Introduction
Macrobrachium amazonicum (Heller, 1862) is a freshwater prawn with a wide distribution in South America and is the native species with the most widespread occurrence in the inland waters of Amazonia (Odinetz-Collart & Moreira, 1993). Despite being endemic to the Amazon region (Odinetz-Collart, 1991), it is also found in the basins of the Paraná and São Francisco rivers (Bialetzki et al., 1997; Sampaio et al., 2007), as well as many other hydrographic basins in South (Kensley & Walker, 1982; Melo, 2003; Valencia & Campos, 2007) and Central America (Vergamini et al., 2011).
Parasitic interactions between isopods of Bopyridae and prawns are common and reasonably well known in the scientific literature, mainly because many parasitic isopods are crustacean-specific and feed on their hemolymph (Boxshall et al., 2005). Generally, the growth of prawns is significantly affected by parasitic infestation, which results in a decrease in the production potential of the prawn (Conner & Bauer, 2010). Bopyrid isopods for instance, are ectoparasites the genus Probopyrus with 46 species widely distributed in tropical and subtropical areas of the globe, commonly found in brackish to freshwater (e.g estuaries) and freshwater (e.g. lakes and rivers) (Jiménez & Vargas, 1990; Jayasree et al., 2001; Chinabut, 2002). They are rarely found under aquaculture conditions but are common in the wild among freshwater organisms with genus Macrobrachium as their definitive hosts (Chaplin-Ebanks & Curran, 2005).
The presence of Probopyrus is easily spotted due to the noticeable swelling in the gills of the prawns (Marina et al., 2011), and it remains until the host dies. In M. amazonicum have been reported, a number of studies have related on the specie Probopyrus bithyni, Richardson, 1904 (Oddinetz-Collart, 1990; Lima-Corrêa et al., 2018) for example, found evidence of a stable interaction between the two species, supported by data on the infestation rates and life cycle of the host, based on specimens collected on the lower Tocantins River, in the Brazilian state of Pará, given that the body length of the female isopods correlated positively with that of the prawn host (Odinetz-Collart, 1991). More recently, Lima-Corrêa et al. (2018) described histopathological alterations in the gills of M. amazonicum specimens collected from the lower Amazon River, in Pará state, caused by P. bithynis infestation. However, such relation for the species Probopyrus floridensis Richardson, 1904 and Probopyrus pandalicola Packard, 1879 has not been done in previous studies.
Information on the intensity of infection, specificity and even the geographic range of ectoparasitic isopods from the family Bopyridae is scarce for most species, compared with the number of reports on their taxonomy. Only six species of Bopyridea and one of Entoniscidae have been recorded from 10 host species (Román-Contreras, 2008; Román-Contreras & Martínez-Mayén, 2011). The apparently low number of bopyrids in the Brazil region is probably due to limited sampling effort (Shields et al., 2015) or to their omission from studies on the ecological aspects of their hosts (Boyko & Williams, 2009). In this paper, we register prevalence, abundance, the parasite’s preference for host sex and evaluate whether parasitism influences the condition facto host and a new locality and host record for ectoparasitic species of the genus Probopyrus, associated with M. amazonicum captured from in the mouth and lower Amazon River.
Material and methods
Prawn study, collection and analysis areas: We conducted captures at two locations: the mouth of the Mazagão River in the Municipality of Mazagão, State of Amapá (00º15’39.9’’ S & 051º20’42.3’’ W) and Marrecas Island in the Municipality of Santarém, State of Pará (02º12’19.3’ S & 054º46’17.9’’ W) as shown in (Fig. 1). The Mazagão municipality region connects to numerous drainage channels of varying sizes and depths that are affected by the daily tide. On Marrecas Island, the hydrodynamics are different as there is no influence from the tide, and the water levels varies only due to seasonal floods. These regions experience temperatures ranging from 24 to 33 °C (Oliveira et al., 2020). The Eastern Amazon has two distinct seasonal periods related to precipitation: the rainy season from December to May and the less rainy season from June to November (Oliveira et al., 2020). These periods coincide with variations in the water level of the Amazon River, which result in floods during the rainy season and low waters in the less rainy season.
