Introduction
The term plant gall has been applied to different systems, and although there is no consensus about the definition of the term, the same has been used as a generalized expression more than a precise scientific term or concept (Williams, 1994). Nonetheless, in general terms, galls could be defined as deviations in the normal plant development pattern, produced by a specific reaction to the presence and activity of a foreign organism (Shorthouse & Rohfritsch, 1992; Inbar et al., 2009; Huang et al., 2015). Gall-inducing insects, also called gall inducers, gall makers, or simply gallers, live within the plant tissue, which supplies food, low levels of potentially harmful chemical substances, protection against unfavorable environmental factors (Nogueira, Costa, Silva, & Isaias, 2018), and shelter against natural enemies (Mani, 1992; Ananthakrishnan, 1998; Raman, Schaefer, & Withers, 2005; Tooker & De Moraes, 2008; Tooker, Rohr, Abrahamson, & De Moraes, 2008; Huang et al., 2015; Isaias et al., 2018).
The meaning of the adaptive value of galls and the kind of biological interaction existing between gall-inducing insects and their host plants is the subject of a continuous debate among the different groups of researchers that work in the field (Nyman & Julkunen 2000; Stone & Schönrogge, 2003). Some groups established that galls originated as a mechanism of defense developed by insects against attack by their natural enemies. Moreover, the main function of the gall is to give shelter and food to the larvae of the galling insect; however, this and other related ideas are still the target of extensive debate (Ananthakrishnan, 1998; Stone & Cook 1998; Price, Waring & Fernández, 1986; Stone & Schönrogge, 2003; Tooker et al., 2008; Giron, Huguet, Stone, & Body, 2016). Different lines of thought relate galls with processes of pathogenesis, symbiosis, and defense mechanisms in plants (Hartnett & Abrahamson, 1979; Price et al., 1986). Regardless of the type of specific interaction between gall-inducing insects and their host plants, natural selection consequently operates on the insect to stimulate the development of protective and/or nutritive tissues in the plant; on the other hand, in the plant, natural selection acts to resist the stimulus generated by the insect (Ananthakrishnan, 1998).
Hymenoptera and Diptera are two orders with a particularly large number of gall inducers, but great diversity can also be found in galls formed by thrips, aphids, and insects from other orders (Ananthakrishnan, 1998; Hanson & Gómez-Laurito, 2005). A large number and diversity of plant gall morphotypes and inducing insects have been reported worldwide (Espírito-Santo & Fernandes, 2007). New species of inducing insects are periodically described, while other studies on the abundance and diversity of gall morphotypes, as well as their corresponding inducers, has helped to broaden the existing knowledge in this field (Shorthouse & Rohfritsch, 1992; Williams, 1994; Ronquist & Liljeblad, 2001; Hanson & Gómez-Laurito, 2005; Dalbem & Mendonça, 2006; Güçlü, Hayat, Shorthouse, & Göksel, 2008; Coelho et al., 2009; Maia, Fernandes, Magalhãcs, & Santos, 2010a; Maia, Fleury, Soares, & Isaias, 2010b; Medianero, Paniagua, & Castaño-Meneses, 2010; Maia & Oliveira, 2010; Santos, Almeida-Cortez & Fernandes, 2011; Sano, Havil, & Ozaki, 2011; Maia, 2014; Santos de Araújo, 2017; Martins dos Santos, Pereira Lima, Souza Suares, & Calado, 2018).
Besides insects, plant galls are also induced by a great variety of organisms such as bacteria, fungi, nematodes, and mites (Leitch, 1994; Williams, 1994; Ananthakrishnan, 1998, Raman, 2011). Galls induced by insects are distinct from those induced by fungi and bacteria in their form, organization, and complexity. More complex and diverse galls are induced by insects such as those of the Cynipidae and Cecidiomyiidae families, which show extreme examples of radial symmetry, belonging to the orders Hymenoptera and Diptera, respectively (Raman, Cruz, Muniappan, & Reddy, 2007; Sinnott, 1960; Raman, 2011). A general scheme for the structural complexity of plant galls and the taxonomic groups of their inducers is proposed by the author from the reviewed literature (Fig. 1) (Rohfritsch & Shorthouse, 1982; Mani, 1992; Davey, Curtis, Gartland, & Power, 1994; Gómez & Kisimova-Horovitz, 1997; Williams, 1994; Valentine, 2003; Sá et al., 2009; Raman 2011; Álvarez, Molist, González-Sierra, Martínez, & Nieto-Nafría, 2014; Formiga, Silveira, Fernandes, & Isaias, 2015; Muñoz-Viveros et al., 2014; Guimarães, Neufeld, Santiago-Fernandes, & Viera, 2015; Hernández-Soto et al., 2015; Suzuki, Moriguchi, & Yamamoto, 2015; Mellah, Enhassaïni, & Álvarez, 2016; Oliveira et al., 2016; Richardson, Body, Warmund, Schultz, & Appel, 2016; Ferreira, Álvarez, Avritzer, & Isaias, 2017; Palomares-Rius, Escobar, Cabrera, Vovlas, & Castillo, 2017; Cotrim Costa, Gonçalves da Silva Carneiro, Santos Silva, & Isaias, 2018; Nogueira, Costa, Silva, & Isaias, 2018); however, a consensus on this approach does not exist, and substantial variation can be observed within each group.
