viperidae) and Xenodon severus (L., 1758) (Squamata: Colubridae), both from São Paulo state, in Brazil (Kohn & Fernandes, 2014).
Currently, there are different studies about the morphology of S. serpentis already highlighted by Prudhoe (1949), Artigas and Perez (1969), Corrêa et al. (1990), Da Silva et al. (1999), Silva and Barrella (2002), Barrella and Silva (2003), Da Silva (2004), Silva (2005) and Silva et al. (2005). Nevertheless, those studies did not include enough morphological aspects based on SEM micrographs, as well as did not offer enough details on the surface ultrastructure. Additionally, a fresh view of the parasite shows the keel dorsal view without histological sections.
In the original description, S. serpentis was characterized by having externally well-developed suckers, with the ventral sucker medial to the body, and a slightly protruding genital pore on the left side. Their life cycle involves two secondary hosts, an invertebrate (gastropod) and a vertebrate (fish or amphibian) (Prudhoe, 1949). Studies on S. serpentis have been limited to the esophagus and oral cavity of snakes (Barrella & Silva, 2003; Prudhoe, 1949; Silva et al., 2005).
The objective of this study is to provide enough data about the characters of S. serpentis using SEM and light microscopy micrographs, as well as, about the parasitism relationship between this trematode and B. atrox collected in the Brazilian Amazon.
Materials and methods
Host collection and capture area: The collections occurred from January to May 2010 in an area of the Amazonian Forest, located in the Tapajós National Forest-FLONA conservation unit, West of Pará State, Brazil (Fig. 1). The captures were made using the active visual search method, limited by time on trails, secondary roads, gallery forests, and residential areas, in addition to occasional encounters as described by Oliveira and Martins (2001).
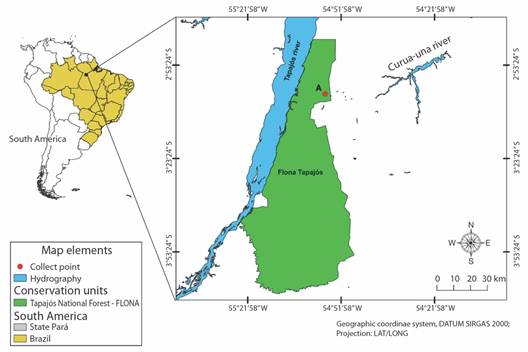
Fig. 1 Map showing the collection area of Bothrops atrox, hosts of Sticolecitha serpentis, in the conservation unit of the Tapajós National Forest, Pará State, Brazil.
The captured specimens were placed in plastic boxes and sent to the Zoological Research Laboratory of the Unama Centro Universitário da Amazônia, in Santarém municipality, Pará State. In the laboratory, the snakes were submitted to physical examination and the parasites observed were collected for identification. After that, the venom was extracted for biochemical studies, the snakes were deposited at the Coleção Herpetológica from Zoological Research Laboratory by accession numbers: HMC 66, 67, 60, 186, 59, 42, 156, 78, 141, and 63.
Collection of parasites and morphological analysis: During this examination, abnormalities in the oral cavity with the parasites were observed collected with tweezers, fixed and stored in an alcoholic solution of 70 %. Sex, weight measurements, snout-vent length (SVL), and tail length (TL) were also determined for the B. atrox specimens.
The trematodes collected were prepared by the Carmine method, using Langeron acid in permanent slides with Canadian balm (Amato & Amato, 2010), and the morphological identification was carried out according to Yamaguti (1971) and Travassos et al. (1969). Two specimens of S. serpentis have been deposited in UFOPA Parasitological Collection, at the Laboratório de Ecologia e Comportamento Animal (LECAn) under access numbers: UFOPA-P(Tre)001 and UFOPA-P(Tre)002. Also, the material was analyzed under light microscopy with a magnification of 100X to 400X at the Microscopy and Sample Collection Laboratory of the Universidade Federal do Oeste do Pará (UFOPA), Pará, Brazil. Samples were photographed using a Zeiss Axioplan optical microscope with an Axiocam ERc 5s camera. The morphometric study was carried out with the Blue Zen software 2nd edition. As well as for the SEM micrographs, were obtained using a Digital Scanning Microscope FEI, Quanta 250, located at the Laboratório de Biologia Celular, Instituto Butantan, São Paulo, Brazil.