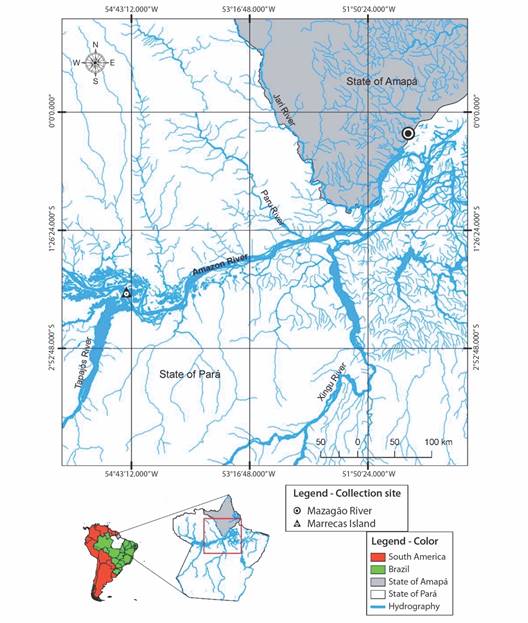
Fig. 1 The location of M. amazonicum captures areas in the mouth and lower Amazon between May 2017 and April 2018, and July 2021 to May. The black circle (l) represents Mazagão, while the green triangle (s) represents Marrecas Island.
Between May 2017 and April 2018, and July to May 2021, 40 pit traps (20 in each study region) known locally as matapi (Vieira, 2003) were used to collect monthly specimens of M. amazonicum. The captured organisms were transported alive in containers with water to the laboratory and stored at -20 °C before use. The identification of the samples was done using the taxonomic keys of Holthuis (1952) and Melo (2003). Sexual differentiation was determined by the presence or absence of the appendix masculine in the second pair of pleopods for males and females, respectively (Ismael & New, 2000). The prawns with large parasites were visually detected by the bulge in the (Carvalho et al., 2014), exoskeleton in the gill chamber. Parasites of small size or immature females were recognized by the pattern of spots on the branchiostegite (Conner & Bauer, 2010).
The parasites were identified based on the original descriptions presented by Lemos de Castro and Silva (1985), Román-Contreras (2004) and Paul et al. (2010). References are provided for the taxonomic authority of all parasitic taxa, but not for those of hosts.
Data analysis: Difference in parasitism levels between male and female prawn using the chi-square (χ2) test (Zar, 1999) to determine if they deviated from the expected 1:1 ratio. The ecological terms of prevalence (P) and mean abundance (MA) followed the definitions of Bush et al. (1997). The monthly prevalence and abundance data were then tested using the Student (t) test to compare the variables between the two areas of study after assessing normality and homoscedasticity. The equation Kn = Wt/We (Le-Cren, 1951) was used to determine the relative condition factor of prawns. Thirty parasitized and thirty non-parasitized prawn were selected from each collection site/month to obtain data on total body mass (Wt) and standard length (Lp). The values were adjusted to the model Wt = a.Lpb using the least squares method after the logarithmic transformation of the values of the total mass/standard length ratio curve. The coefficients “a” and “b” were estimated as they were used in the calculation of the theoretically expected values of total weight (We) for a given value of Lt through the equation: We = a.Lpb. The Mann-Whitney (U) test compared the mean Kn values of parasitized and non-parasitized prawn to the theoretical value 1 (Le-Cren, 1951). The test was also used to determine any differences in the mean Kn between parasitized and non-parasitized prawn in both study regions, with significant differences being considered when P < 0.05.
Results
Total of 6 796 prawns were caught during the study, with 4 163 females and 2 633 males. The majority of the prawn, 4 132, were obtained in Mazagão with the remaining 2 664 found in Marrecas Island. The average monthly catch was 531.66 specimens. The sex ratio in both areas was skewed towards females, with ratios of 1.32 females to 1 male in Mazagão and 2.34 females to 1 male on the Marrecas island. Among the females caught, 1 579 were carrying eggs, with 871 found in Mazagão and 708 in Marrecas Island. The ovigerous females in Mazagão were larger in size (56.85 ± 11.34) than those found on Marrecas Island (43.57 ± 10.48).