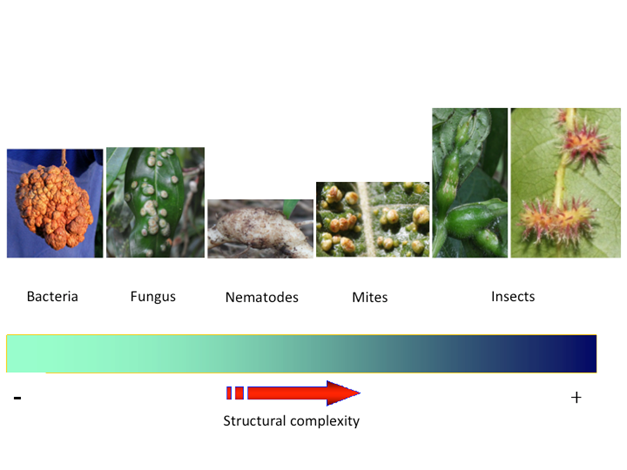
Fig. 1 Proposed general scheme for the structural complexity of plant galls and the taxonomic groups of gall inducers. Images show some examples of galls. Bacteria crown gall found on Pittosporum sp. (Pittosporaceae), induced by Agrobacterium tumefaciens. Fungus gall on Satyria warszewiczii (Ericaceae), induced by Exobasidium emeritense. Nematode gall induced by Meloidogyne incognita on Solanum lycopersicum (tomato, Solanaceae). Mite gall induced on Acnistus arboresens (Solanaceae). Insect gall induced on Cissus fuliginea (Vitaceae) by an unknown diptera Cecidomyiidae and on Hirtella racemosa (Chrysobalanaceae) by an unknown diptera Cecidomyiidae. Taxonomic identification of host plants of insect galls was performed by Roberto Espinoza, and inductor insects were identified by Paul Hanson. Photo credit: taken from Patrick Roper (bacteria crown gall), Omar Gätjens-Boniche (fungus gall, nematode gall, mite gall and insect galls).
The fact that different groups of insects possess the capacity to form galls in a wide variety of plants has motivated a great number of investigations attempting to elucidate the mechanism of induction of this type of structure. Nevertheless, considering the importance of galls as models for understanding a series of fundamental processes in the development of plants, the induction mechanisms and the evolutionary context of this type of structure is still poorly understood (Stone & Schönrogge, 2003, Raman, 2011; Oates, Denby, Myburg, Slippers, & Naidoo, 2016).
The aim of this paper is to provide an updated general description of plant galls induced by insects, focused on the induction process as well as how, according to an integrated interpretation by the author, the associated characteristics of these structures and the biological processes they regulate could be the basis for an alternative induction hypothesis mediated by the insertion of exogenous genetic elements into the plant gall cells through some endosymbiotic bacteria originating from the insect.
Plant gall development and diversity of plant gall-inducing insects
The association between galls and their inducing organisms has likely been recognized since the study of these systems began (Mani, 1992). However, it was not until the 17th century that Malpighi described, in the Western World, that the growth and development of these structures was correlated to the activity of feeding, oviposition, and particular nutritional requirements of the inducing insect (Fagan, 1918; Hough, 1953).