Scanning electron microscopy: External morphology was evaluated by scanning electron microscopy (SEM) previously fixed in ethyl alcohol (70 %), transferred to glutaraldehyde solution (2.5 %) in 0.15 M phosphate buffer (pH 7.3), and then subsequently fixed in osmium tetroxide (1 %) in the same buffer, for 2 h. The samples were dehydrated in an increasing sequence of ethyl alcohol solutions and washed in a solution of distilled water and filtered water (1:1). Dehydration was carried out with an increasing sequence of ethyl alcohol solutions, and drying was carried out employing a critical point in CPD 020 (Balzer Union), with liquid CO2. The samples were placed on double-sided tape in Stub and covered with a gold-palladium jet.
Data analysis: The ecological terms percent infected (%), mean intensity, and mean abundance (IC = confidence intervals) were used according to Bush et al. (1997). These parameters were calculated using Quantitative Parasitology 3.0 software (Reiczigel et al., 2019).
Results
Ten specimens of B. atrox were examined, seven females and three males with SVL: 580.8 mm ± 118.52 mm, and TL: 97.6 mm ± 63.68 mm. During the physical examination, three specimens presented parasitism by trematodes: HMC186 = 21 parasites in the mouth; HMC156 = 26 parasites; and HMC59 = 53 in the mouth and 19 in the esophagus. All the parasites found were adults and the morphometric measurements are shown in Table 1.
Table 1 Morphometry of the external and internal structures of adult individuals of Sticholecitha serpentis.
| Measured structures | Sticholecitha serpentis (n = 25) Present study | Sticholecitha serpentis (n = 25) Silva et al. 2005 | Sticholecitha serpentis (n = 25) Prudhoe, 1949 | ||
| Body | L | 3.30 (2.9-3.5) | 7.41 (6.23-8.12) | 6.8 (5.20-8.40) | |
| W | 1.00 (0.80-1.2) | 1.58 (1.39-1.68) | 1.6 (1.20-2.0) | ||
| Oral sucker | L | 0.38 (0.32-0.45) | 0.62 (0.56-0.71) | 0.56-0.65 (in diameter) | |
| W | 0.37 (0.31-0.42) | 0.63 (0.61-0.66) | |||
| Acetabulum | L | 0.33 (0.28-0.40) | 0.79 (0.70-0.85) | - | |
| W | 0.31 (0.27-0.35) | 0.59 (0.53-0.63) | - | ||
| Pharynx | L | 0.19 (0.15-0.22) | 0.25 (0.21-0.30) | 0.21-0.25 (in diameter) | |
| W | 0.21 (0.17-0.24) | 0.28 (0.26-0.30) | |||
| Right testes | L | 0.26 (0.23-0.29) | 0.47 (0.43-0.51) | 0.62 (0.5-0.75) | |
| W | 0.30 (0.26-0.34) | 0.52 (0.46-0.56) | 0.47 (0.45-0.50) | ||
| Left testes | L | 0.25 (0.19-0.29) | 0.47 (0.42-0.55) | 0.45 (0.40-0.50) | |
| W | 0.23 (0.19-0.27) | 0.42 (0.38-0.44) | 0.47 (0.46-048) | ||
| Ovary | L | 0.20 (0.17-0.23) | 0.34 (0.29-0.38) | 0.48-0.50 (in diameter) | |
| W | 0.22 (0.18-0.24) | 0.34 (0.32-0.37) | |||
L: Length, W: Width; the values in parentheses (in mm) represent the range).
The preparations described in the Methodology were made and the 25 trematodes were identified as S. serpentis (Trematoda: Plagiorchiidae). More details are in the ‘Taxonomic Summary’ section. The percent infected of S. serpentis on their hosts was 30 %, with a mean infection intensity of 39.67 (IC = 21.0-56.67) worms/host and a mean abundance of 11.90 (IC = 2.10-33.50).