Out of the total catch, 216 prawn (3.7 %) were found to be parasitized, with prevalence of 3.2 % in Mazagão and 3.1 % in Marrecas Island. The majority of the parasitized prawn were female (163) with the rest being male (53). None of the ovigerous females were found to be parasitized. Most of the parasitized prawn had only one gill chamber infected (98.1 %). The parasites were always found in pairs, with a 1 female to 1 male sex ratio.
Examining the parasites’ morphology allowed for identifying the three species P. pandalicola (Fig. 2), P. bithynis (Fig. 3), and P. floridensis (Fig. 4). As shown in Mazagão, 133 parasites were collected, with 53 showing morphological traits consistent with P. pandalicola, 48 with P. bithynis, and 32 with P. floridensis. On the Marrecas Island, 83 parasites were collected, with 45 displaying morphological characteristics of P. pandalicola and 38 of P. bithynis; P. floridensis was not found in this area.
The specimens P. pandalicola the pereomeres are distinct dorsally and separated by notches laterally; lateral margins of all pereomeres are greatly reflexed ventrally. Abdomen with all somites well developed, fused in the center but separated laterally by deep incisions. Telson with rounded contour and pointed distal end. The pleopods form five pairs of rounded tubercles, one for each pair. Pleopoda are present in pairs of small, rounded processes, one pair on each segment of the abdomen. Eyes are present. All regions and segments of the male body are distinct dorsally and separated laterally (Fig. 2C). The sides of the pereon are subparallel, but the pleon suddenly becomes broader than the pereon. Most of the dorsal surface of the body has dark brown pigment.
The specimens P. bithynis, based on the illustrations and descriptions presented by Richardson in 1904, we propose that the specimens that differed from the type P. bithynis are different species. We observed that the absence of anterolateral processes on the head of the female, the presence of patches of black on the lateral margins of all the segments of the thorax on one side of the body, and the presence of a bilobed telson are morphologically similar to what was observed in specimens later designated by Lemos de Castro & Brasil-Lima (1974).
The specimens P. floridensis, based on the illustrations the female’s body is light brown and has distinct parts such as the head, abdomen, ovarian bosses and light yellow epimera. There are dark markings all over the thorax and a few black lines on the abdomen. The incubatory lamellae are almost entirely covered with black markings, giving a uniformly dark color. On the ventral side of the thorax, the lateral parts have black markings with yellow areas separating them, and all the legs on this side are yellow (Fig. 4). The legs on the opposite side are dark.
Based on grouped species, there was no significant difference in the monthly prevalence of Probopyrus-infected prawn in the two locations (t = 0.33; p = 0.738). In Mazagão, the highest prevalence rates were observed for P. floridensis in May (1.9 %), P. bithynis in July (2.1 %), and P. pandalicola in March (1.9 %) (Table 1). On the Marrecas Island, the highest prevalences were observed in February, with 3.4 % for P. pandalicola and 2.8 % for P. bithynis (Table 1).
Table 1 Monthly prevalence of the parasitized Probopyrus pandalicola, Probopyrus bithynis, and Probopyrus floridensis sampled in the shrimp Macrobrachium amazonicum from regions of Mazagão-AP and Marrecas Island-PA from April 2017 to March 2018.