Gall morphogenesis is a complex phenomenon, which involves reorientation of the plant’s development by the inducing insect (Ananthakrishnan, 1998; Raman, 2011; Oates, Külheim, Myburg, Slippers, & Naidoo, 2015; Agudelo et al., 2018). The degree to which the insect manipulates the plant’s growth to form the gall varies considerably and involves changes ranging from the induction of cell proliferation (Agudelo et al., 2018) to the formation of a complex structure that the plant does not produce under normal conditions. Just like normal plant organs and structures, galls induced by insects present anatomic and histologic characteristics of their own, which vary greatly in their diversity and degree of complexity (Fig. 2) (Nyman & Julkunen, 2000; Mani, 1992; Ananthakrishnan, 1998; Stone & Schönrogge, 2003; Oliveira & Isaias, 2010; Raman, 2011; Oliveira, Carneiro, Magalhães, & Isaias, 2011; Oliveira et al., 2016). Tissues near the inducing insect show cytological and morphological changes that benefit its feeding process and development. This tissue, also known as “nutritive tissue”, commonly presents high concentrations of sugar (Nogueira et al., 2018), lipids, proteins, nitrogen, and other nutrients that provide a continuous source of food for the insect and show intense phosphatase activity (Miles, 1968; Rohfritsch & Shorthouse, 1982; Shorthouse & Rohfritsch, 1992; Raman, 2011, Oliveira & Isaias, 2010; Oliveira et al., 2011; Nabity, Haus, Berenbaum, & Delucia, 2013; Huang et al., 2015; Oates et al., 2016; Ferreira et al., 2017; Isaias et al., 2018). Typical nutritive cells show a dense cytoplasm with abundant cell organelles, fragmented vacuoles, a hypertrophied nucleus and nucleolus, and dedifferentiated plastids clustered around the nucleus, as well as chloroplasts modified to varying degrees and modified cell walls (Shorthouse & Rohfritsch, 1992; Raman, 2011; Carneiro & Isaias, 2015). Ferreira et al. (2017) compared six gall systems with different levels of structural complexity (a phids, mites, and Nematoda), using histometric and histochemical analyses. Based on the types of storage tissue, the authors proposed a classification of three types of storage tissues: typical nutritive tissues (TNT), common storage tissues (CST), and nutritive-like tissues (NLT). TNT and NLT present cells with a dense cytoplasm and a large nucleus; TNT serve as a direct food source for gall inducers. CST have vacuolated cells, and may store starch and other types of energy-rich molecules, as do the cells of NLT. Likewise, several studies have demonstrated that insects generally feed on a reduced area of the gall (Nyman & Julkunen, 2000).

Fig. 2 Some plant galls from Costa Rican flora. A) Gall induced on Pisonia macranthocarpa (Nyctaginaceae) by an unidentified insect species (Diptera, Cecidomyiidae). B) Gall induced on Vitis tiliifolia (Vitaceae) by an unidentified insect species (Diptera, Cecidomyiidae). C) Gall induced on Hirtella racemosa (Chrysobalanaceae) by an unidentified insect species (Diptera, Cecidomyiidae). D) Gall induced on Semialarium mexicanum (Hippocrateaceae) by an unknown inducer. Taxonomic identification of the host plants was carried out by Roberto Espinoza, and the inductor insects were identified by Paul Hanson. Photo credit: Omar Gätjens-Boniche (A, C, and D) and Gregorio Dauphin (B).
The inducing insect can modify the expression of genes within restricted areas of the host plant, thereby producing new developmental events in the tissues under its influence. Gall morphogenesis occurs in a relatively short time; however, this fact apparently does not influence the complexity observed in such morphological entities (Ananthakrishnan, 1998, Nabity et al., 2013; Oates et al., 2015).
In many kinds of abnormal growth or deviation from normal organismal development, there are alterations in the mechanisms that regulate cell proliferation and differentiation. Within this context, “crown galls” induced by the genus Agrobacterium are an example of structures formed due to the proliferation of cells with a low level of differentiation; hence, they are considered the simplest and least derived plant gall within the wide variety of these structures found in Nature. On the other hand, galls induced by insects are very well-organized structures showing different degrees of differentiation, the reason why they are considered as the most complex and derived structures. Nonetheless, in spite of the clear differences between these two extremely diverse groups of plant galls, they show important similarities. For instance, both systems require a previous state of “conditioning” towards the development of the structure. In the case of insect galls, the “conditioner” is the insect itself, which modulates the tissue that will form the structure through mechanical action and the secretion of chemical substances. In crown galls the conditioning factor is given by a series of metabolic events prior to the genetic transformation of plant cells by the bacterium (Rohfritsch & Shorthouse, 1982; Davey et al., 1994; Piñol, Palazón, Cusidó & Serrano, 1996; Valentine, 2003; Suzuki et al., 2015).