Taxonomic Summary
Class Trematoda Rudolphi, 1808
Subclass Digenea
Family Plagiorchiidae Lühe, 1901
Sticholecitha serpentisPrudhoe, 1949
Host: Bothrops atrox (Squamata: Viperidae).
Geographical location: Amazonian Forest, Pará State, Brazil (3º 02’ 39.07” S & 54º 57’ 04.24” W).
Infection site: Oral cavity and esophagus.
Morphological description: The description presented here is based on the analysis of 25 specimens of S. serpentis examined. In nature, it has a milky color with a well-highlighted cup-shaped oral sucker in the anterior region and a cup-shaped ventral sucker located in the equatorial region. The genital pore stands out with a slightly lighter placement than the body on the left lateral extremity. The body has a navicular shape, being flat or slightly concave ventrally, with an evident elevation of a keel dorsally, having a thin cuticle armed with sharp spines.
The large leafy-shaped uterus, when considered its proportion to the total body size, can occupy up to one-third of the total size, presenting a slightly yellowish color (Fig. 2A). In Carmine, the pharynx stands out wider than long, and the testicles can present rounded shapes varying the globose with a smooth appearance. The cirrus pouch is elongated in shape and can extend from the ventral sucker region to the genital pore.
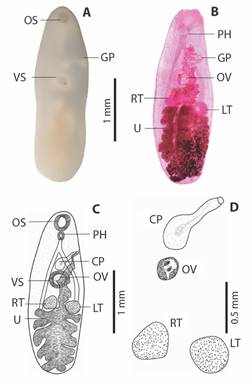
Fig. 2 Adult specimen of Sticolecitha serpentis. A. External structures observed in natura (OS-Oral Sucker Cup Shaped, GP-Genital Pore, AC-Acetabulum), B. Internal structures observed after staining in Carmine (PH-Pharynx, GP-Genital Pore, OV-Ovary, RT-Right testes, LT-Left testes, U-Leaf Shaped Uterus), C. Schematic drawing (OS-Oral Sucker Cup Shaped, GP-Genital Pore, VS-Ventral Sucker, PH-Pharynx, CP-Cirrus Pouch, GP-Genital Pore, OV-Ovary, RT-Right testes, LT-Left testes, U-Uterus), D. Reproductive organs (CP-Cirrus Pouch, OV-Ovary, RT-Right testes, LT-Left testes).
The lobous ovary located close to the ventral sucker, with small vitellogenic glands in granular-lobed form, lateralized to the body (Fig. 2B). It was possible to compare the variations with parasites from other hosts (Table 1), demonstrating that the parasite in this study is smaller in comparison to others.
SEM photographs are displayed an elongated body in boat-shaped (Fig. 3), with details of the oral sucker (Fig. 3A) and the acetabulum (Fig. 3B), presented body longer than wide, with tegument covered by microspines, and two well-defined spines on the ventral and posterior regions of the body (Fig. 3C). The oral sucker is oval, with circular striated grooves at the base, and a robust labial portion protruding at the apical region, which is longer than it is wide. The oral sucker measures 160 µm long by 150 µm wide, and there are no spines along the entire edge (Fig. 3A). In addition, the dermis has a raised, volcano-like area with spines at the base and center, but none at the apical margins (Fig. 3B). The dermal border is circular and has grooves due to its muscular origin and fixation, making it a muscularized structure. The cavity on the inner edge’s measures 109 µm in length by 85 µm in width, while on the outer edges, it measures 91 µm in length by 98 µm in width (Fig. 3B).
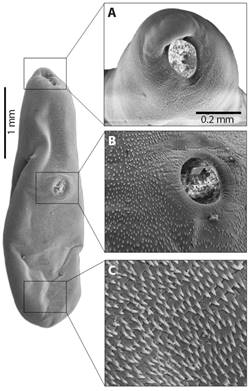
Fig. 3 Photographs on SEM of adult specimen of Sticolecitha serpentis. A. Oral sucker, B. Ventral Sucker, C. Details of tegument with microspines.
The dorsal keel appears as a prominent elevation from a horizontal view of the parasite’s body, with discrete undulations in the median to caudal region, with a marked lowering in the first anterior third (Fig. 4). In addition, the first anterior third is marked by the presence of rugosities and furrows in a longitudinal direction. In the second third, from the median region to the end of the distal caudal region of the parasite body, the roughness and furrows are horizontal (Fig. 4).