| Months | Site | Prevalence (%) | |||||
| P. pandalicola | P. bithynis | P. floridensis | Grouped | ||||
| April | M | 1.08 | 1.08 | 0.36 | 2.53 | ||
| MI | 0.71 | 0 | 0 | 0.71 | |||
| May | M | 0.99 | 1.98 | 1.98 | 4.55 | ||
| MI | 2.04 | 2.04 | 0 | 4.08 | |||
| June | M | 1.12 | 1.30 | 0.93 | 3.35 | ||
| MI | 1.68 | 2.35 | 0 | 4.03 | |||
| July | M | 1.09 | 2.18 | 0.66 | 3.93 | ||
| MI | 0.86 | 0.22 | 0 | 1.08 | |||
| August | M | 1.07 | 1.43 | 0.54 | 3.04 | ||
| MI | 1.17 | 1.95 | 0 | 3.13 | |||
| September | M | 1.12 | 1.50 | 0.75 | 3.36 | ||
| MI | 2.48 | 1.42 | 0 | 3.90 | |||
| October | M | 0.72 | 2.88 | 0 | 3.60 | ||
| MI | 1.49 | 1.98 | 0 | 3.47 | |||
| November | M | 0.89 | 1.12 | 0.22 | 2.23 | ||
| MI | 0.56 | 0.37 | 0 | 1.12 | |||
| December | M | 0.76 | 0.76 | 1.53 | 3.05 | ||
| MI | 3.48 | 1.74 | 0 | 5.22 | |||
| January | M | 1.25 | 0.93 | 0.62 | 2.80 | ||
| MI | 1.55 | 2.58 | 0 | 4.12 | |||
| February | M | 0.67 | 2.0 | 1.0 | 3.67 | ||
| MI | 3.47 | 2.89 | 0 | 6.36 | |||
| March | M | 1.93 | 0.97 | 0.48 | 3.67 | ||
| MI | 2.11 | 1.05 | 0 | 3.16 | |||
| M: mean and sd | 1.05 ± 0.31 | 1.51 ± 0.60 | 0.75 ± 0.52 | 3.32 ± 0.67 | |||
| MI: mean and sd | 1.80 ± 0.93 | 1.55 ± 0.91 | 0 | 3.36 ± 1.62 | |||
Abbreviations: M - Mazagão, MI - Marrecas Island.
There was no significant difference in the monthly abundance of grouped parasite species between the various locations (t = 0.32, p = 0.739). In Mazagão, P. pandalicola and P. bithynis had the highest average abundance values in the region, with values of 0.0105 ± 0.0031 for each species (Table 2). On Marrecas Island, the species P. pandalicola had the highest average abundance value of 0.0179 ± 0.0093 (Table 2).
Table 2 Monthly abundance of the parasitized Probopyrus pandalicola, Probopyrus bithynis, and Probopyrus floridensis sampled in the shrimp Macrobrachium amazonicum from regions of Mazagão-AP and Marrecas Island-PA from April 2017 to March 2018.
| Months | Site | Abundance mean | |||||
| P. pandalicola | P. bithynis | P. floridensis | Grouped | ||||
| April | M | 0.0108 | 0.0108 | 0.0036 | 0.0252 | ||
| MI | 0.0071 | 0 | 0 | 0.0071 | |||
| May | M | 0.0099 | 0.0099 | 0.0198 | 0.0495 | ||
| MI | 0.0204 | 0.0204 | 0 | 0.0408 | |||
| June | M | 0.0111 | 0.0111 | 0.0093 | 0.0335 | ||
| MI | 0.0167 | 0.0234 | 0 | 0.0402 | |||
| July | M | 0.0109 | 0.0109 | 0.0065 | 0.0393 | ||
| MI | 0.0086 | 0.0021 | 0 | 0.0107 | |||
| August | M | 0.0107 | 0.0107 | 0.0053 | 0.0304 | ||
| MI | 0.0117 | 0.0195 | 0 | 0.0312 | |||
| September | M | 0.0112 | 0.0112 | 0.0074 | 0.0336 | ||
| MI | 0.0248 | 0.0141 | 0 | 0.0390 | |||
| October | M | 0.0071 | 0.0071 | 0 | 0.0359 | ||
| MI | 0.0148 | 0.0198 | 0 | 0.0390 | |||
| November | M | 0.0089 | 0.0089 | 0.0022 | 0.0223 | ||
| MI | 0.0055 | 0.0198 | 0 | 0.0346 | |||
| December | M | 0.0076 | 0.0076 | 0.0152 | 0.0305 | ||
| MI | 0.0347 | 0.0173 | 0 | 0.0521 | |||
| January | M | 0.0124 | 0.0124 | 0.0062 | 0.0280 | ||
| MI | 0.0154 | 0.0257 | 0 | 0.0413 | |||
| February | M | 0.0066 | 0.0066 | 0.0100 | 0.0366 | ||
| MI | 0.0346 | 0.0289 | 0 | 0.0635 | |||
| March | M | 0.0193 | 0.0193 | 0.0048 | 0.0338 | ||
| MI | 0.0210 | 0.0105 | 0 | 0.0315 | |||
| M: mean and sd | 0.0105 ± 0.0031 | 0.0105 ± 0.0031 | 0.0075 ± 0.0052 | 0.03325 ± 0.0067 | |||
| MI: mean and sd | 0.0179 ± 0.0093 | 0.0154 ± 0.0091 | 0 | 0.0336 ± 0.0162 | |||
Abbreviations: M - Mazagão, MI - Marrecas Island.