In a similar manner as in so-called “tumor cells” of crown galls, cells from insect-induced galls acquire a certain autonomy and independence from their normal tissue development pattern. From the induction process, cell development is redirected due to the influence of the inducing stimulus. However, unlike insect galls, crown galls have an unlimited capacity to grow without a defined pattern of development. After the initial stimulus, cell proliferation in both systems develops in a different way; in the case of bacteria-induced crown galls, cell proliferation occurs in an uncontrolled way and does not require the continuous presence of bacteria once the process is initiated. In contrast, for adequate and complete development of galls induced by insects, in general, the continuous, active presence of the insect is required (Rohfritsch & Shorthouse, 1982; Davey et al., 1994; Valentine, 2003; Suzuki et al., 2015).
According to Rohfritsch & Shorthouse (1992), Arduin & Kraus (1995), and Sá et al. (2009), plant galls present four basic stages of development, but significant differences between gall inducers can occur. These stages of development involve the processes of initiation, growth and differentiation, maturation, and finally, dehiscence (Fig. 3). The state of growth and development is a continuous process of cell division and differentiation that generally depends on the feeding activity of the larva, which in turn is mainly responsible for molding the shape of the gall’s inner chamber. After the nutritive tissue is formed around the inner chamber, a mass of cells binds the vascular tissue of the gall to the plant. Frequently, sclerenchymatous tissue is also formed around the inner chamber and near the vascular tissue of the gall, which causes its separation into internal and external regions. The internal region is considered to be influenced by the activity of the larva, whereas the external region or outer cortex of the gall is under the influence of the plant. The opening and dehiscence of galls occurs towards the end of the maturation stage and represents the period of greatest chemical and physiological change in the tissues that comprise it. Not only has it been demonstrated that the meristematic tissues react to the stimuli but also that young stems of various species of plants can be stimulated and modified to make these structures.
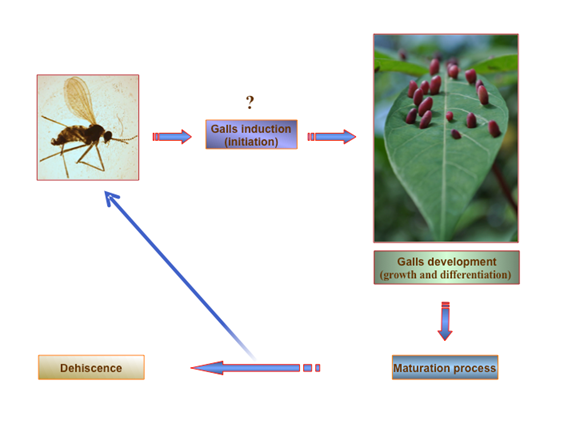
Fig. 3 General scheme for the life cycle of insect-induced galls. This figure is based on the gall induced by the Cecidomyiidae Latrophobia brasiliensis in Manihot esculenta Crantz (Cassava). The scheme was synthesized from the reviewed literature. Photo credit: Omar Gätjens-Boniche.
Shorthouse & Rohfritsch (1992) separate the morphogenesis of galls induced by insects into two processes. The first is a permanent effect, which remains even when the corresponding insect is removed or dies. A second process implies that the effect is generated through continuous stimulation by the inducing insect, which disappears if the insect is removed from the gall or if it dies.
Several authors have tried to classify galls according to a series of morphological criteria that have, in spite of being arbitrary, established the groundwork for the development of a great number of studies. Shorthouse & Rohfritsch (1992) and Williams (1994) distinguished two basic types of galls: organoids and histoids. The first one results from organ proliferation or modification, maintaining the basic organ structure. Histoid galls, in contrast, originate from the proliferation of modified cells leading to the formation of new tissue. Plant galls are also classified according to more strict morphological criteria. Galls called “kataplasmic” are irregular in size and shape, presenting an irregular growth pattern with little differentiation in their tissues. On the other hand, “prosoplasmic” galls are more complex and differentiated and are formed as a result of the formation of a new structure (Miles, 1968; Williams, 1994). Nonetheless, it is important to emphasize that any attempt at classification turns into a difficult task, mainly because of the great quantity of existing intermediate states and shapes among the different gall morphotypes. Therefore, the shape and structure of galls depends on a large number of factors, including the species to which the host plants belong, the species of the inducing insects, the type of organ attacked, the state of development of the plant and, in some cases, even the sex of the insect. According to Raman (2011), about 90 % of all the known galls show bilateral or radial symmetry. As reported by the same authors, in the specific case of galls formed by insects belonging to the families of Cecidomyiidae (Diptera) and Cynipidae (Hymenoptera), they show surprising levels of radial symmetry. Moreover, some factors that affect gall size include the number of larvae present, the structural diversity of the galls, the percentage of tissue infected, the physiological state of the host plant, environmental conditions, and the genotype of the plant (Mani, 1992; Ananthakrishnan, 1998; Stone & Schönrogge, 2003; Raman, 2011).
