Introduction
Amphibia is composed of three orders: Caudata (salamanders and newts), Gymnophiona (caecilians), and Anura (frogs and toads); each has different organs associated with the hematopoietic process. In Gymnophiona (like Ichthyophis kohtaoensis), erythropoiesis and thrombopoiesis are restricted to the spleen, and granulocytopoiesis occurs in the liver (Zapata, Gomariz, Garrido, & Cooper, 1982). For salamanders, information about the source of the red blood cells is poor; bone marrow, at least during their adult life, and organs like the liver may act as secondary hematopoietic organs (Arikan & Çiçek, 2014). In frogs and toads, there is no consensus about the hematopoietic organs. Nonetheless, the liver, spleen, kidney, thymus, and bone marrow are suggested as the main hematopoietic organs (Jordan & Speidel 1930; Glomski, Tamburlin, Hard, & Chainani, 1997; Cumano & Godin, 2007; Akulenko, 2012). During the tadpole stage, the kidney is the organ involved in the hematopoiesis process (Jordan & Speidel, 1930; Arikan & Cicek, 2014). This characteristic may be species-dependent and could be associated with metamorphosis and the environmental shift during their life-time. In Xenopus laevis, the aquatic toad, vascularization in bone marrow is rudimentary. In this species, erythropoiesis occurs in the liver (Nogawa-Kosaka et al., 2011; Okui et al., 2013), and myelopoiesis is restricted to the bone marrow (Yaparla, Reeves, & Grayfer, 2020). In terrestrial amphibians, as Lithobates catesbeianus, the bone marrow vascularization is more complex, similar to mammalian vascularization (Tanaka, 1976). In this species, erythropoiesis does not occur in the liver or spleen (Cumano & Godin, 2007) but in bone marrow, and the kidney (de Abreu Manso, de Brito-Gitirana, & Pelajo-Machado, 2009).
Amphibian red blood cells (RBCs) are nucleated, as in fish, reptiles, and birds. Amphibian RBCs’ lifespans range from 200 to 1 400 days (Altland & Brace, 1962); their RBCs (mainly in salamanders) are the largest among vertebrates, e.g., Amphiuma tridactylum RBCs sizes are approximately 70 μm length and 40 μm width. In contrast, frogs and toads, have the smallest RBCs of amphibians, 22 μm length and 15 μm width on average (Mitsuru, 1981; Claver & Quaglia, 2009; Arikan & Cicek, 2014).
Red blood cell maturation in peripheral blood has been reported in other vertebrates such as Cyclostomi, Chondrichthyes, Teleosts (Glomski et al., 1997), as well as in amphibians like Pelophylax saharicus, Bufo bufo, Epidalea calamita, Bufotes viridis, and Xenopus laevis (Plum, 1953; Thomas & Maclean, 1974; Bouhafs, Berrebbah, Devaux, Rouabhi, & Djebar, 2009). This could be accompanied by mitosis of immature RBCs (Dawson, 1930), but it is an unusual phenomenon that has been barely reported in some amphibians, reptiles, and occasionally in birds. Seasonal changes and heavy metal contamination can induce RBC maturation in peripheral blood and immature RBCs’ mitosis (Bouhafs et al., 2009; Akulenko, 2012; González-Mille et al., 2019). Other factors that may be related to the increase of immature RBCs in peripheral blood are the reduction of oxygen levels due to anemia, hemolysis, or hypoxia; these conditions promote a regenerative physiological response that increases the RBCs count (Martinho, 2012; Maceda-Veiga et al., 2015; Dissanayake et al., 2017). Thomas and Maclean (1974) and Nogawa-Kosaka et al. (2011) showed that, after anemia induction, Xenopus laevis increases the immature RBCs in peripheral blood by raising the erythropoietin levels, and in newts (Family: Salamandridae), spleen ablation could be a triggering factor for this too (Dawson, 1933).
Amphibians are commonly infected by intracellular blood parasites like Dactylosoma, Hepatozoon, Hemolivia, or Karyolysus (Petit, Landau, Baccam, & Lainson, 1990; Barta, 1991; Haklová-Kočíková et al., 2014; Netherlands, Cook, & Smit, 2014) and extracellular blood parasites such as Filarioidea and Trypanosoma (Desser, 2001; Žičkus 2002; Ferreira et al., 2015; Nguete, Wondji, Pone, & Mpoame, 2019). Parasitic infection by Plasmodium and Hepatozoon, among others, may induce anemia in reptiles, birds, and amphibians (Schall, 2002; Vardo-Zalik & Schall, 2008; Saggese, 2009; Motz, Lewis, & Vardo-Zalik, 2014; Forzán, Heatley, Russell, & Horney, 2017; Stijlemans, De Baetselier, Magez, Van Ginderachter, & De Trez, 2018).