Discussion
This is the first record of the association of S. serpentis and B. atrox. Until now, the records of parasitism by this trematode are restricted to Surinam, parasitizing the C. carinatus (Prudhoe, 1949); and in Porto Primavera, São Paulo State, Brazil, parasitizing B. moojeni (Barrella & Silva, 2003; Silva et al., 2005) and unidentified locality in Brazil, parasitizing X. severus (Freitas, 1956). So, this study reports the new host to S. serpentis and the first record of this species parasitizing a viperid snake in the Brazilian Amazon.
As shown in other studies involving Plagiorchiidae and other trematodes (Byrd & Scofield, 1952; Byrd & Scofield, 1954; Corrêa et al., 1990; Pinto & De Melo, 2012; Sogandares-Bernal & Grenier, 1971; Sue & Platt, 1999), the life cycle of S. serpentis in B. atrox, can be considered invertebrate and vertebrate intermediate hosts. We conjecture that the eggs of the parasite are dispersed in the aquatic or terrestrial habitat, where they are consumed by snails, which are considered to be the first hosts. The secondary intermediate host, which in this case could be an anuran, can be infected in two different ways: by ingestion of invertebrates infected with metacercariae or by ingestion, aspiration, or coming into direct contact with infective metacercariae in their anuran larval forms (tadpoles). Consequently, considering the feeding habits of B. atrox, which includes amphibians in its diet (Bisneto & Kaefer, 2019), the intermediate host is preyed upon and thus the parasite completes its life cycle in its definitive host.
Ingesting parasitized adult amphibians is suggested as a hypothesis of infection in the studies of Corrêa et al. (1990), where a Bothrops insularis (Amaral, 1922) (Squamata: Viperidae) kept in captivity with controlled diet became infected with Ochetosoma heterocoelium (Travassos, 1921) after feeding on a Hyla sp. amphibian, suggesting this as a probable secondary host. Anurans are predators of gastropods and are known components of the diet of B. atrox (Bisneto & Kaefer, 2019; Da Silva et al., 2010; Solé et al., 2017; Solé et al., 2019).
Our study found a lower infection frequency compared to Barrella and Silva (2003) results. They reported a total prevalence of 68 % in fifty analyzed B. moojeni, with 1 to 15 trematodes per animal being the most common finding. Only one snake had a total of 51 parasites. We analyzed the same infection sites as Barrella and Silva (2003) and found 21 to 51 parasites in the mouths of three B. atrox. Only one individual was infected with 19 S. serpentis in the esophagus. These parasites are typically present in high numbers within their hosts. Due to the low number of studies involving S. serpentis, there is limited integrated information regarding its distribution, taxonomy, parasite ecology, and molecular characterization. As a result, the systematic of this species is poorly known (Artigas & Perez, 1969; Prudhoe, 1949). It should be emphasized that there are currently no DNA sequences available for S. serpentis in any nucleotide database.
Also, the present study aimed to contribute to the knowledge of the species S. serpentis, with detailed morphological descriptions and SEM images that had never been done before. The new aspects presented, demonstrate that through the usual technique for trematodes, unfortunately, the dorsal keel is pressed, making its visualization difficult. However, in histological study, the dorsal keel is visualized, as presented by Silva (2005). Nevertheless, in our study, we present a fresh parasite visualization, as well as SEM photographs that demonstrate the well-defined dorsal keel. Therefore, we are showing, through different techniques, information that will help in the identification of S. serpentis in further studies. Furthermore, we emphasize the need for molecular studies to better understand the phylogenetic position of the group and the relationship with their vertebrate hosts.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives. Ethics approval: This study was approved by the Animal Use Committee from the Federal University of Western Pará (Authorization #1020180044). Wild animals were collected with the approval from the Brazilian Institute of Environment and Renewable Natural Resources, authorized through the Biodiversity Information and Authorization System-SISBIO nº. 66047-4.












 uBio
uBio 