Some galls are simply “swellings”-undifferentiated cell masses or those with a low level of differentiation, while others show a surprisingly high degree of differentiation, organization and specialization within their cells and tissues, frequently with characteristics exclusively associated with the gall from which they originate. Based on the above, this last type of gall, called “prosoplasmic”, presents an anatomy and histology very characteristic of its own. Prosoplasmic galls induced by some families of insects are, due to their high degree of complexity and organization, the ones that generate a higher interest (Mani, 1992).
The order Hymenoptera includes the most complex and organized galls described so far for the Class Insecta. In this group, gall-inducing insects are classified in the Suborder Symphyta, family Tenthredinidae and the Suborder Apocrita, with two Superfamilies: Chalcidoidea, which includes the families Pteromalidae, Eurytomidae, and Agaonidae and the superfamily Cynipoidea, represented by the family Cynipidae. Gall-inducing Hymenoptera species present a wide distribution over several areas of the planet and can be found in various groups of dicotyledonous plants and even in monocotyledonous plants, especially in gramineous species (Shorthouse & Rohfritsch, 1992). According to Rohfritsch & Shorthouse (1982) and Shorthouse & Rohfritsch (1992), Cynipidae contains the main gall inducers of the order Hymenoptera, and they can cause the development of different kinds of galls in distinct organs of the plant. Most representatives of this family induce the formation of galls in leaves, sprouts, stems, and roots. Females lay eggs on the surface of the tissue, and the egg itself induces an initial gall, even though the larvae of these insects are the main inducing agents of these structures. Cynipidae eggs have a lytic effect on the cells that surround them, which leads to the formation of a chamber that protects the young larva. Larvae have mouth structures that allow them to break the plant cell wall to suck on the contents of the nutritive cells. Many species of Cynipids are characterized by complex life cycles, commonly accompanied by an alternation of generations. Individuals of both generations can attack the same organ or different types of organs in the plant, resulting in the formation of radically different galls, and in some cases, even the individuals of the two generations are morphologically different. The surface of the galls formed by the Cynipids can be coated by trichomes, scales, thorns, or other types of outgrowths. Nevertheless, one of the most important characteristics of these structures is the formation of concentric areas of differentiation and an area of sclerenchymatous cells around the larval chamber.
The order Diptera grouped in the suborder Nematocera, which includes the family Cecidomyiidae (gall midges), and the suborder Brachycera, which includes Tephritidae (fruit flies) and Agromyzidae. Gall inducers belonging to this order present a broad global distribution, and in contrast to other orders of gall- inducing insects, they can also be found in monocotyledonous plants, especially grasses. Nonetheless, the majority of arthropod-induced galls occur on dicotyledons, and at least 66 % of the dicotyledon families harbor galls (Shorthouse & Rohfritsch, 1992; Hanson & Gómez-Laurito, 2005). Hanson & Gómez-Laurito (2005) suggested that possibly more than 90 % of dicotyledon species in all major biogeographic regions harbor gall-inducing by cecidomyiids. Around 80 % of plant galls induced by insects from the Neotropical region are induced by the Cecidomyiidae family, and new species of inducing insects belonging to this family are constantly being described around the world. Moreover, according to Hanson & Gómez-Laurito (2005), about 70 % of the gall-inducing arthropods in Costa Rica are Cecidomyiidae.
Galls formed by Diptera, especially those induced by individuals of the family Cecidomyiidae, are characterized by a high degree of tissue differentiation; on the other hand, the insects are characterized by the complexity of their life cycles. Another important characteristic present in gall-inducing Diptera species is their capacity to pupate inside the gall. In the family Cecidomyiidae only the larvae have the capacity to induce galls; these have a poorly developed mouth structure and feed by sucking on fluids exuded from the gall cells, without causing any damage or necrosis (Rohfritsch & Shorthouse, 1982; Shorthouse & Rohfritsch, 1992). In those galls, nutritive tissue is present throughout the development of the structure. At the same time, for the development and maintenance of nutritive tissue, the active presence of the larva of the inducing insect is necessary (Ananthakrishnan, 1998).