In this study, we describe the presence of mitosis in the peripheral blood of amphibians from different Colombian localities and evaluate the possible association of this process with the increase of immature RBCs in peripheral blood and the presence of blood parasites. We hypothesize that infection by blood parasites may influence the presence of mitosis in peripheral blood since some blood parasites could induce anemia in their hosts, triggering an increase of immature RBCs in the peripheral blood where they mature and carry out mitosis. This is the first study about the potential influence of blood parasites on hematopoiesis in peripheral blood in several wild species of anurans occurring in Andean, pacific and Amazonian regions in Colombia. Besides, it is the study, around this topic, with the largest number of species sampled in the world. Not leaving aside, that this topic has not been treated for several years.
Materials and methods
Sampling: A total of 116 amphibians (31 species) blood smears were analyzed (Table 1). Blood was withdrawn by maxillary vein puncture or by cardiac puncture just after euthanasia (American Veterinary Medical Association, 2020), carried out for taxonomic purposes. Blood smears (two or three replicas per individual) were fixed with methanol for five minutes and stained with 4 % Giemsa (pH 7.2) for 45 minutes (Valkiūnas, 2005). Six localities in Colombia were sampled: San José del Guaviare-Guaviare (N = 6 individuals sampled), Tumaco-Nariño (N = 8), Santa María-Boyacá (N = 8), Medina-Cundinamarca (N = 51), San Gil-Santander (N = 15) and the campus of the Universidad Nacional de Colombia-Bogotá (N = 28), all sampled individuals are specified in the Digital Appendix. Smears were deposited into the biological collection GERPH of the Universidad Nacional de Colombia. All animal procedures were conducted with the Bioethics Committee’s approval, Fundación Universitaria Internacional del Trópico Americano-Unitrópico (Act May 18, 2020), and the Science Faculty’s Ethics Committee, Universidad Nacional de Colombia (Act 06, 2019). This investigation was developed following the Colombian Congress’ 84 th law of 1989, which is the current national statute for animal protection, and the resolution 8 430 of 1993 from the Ministry of Health, which regulates the biomedical investigation with animals.
Table 1 Amphibian species included in this study, common name, sampling location, and N (number of individuals sampled per species) are depicted
| Family | Species | English name | N |
| Microhylidae | Elachistocleis ovalis (Schneider, 1799) 1 | Narrow-mouthed frog | 1 |
| Ranidae | Lithobates palmipes (Spix, 1824) 3 | Amazon Waterfrog | 1 |
| Brachycephaloidea | Pristimantis cf. savagei (Pyburn & Lynch, 1981) 2 | Pyburn’s Robber Frog | 1 |
| Pristimantis vilarsi (Melin, 1941) 1 | Rio Uaupes Robber Frog | 1 | |
| Pristimantis sp. 3 | 1 | ||
| Rhaebo glaberrimus (Günther, 1868) 3 | Cundinamarca Toad | 2 | |
| Rhinella gr. margaritifera (Laurenti, 1768) 2, 3 | 2 | ||
| Rhinella beebei (Gallardo, 1965) 3 | North Coastal Granular Toad | 5 | |
| Rhinella marina (Linnaeus, 1758) 2, 3, 4 | South American Cane Toad | 37 | |
| Hylidae | Boana lanciformis (Cope, 1870) 3 | Rocket Treefrog | 1 |
| Boana boans (Linnaeus, 1758) 2, 3 | Giant Gladiator Treefrog | 2 | |
| Boana xerophylla (Duméril & Bibron, 1841) 2, 3 | Emerald-eyed Treefrog | 2 | |
| Boana maculateralis (Caminer and Ron, 2014) 1 | Stained Treefrog | 1 | |
| Boana pellucens (Werner, 1901) 5 | Palm Treefrog | 1 | |
| Boana rosenbergi (Boulenger, 1898) 5 | Rosenberg’s Gladiator Frog | 1 | |
| Dendropsophus minutus (Peter 1872) 2 | Lesser Treefrog | 1 | |
| Scinax elaeochroa (Cope, 1875) 5 | Olive Snouted Treefrog | 1 | |
| Scinax quinquefasciatus (Fowler, 1913) 5 | Fowler’s Snouted Treefrog | 1 | |
| Scinax rostratus (Peters, 1863) 1, 3 | Caracas Snouted Treefrog | 3 | |
| Scinax ruber (Laurenti, 1768) 2 | Common Snouted Treefrog | 1 | |
| Smilisca phaeota (Cope, 1862) 5 | New Granada Cross-banded Treefrog | 1 | |
| Sphaenorhynchus lacteus (Daudin, 1800) 1 | Orinoco Lime Treefrog | 1 | |