The prawn parasitized in Mazagão and Marrecas Island had lower than expected condition factors, with a mean of 0.85 ± 0.55 for Ilha de Marrecas and 0.88 ± 0.44 for Mazagão. The condition factor of shrimp infested with Probopyrus (grouped species) differed significantly from those not infested in both locations, with a t-value of -2.76 and P-value of 0.006 in Mazagão (Fig. 5A) and a t-value of -2.27 and P-value of 0.024 in Marrecas Island (Fig. 5B).
Discussion
The M. amazonicum females were more abundant than males in both areas of study, corroborating to be expected for estuarine and coastal populations of this species in the Amazon region (Freire et al., 2012; Lima et al., 2014; Sampaio et al., 2007). Regarding ovigerous females’ standard length, it was found that female M. amazonicum was larger in Mazagão than in Marrecas. This difference in length may be associated with the characteristic of the Mazagão region, with proximity to the mouth of the Amazon River and an area of intense tidal activity and daily transport of organic matter, which may be contributing to an ‘enrichment’ of this region. Then promoting better nutritional conditions for the growth of females. Thus, the standard length of females collected in Mazagão was similar to that reported in studies involving other estuarine areas in the Amazon region (Freire et al., 2012; Hayd & Anger, 2013). Likewise, the females collected in the Marrecas Island had similar lengths to the shrimps collected of Amazon River in continental areas (da Silva et al., 2004).
Female shrimp had a significantly higher prevalence of parasites compared to male shrimp. This is a common characteristic observed in the genus Probopyrus according to several sources (Truesdale & Mermilliod, 1977; Oddinetz-Collart, 1990; Lima-Corrêa et al., 2018). Additionally, all the infested females were found to be non-ovigerous, as reported by Vargas-Ceballos et al. (2016). This may be due to isopods, which act as castrating parasites that inhibit gonadal development, negatively affecting reproductive success (Conner & Bauer, 2010; Sánchez-Murillo, 2016). In the case of M. amazonicum, energy and nutrients that should be used for reproduction are instead diverted to the growth and reproduction of the parasite interrupting the gonadal maturation of the shrimp (Conner & Bauer, 2010).
In Mazagão and Marrecas Island was it possible to distinguish three clear morphological patterns that indicate the species P. pandalicola, P. bithynis, P. floridensis, based in original descriptions and illustrations are recognized as valid by the World Register of Marine Species (World Register of Marine Species [WoRMS], 2023). According to Nunes-Pralon et al. (2018), infestations of Probopyrus species are not limited to specific host species or genera, supported by the present study that reports three Probopyrus species infesting M. amazonicum. Thes Parasites of the genus Probopyrus have a well-established relationship with a number of different representatives of the family Palaemonidae, in particular, the prawns of the genus Macrobrachium (Markham, 1985; Saito et al., 2010). In the specific case of M. amazonicum, records of infestation by P. bithynis are restricted to the coastal region of the Amazon basin, in areas near the Tucuruí hydroelectric dam on the Tocantins River, which is approximately 300 km from the Atlantic Ocean (Odinetz-Collart, 1988; Odinetz-Collart, 1990). More recently, records of this parasitism were obtained from the lower Amazon, near the community of Maruim, in the municipality of Gurupá, around 400 km from the Atlantic Ocean, in the Brazilian state of Pará (Lima-Corrêa et al., 2018; Pereira et al., 2022).