Galls induced by the orders Thysanoptera and Hemiptera appear as small bumps or abnormal growths, whose tissues are essentially made of parenchymatous cells. Some species can also cause a leaf roll accompanied by cellular hypertrophy. In the case of the hemipterans, they induce a variety galls types, which vary from simple forms to very sophisticated complex structures. Several species of coleopteran gall-inducing larvae produce tunnels in different parts of the plants, and the eggs are placed in the interior of cavities prepared by insect females. Although plant galls induced by Coleoptera have been described as characterized by the absence of nutritive tissue (Shorthouse & Rohfritsch, 1992), there are descriptions detailing the presence of this type of tissue or nutritive-like tissue in coleopteran galls (Raman et al., 2007; Barnewall & De Clerck- Floate, 2012). Nevertheless, little is known about gall-inducing Coleoptera, especially in tropical ecosystems (Korotyaev, Konstantinov, Lingafelter, Mandelshtam, & Volkovitsh, 2005). Many galls formed by Lepidoptera do not develop nutritive tissue, and larvae of these insects are fed by chewing the tissue that surrounds the internal chamber, producing a large amount of detritus (Rohfritsch & Shorthouse, 1982; Shorthouse & Rohfritsch, 1992). However, recent studies on lepidopteran-induced galls suggest that these structures may also present nutritive tissue and are not as simple as they have traditionally been described. For example, a true nutritive tissue that showed metabolite concentration gradients, which seem to be specific for lepidopteran galls, was described by Ferreira & Isaias (2013). Nutritive tissue was described in Bauhinia ungulata L. (Fabaceae) by Bedetti, Ferreira, de Castro, and dos Santos Isaias (2013). Moreover, nutritive cells in the galls induced on the leaves of Tibouchina pulchra (Cham.) Cogn. (Melastomataceae) have a large amount of rough endoplasmic reticulum, ribosomes, polyribosomes, and mitochondria, which are evidence of the high metabolic status of these cells. Likewise, vascular cambium-like, with high protein synthesis and lipid storage, are characteristic of that nutritious tissue. The nutritive cells are stimulated by the activity of galling larvae, consequently generating a new tissue type (Vecchi, Menezes, Oliveira, Ferreira, & Isaias, 2013).
The induction mechanism of plant galls by insects: What do we know?
The capacity of a large number of insects to form galls in different groups of plants has motivated a great deal of research with the aim of elucidating the mechanism of induction of this type of structure. Hori (1992) describes four main hypotheses that could explain the formation of plant galls. The first of these hypotheses suggests the injection of a fluid from the insect during the oviposition process, which would mediate gall induction. A second hypothesis proposes that gall formation is the result of mechanical irritation due to the presence of a foreign body on the plant tissue. An extension of this hypothesis suggests that galls are induced at a "reactive site" with particular traits of available meristematic regions by the action of the inductor insect, probably in stem cell areas (Weis, Waltonanand, & Crego, 1988; Abrahamson & Weis, 1997; Espírito-Santo, Neves, Andrade-Neto, & Fernandes, 2007; Silvia & Connor, 2017). The third hypothesis proposes that the formation of galls is induced by the secretion of active components from the saliva of the insect. Finally, a fourth hypothesis purports that the formation of galls is mediated by the excretion of metabolic products from the insect.
For simplicity, the morphogenic process of plant gall induction by insects can be divided into three main phases. The first one involves “conditioning” of the cells of the corresponding plant tissue by the insect, to make them more susceptible and suited to its action as inductor. In the following phase, induction of the gall as such takes place, whereby cell division and elongation results in the formation of a “primary” gall. The final phase consists of gall maturation, in which the primary gall grows to complete its morphogenesis (Shorthouse & Rohfritsch, 1992; Raman, 2011).