| Trachycephalus jordani (Stejneger & Test, 1891) 5 | Jordan’s Casque-headed Treefrog | 1 | |
| Dendropsophus molitor (Schimdt, 1857) 3 | Green Dotted Treefrog | 28 | |
| Leptodactylidae | Adenomera hylaedactyla (Cope 1868) 2, 3 | Napo Tropical Bullfrog | 2 |
| Physalaemus fischeri (Boulenger, 1890) 3 | Fischer’s Dwarf Frog | 1 | |
| Engystomops pustulosus (Cope, 1864) 4 | Túngara Frog | 3 | |
| Leptodactylus colombiensis (Heyer, 1994) 3 | 2 | ||
| Leptodactylus fuscus (Schneider, 1799) 3 | Rufous frog | 7 | |
| Leptodactylus ventrimaculatus (Boulenger, 1902) 5 | Spotted-vented Thin-toed Frog | 2 | |
| Lithodytes lineatus (Schneider, 1799) 1, 3 | Painted Antnest Frog | 2 |
Sampling location: 1 Guaviare, 2 Boyacá, 3 Cundinamarca, 4 Santander, 5 Nariño.
Blood smear examination: smears were examined and photographed using an Olympus CX41 microscope (Olympus Corporation, Tokyo, Japan), equipped with an integrated camera and a Leica DM750e microscope (Leica Microsystems, Heerbrugg, Switzerland), equipped with a Leica EC3 digital camera. The parasites identified were classified as extra- and intracellular and identified to the genus level. There is no established protocol for the estimation of immature RBCs in amphibians. We obtained the percentage of immature RBCs by counting 50 ideal fields at x400 magnification; each field had an average of 100 RBCs for approximately 5 000 RBCs; it represents a modification of the method described by Collicutt, Grindem, and Neel (2012). For parasite detection the whole blood smear and all replicas were screened, then the parasitemia was estimated as the number of parasites on 10 000 RBCs, for intracellular parasites, and for extracellular parasites as the number of parasites on 100 fields (Valkiūnas, 2005; Benedikt, Barus, Capek, Havlicek, & Literak, 2009).
Statistical analysis: Statistical analysis was carried out using the R commander (Fox & Bouchet-Valat, 2019) package for R software (R Core Team, 2020). Variables evaluated were: the presence or absence of mitosis in peripheral blood, the percentage of immature RBCs, the presence or absence of blood parasites, and the type of parasite. A Kolmogorov-Smirnov test was used to evaluate the normal distribution of the data. A common feature of the data was a non-normal distribution, so a Wilcoxon’s rank test for two variables was calculated to compare quantitative variables, and a Fisher exact test was used to compare qualitative variables. For the hypothesis test, P values less than 0.05 were considered significant.
Results
A total of 116 amphibia were analyzed; 53.5 % of the samples showed mitotic cells in different phases in the peripheral blood (Fig. 1). The mitotic index observed was low; overall, less than two mitotic cells per thousand RBCs. The prevalence of blood parasites in the sample was 30.1 %, being 70.3 % with single infections and 29.7 % with mixed infections. The identified blood parasites were Trypanosoma 24.1 % (28/116), Hepatozoon-like (Named as Hepatozoon-like and Karyolysus-like due to the lack of molecular diagnosis that does not allow accurate classification of those parasites) 6 % (7/116), Dactylosoma 4.3 % (5/116), Karyolysus-like1 0.9 % (1/116), and Filarioidea 2.6 % (3/116) (Fig. 2; Digital Appendix). The percentage of immature RBCs in individuals with mitosis ranged between 0.86-80.08 % (15.76 ± 15.99), while individuals without mitosis had percentages less than 8.98 % (2.1 ± 2.3) (Digital Appendix). All the infected samples had parasitemias that ranged between 0.01 and 3 %, except for one Rhinella beebei whose parasitemia for Dactylosoma sp. was 16 % (Digital Appendix). Given that most of the samples were collected at low altitudes (75.9 % of the samples were collected between 0 and 1 117 m.a.s.l; Digital Appendix) and some species have a low number of individuals sampled (e.g., Lithodytes lineatus N = 2), there was no possibility to statistically analyze a tendency of parasitic altitudinal distribution nor the host species and its association with mitosis in peripheral blood.