According to Ribeiro & Horch (2023), P. pandalicola has widespread distribution in Brazil. See Ribeiro et al. (2019) for a discussion on the distribution and taxonomic history of this widespread species. Several studies have cited the Southern edge of P. pandalicola distribution as the state of São Paulo, Brazil, following Beck (1979). However, it should be noted that Beck (1979) considered P. floridensis to be a synonym of P. pandalicola and combined their distributions when discussing the species. As far as we have been able to discern through separating the two species in the literature, the southernmost location of P. pandalicola is in the state of Rio de Janeiro and the northernmost is in the state of Amapá, Brazil.
Recently, Pereira et al. (2022) conducted a study on Probopyrus sp. populations that parasitize M. amazonicum, which were sampled in coastal (Abaetetuba, Afuá, Augusto Corrêa and Breves-state of Pará, Brazil) areas of the Amazon and continental (Santarém-state of Pará, Brazil). They concluded that the coastal populations analyzed could be assigned to P. bithynis, while the inland populations consisted of a different species, probably P. palaemoni.
P. floridensis, like P. bithynis, was initially considered to be a synonym of P. pandalicola, by Markham (1985). However, it was later established as a distinct species by Román-Contreras (1993), in agreement with Dale & Anderson (1982). Two studies have been conducted on the population structure and prevalence of this species on the host Macrobrachium potiuna in Brazil. One was conducted in Paraná (Masunari et al., 2000), while the other was conducted in São Paulo (Rocha & Bueno, 2000). The study by Masunari et al. (2000) registered the southernmost location known for this species, while Rocha & Bueno (2000) discussed the taxonomic validity of the species. The present study provides the first report of this parasite in M. amazonicum from Brazil.
In the present study, infestation in just one-gill chamber were frequent. This is consistent with observations in many other palaemonid species (Jiménez & Vargas 1990; Oddinetz-Collart, 1990; Román-Contreras, 2004; Hassan et al., 2017; Lima-Corrêa et al., 2018; de Barros et al., 2021) being strategy de survival of parasite to keep the host alive (Lima-Corrêa et al., 2018). A cryptoniscus larva initially infects one of the shrimp’s gill chambers, inhibiting another pair of parasites’ presence in one of the gill cavities (Oddinetz-Collart, 1990). However, the present study observed several cases of double parasitism of the same species in one host. This occurrence had already been noted in M. amazonicum (Odinetz-Collart, 1990) and Macrobrachium ohione (Truesdale & Mermilliod, 1977).
In the present study, prevalence and abundance there was no significant difference of grouped parasite species between Mazagão and Marrecas Island. It is noteworthy that P. floridensis was not found on Marrecas Island. In prevalence and abundance may be attributed to variations in environmental conditions such as salinity, which can interfere with the life cycle of intermediate hosts and the varying abundance of intermediate hosts can have a direct impact on the reproductive cycle of the parasites. This suggests that intermediate hosts of the parasites observed in this work can be more commonly found in estuarine areas than in continental ones.
In both Mazagão and Marrecas Island, prawn infested with all three species of Probopyrus had a Kn value of less than 1.00. In contrast, non-infested prawn had values similar to the standard value for Macrobrachium prawn (Kn = 1.00). Infected samples of M. amazonicum may have experienced nutritional status retardation due to stress and respiration distortion. This is evident in the condition factor values of the prawns as compared to the non-parasitized prawns. The hypothesized nutritional status retardation may have resulted from the nutrient absorption of the parasite through the hemolymph (Romero-Rodríguez et al., 2016; Romero-Rodríguez et al., 2017). The branchial chamber of the host, being the primary site of infection for the bopyrid isopods has serious consequences for gaseous exchange as well as the effectiveness of the host metabolic rate. These actions eventually lead to stress and affect the growth rate of the infected prawn. According to Conner & Bauer (2010), the parasite is also capable of meddling with the feeding abilities of the host. Hence, the energy and nutrients that the host normally channels towards their reproduction and growth appear to be used for survival instead (Boxshall, 2005).
During the study. it was found that P. pandalicola. P. bithynis. and P. floridensis were present in M. amazonicum throughout the year. However, the levels of prevalence and abundance were low. Additionally, female shrimp were found to be more susceptible to the parasites. The condition factor (Kn) of M. amazonicum showed adverse effects due to the parasites. This study provides essential data on the prevalence, abundance, and occurrence of these three parasite species in M. amazonicum across different areas.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio 