As mentioned above, in the plant gall induction process, plant cells should be conditioned to produce a particular physiological state (Raman, 2011). In this respect, different studies have mentioned that the amino acids present in the salivary secretions of gall-inducing insects, essentially lysine, histidine, and tryptophan, could function as “preconditioners” for gall induction. It seems that these amino acids could cause major plasticity and would increase the sensitivity of the plant tissue to the action of the corresponding inducing insect. Although the presence of pectinase in the saliva of insects has not been correlated with gall induction, such enzymes could degrade the cell walls and in turn contribute to tissue preconditioning to the action of the insect. Likewise, it has been speculated that polyphenol oxidase (PPO), also present in the saliva secretions of insects and the phenolic compounds derived from its enzymatic action, could increase plant tissue sensitivity to the stimulus of the inductor insect. It has also been suggested that the complex interaction between the host plant tissue and polyphenol oxidase might be of fundamental value in gall formation (Miles, 1968; Hori, 1992; Ananthakrishnan, 1998; Saltzmann, Giovanini, Zheng, & Williams, 2008). In this respect, Miles (1968) indicated that interactions and the balance between insect polyphenol oxidase and the host plant could determine whether the “attack” of an insect causes injury (necrosis) or gall development. Moreover, the modulation of redox potential has been related to gall initiation and establishment, especially concerning the accumulation of reactive oxygen species (Isaias, Oliveira, Moreira, Soares, & Carneiro, 2015).
Different studies have reported that indoleacetic acid (IAA) could be a powerful gall- inducing agent, and it has also been speculated that this compound could interact with other plant growth regulators, like cytokinins and gibberellins, or in a synergistic way with other chemical substances, to promote the induction and maturation of these structures (McCalla, Genthe, & Hovanitz, 1961; Miles, 1968; Hori, 1992; Leitch, 1994; Ananthakrishnan, 1998; Mapes & Davies, 2001; Stone & Schönrogge, 2003; Raman, 2011, Tooker, & Helms, 2014; Bartlett & Connor, 2014; Bedetti, Modolo, & dos Santos, 2014; Bailey, Percy, Hefer, & Cronk, 2015). However, the mechanism of action through which these substances act to promote the development of plant galls is very poorly understood and is currently a subject of discussion. This scenario becomes more complicated in the case of prosoplasmic galls, like the ones formed by insects of the Cecidomyiidae and Cynipidae families, because of the complexity of their development and structure.
Symbiotic relationships between gall-inducing insects and microorganisms have been hypothesized to be involved in plant gall development (Hansen & Moran, 2014; Tooker and Helms, 2014). Several studies have demonstrated the presence of a great number of endosymbiotic bacteria in different insect groups (Degnan, Lazarus, & Wernegreen, 2005; Kikuchi, Meng & Fukatsu, 2005; Delmotte, Rispe, Schaber, Silva, & Moya, 2006; Fukatsu et al., 2007; Jaenike, Polak, Fiskin, Helou, & Minhas, 2007; Goto, Anbutsu, & Fukutsa, 2006; Xi, Gavotte, Xie, & Dobson, 2008; Toft, Williams, & Fares, 2009; Gutzwiller, Dedeine, Káiser, & Giron, 2015; Krawczyk, Szymańczyk, & Obrępalska-Stęplowska, 2015; El-Sayed & Ibrahim, 2015; Campbell et al., 2015), as well as bacteriocytes (Nikoh & Nakabachi, 2009; Braendle et al., 2009). Some of these symbiont microorganisms are mutualistic and contribute to the viability of their hosts, while others are parasites, which tend to affect their corresponding hosts in a negative way. Insect-associated microorganisms could be important mediators of interactions between insects and plants (Sugio, Dubreuil, Giron, & Simon, 2015; Hammer & Bowers, 2015, Wielkopolan & Obrępalska-Stęplowska, 2016). Some researchers have reported that simultaneous infection with different species of endosymbionts in the same host organism is a common phenomenon in several insect groups (Thao et al., 2000; Thao, Gullan, & Baumann, 2002; Russell et al., 2003; Ishii, Matsuura, Kakizawa, Nikoh, & Fukatsu, 2013; Krawczyk et al., 2015; El-Sayed et al., 2015; Ghosh, Bouvaine, & Maruthi, 2015; Brentassi et al., 2017). Different tissues in the body of the same host constitute different microenvironments for endosymbiont organisms. Some tissues could be, for instance, nutritionally favorable, immunotolerant, or simply easy to colonize (Mouton, Henri, Bouletreau, & Vavre, 2003; Kondo, Shimada, & Fukatsu, 2005; Koga, Meng, Tsuchida, & Fukatsu, 2012; Hansen & Moran, 2014; Sugio et al., 2015).