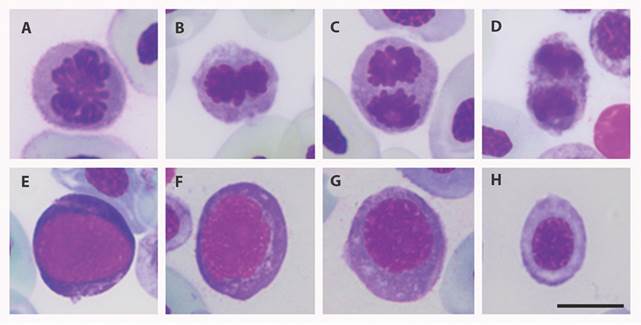
Fig. 1 Phases of mitosis and immature RBCs observed in peripheral blood smears of Colombian amphibians included in the study. A. Mitotic cell in prophase; B. Mitotic cell in metaphase; C. Mitotic cell in anaphase; D. Mitotic cell in telophase; E-H. Immature Red blood cells at different maturation stages. Giemsa stain. Scale bar: 10 μm.
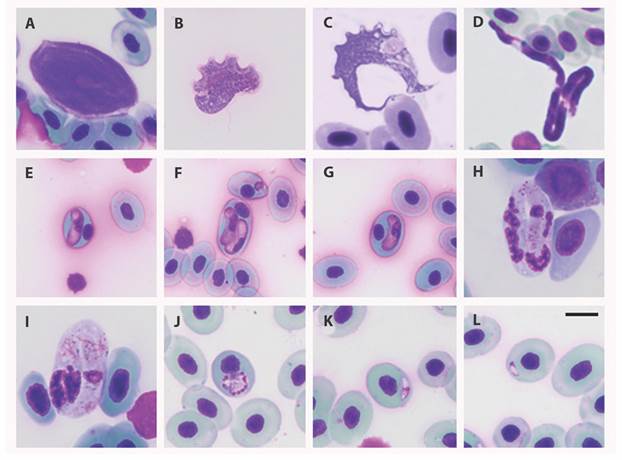
Fig. 2 Blood parasites infecting Colombian amphibians analyzed in the study A-C. Trypanosoma; D. Microfilaria; E-G. Hepatozoon-like; H-I. Karyolysus-like; J-L. Dactylosoma. Giemsa stain. Scale bar: 10 μm; Differences in the sizes of RBCs are due to their belonging to different host species.
Percentage of immature RBCs and mitosis: By carrying out a morphological comparison between cells in mitosis and immature RBCs, some standard features were found: both cell types showed a roundish form and a basophilic cytoplasm, as demonstrated by the Giemsa stain (Fig. 1). Due to these morphological similarities and literature reports, we propose that those mitotic processes may be occurring in immature cells, thereby, an increase in the percentage of immature RBCs in peripheral blood could be related to the presence of mitotic cells. Comparing samples that show mitosis with those without mitosis, using a Wilcoxon’s rank test, a significant difference in the percentage of immature cells in peripheral blood was found (P < 0.0001).
Percentage of immature RBCs and blood parasites: The comparison of the percentage of immature RBCs between samples with a positive and negative diagnosis for blood parasites also showed a significant difference (Wilcoxon’s rank test, P < 0.001). However, the percentage of parasitemia and immature RBCs did not correlate (data not shown).
Mitosis and blood parasites: An association between mitosis and blood parasites were assessed; 25 out of 35 infected samples show mitosis, and by applying a Fisher exact test, we identified an association between the infection by blood parasites and the presence of mitosis in peripheral blood (χ2 1 = 12.46 and P < 0.0001); although, there is no association between parasitemia and presence of mitosis (Wilcoxon’s rank test, P > 0.05).
Discussion
There are limited reports on erythrocytic mitosis in amphibian’s peripheral blood (Thomas & Maclean, 1974; Glomski et al., 1997). This is the first report of this subject in Colombia and for the species analyzed here; it also represents the study of erythrocytic mitosis in the largest sample of amphibians. Our results evidentiate how often this phenomenon occurs in amphibians and discuss its possible causes.
Previous reports have shown that mitotic RBCs in peripheral blood occur in immature RBCs, but not in mature RBCs (Chegini, Aleporou, Bell, Hilder, & Maclean, 1979). Our results also show that mitosis is present in immature RBCs due to the association evidenced between the presence of mitosis and the percentage of immature RBCs in peripheral blood and the morphological features shared between them (Fig. 1). The presence of a high number of immature RBCs at different stages of maturation (as determined by their basophilic cytoplasm, roundish form, nucleus occupying the best part of the cellular body, and the less condensed chromatin) (Fig. 1) is an indicator of active erythropoiesis (Crouch & Kaplow, 1985) that can be triggered by respiratory stress or anemia (Nogawa-Kosaka et al., 2011).
The presence of blood parasites has been associated with physiological conditions, such as anemia (Saggese, 2009), and a regenerative response to this stress induces the early release of immature RBCs to peripheral blood. Our results suggest that this phenomenon is accompanied by mitosis of these immature RBCs in peripheral blood in amphibians. Regenerative anemia has been shown to induce increased immature RBCs in birds (Campbell, 2004), reptiles (Saggese, 2009), and fish (Clauss, Dove, & Arnold, 2008). The data obtained here do not allow us to determine the presence of anemia in the sampled animals because of the absence of a physical evaluation of them and the lack of hemoglobin data concentrations. Nonetheless, the high percentage of immature RBCs found in the organisms allows us to consider that perhaps anemia was present in the individuals sampled since the presence of such cells in peripheral blood and anemia have been correlated before (Thomas & Maclean, 1974; Saggese, 2009).
Blood parasites were found infecting 30.1 % of the sampled individuals with parasitemias ranged between 0.01 and 16 % (Digital Appendix). Trypanosoma was the most prevalent genus found, infecting 24.1 % of the animals sampled, while Filarioidea was the less prevalent type of parasite (2.6 %). It is also important to highlight the diversity of intracellular parasites found, parasites tentatively belonging to the genus Hepatozoon, Dactylosoma, and Karyolisus, which were diagnosed by microscopic analysis. Amphibians are a group of vertebrates highly infected by parasites, bacteria, fungus, and viruses; due to their habits, which often combine terrestrial and aquatic environments, they are usually exposed to different conditions that ease those infections. Additionally, their habits, for example, promote the interaction with hematophagous insects and leeches, both vectors for the transmission of different blood parasites (Ferreira, Perles, Machado, Prado, & André, 2020). This study presents some insights regarding blood parasite prevalence and diversity in Colombian amphibians, although it is important to complement this information reported by microscopic analysis with molecular approaches that allow a complete characterization of the parasites found.
Some reports in lizards show the presence of high numbers of immature RBCs in peripheral blood associated with blood parasite infections (Martínez-Silvestre, Mateo, Silveira, & Bannert, 2001; Martinez-Silvestre & Arribas, 2014). Intracellular blood parasites, like Hepatozoon, Dactylosoma, Karyolysus, and Plasmodium, may be related to anemia due to the disruption of the RBCs caused by some of the parasites’ life stages. This phenomenon has been reported in several species like Sceloporus occidentalis and other lizards (Schall 1982; Barta, 1991; Peirce & Adlard, 2004). Extracellular parasites, like Trypanosoma or Filarioidea, are associated with a decrease in RBCs count, hematocrit and hemoglobin levels (Maqbool & Ahmed, 2016), inflammation, and tissue degeneration of skeletal heart muscle in Australian mammals (Thompson, Godfrey, & Thompson, 2014). Trypanosoma experimental infection has been associated with food refusal resulting in death in European green frogs and Canadian frogs (Reichenbach-Klink & Elkan, 1965). Extracellular hemoparasitic infections have also been associated with anemia (Amole, Clarkson, & Shear 1982; Silva, Herrera, Domingos, Ximenes, & Dávila, 1995; Stijlemans et al., 2018).
Our possible explanation for the presence of mitotic cells in peripheral blood in an infected organism is that those parasitic organisms could induce anemia in their host. As a response, immature RBCs are released to the peripheral blood. Those cells would finish their maturation in the peripheral blood and could divide to increase the number of RBCs to offset anemia. This explanation could be supported by the association found between the presence of mitotic RBCs and the percentage of immature RBCs in peripheral blood, and by the association between the percentage of immature RBCs and the presence of blood parasites.
Further research based on experimental trials is needed to confirm the hypothesis here proposed and to evaluate other factors that can also induce similar physiological responses to heavy metal pollutants, antifungals, or other toxic agents (Preetpal, 2014).
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