In recent years, a growing interest has emerged regarding the reproductive biology of endosymbiont parasites that are transmitted through the mother and manipulate the reproduction of their host organism. Accumulated evidence shows that many species of arthropod are infected by different kinds endosymbiont organisms transmitted from the mother through vertical transmission, which have a great influence on the biology of their hosts. Some of these endosymbiont microorganisms includeWolbachia,Spiroplasma,Rickettsia,Arsenophonus, and Cardinium, among others (Weeks, Velten & Stouthamer, 2003; Zchori-Fein & Perlman, 2004; Goto et al., 2006; Casper- Lindley et al., 2011; Goodacre & Martin, 2012; Kageyama, Narita, & Watanabe, 2012; Koga et al., 2012; Kremer et al., 2012; Herren, Paredes, Schüpfer, & Lemaitre, 2013; Ma, Vavre & Beukeboom, 2014; Boivin et al., 2014; Ma et al., 2015; Sugio et al., 2015; Brentassi et al., 2017; Mariño, Verle Rodrigues, & Bayman, 2017; Ma & Schwander, 2017).
Many galling insects are known to have microbial associates that may be involved in gall development or could facilitate herbivory, such as Ambrosia gall midges associated with fungal symbionts, but studies exploring the role of microbial associates in the lifecycles of insect gallers are scarce (Hansen & Moran, 2014; Tooker & Helms, 2014; Huang et al., 2015). Bacteria of the genus Wolbachia have been associated with green-island formation by the apple leaf-mining moth Phyllonorycter blancardella, a similar phenomenon to the one observed in some types of plant galls induced by insects (Kaiser, Huguet, Casas, Commin, & Giron, 2010; Gutzwiller et al., 2015). Their results suggest that bacteria impact green-island induction by manipulating cytokinin levels. In addition, secretions of phytohormones, such as cytokinins, by endosymbiotic microorganisms have also been associated with the plant-galling insect interaction (Spíchal, 2012). Likewise, Bartlett and Connor (2014) hypothesized that the inducing insects obtained their ability to induce galls via endosymbiotic microbes, which have acquired the biosynthetic pathways to produce IAA and trans-zeatin family cytokinins from plants. It is not surprising then that the control of cytokinins constitutes an important selection factor for arthropods and pathogens because of the importance of these phytohormones in the regulation of plant morphology, senescence, and defense, especially with regard to the mobilization of nutrients in each of these processes (Giron, Frago, Glevarec, Pieterse, & Dicke, 2013; Tooker & Helms, 2014; Naseem, Wölfling, & Dandekar, 2014; Giron et al., 2016).
It has been proposed that galling insects acquired genes from symbiotic microorganisms through horizontal gene transfer (Giron & Glevarec, 2014; Bartlett & Connor, 2014). Horizontal gene transfer (HGT) is the movement and transference of genetic information between different organisms, and it is a common phenomenon between pathogens of animals and plants, and between symbionts and pathogens (De la Cruz & Davies, 2000; Suzuki et al., 2015). Indirect evidence supporting the previous hypothesis is provided by works such as those carried out by Nikoh et al. (2008). Molecular analyses performed by these authors in Wolbachia, one of the most abundant intracellular bacteria described in arthropods, as well as nematodes, suggested that approximately 30 % of Wolbachia genes are present in the nuclear genome of host insects. In this study, through fluorescence in situ hybridization techniques, they located the transferred genes of Wolbachia in the proximal region of the short arm of the insect X chromosome. The collected evidence indicated that this process of horizontal gene transfer was probably generated from an individual event. In another study, it was determined that the genome ofWolbachia pipientis contains high levels of repetitive sequences of DNA and also mobile genetic elements (Wu et al., 2004). In spite of its wide distribution and the effects of Wolbachia on the biology of its hosts, little is known about the molecular mechanisms that mediate the interaction between this bacterium and its invertebrate hosts (Wu et al., 2004; Chrostek & Teixeira, 2015).
Therefore, under the previous scenario, horizontal gene transfer (HGT) could play a fundamental roll in plant gall induction and evolution. In recent years, more evidence has shown that the molecular mechanisms involved in the different processes of symbiosis and pathogenesis present a series of common pathways which have revealed existing similarities in the modulation and interactions between pathogens and symbionts with their hosts (De la Cruz & Davies, 2000; Hentschel, Steiner, & Hacker, 2000; Rankin, Rocha, & Brown, 2011; Suzuki et al., 2015). Furthermore, the information generated by microbial genome sequencing studies has demonstrated that horizontal transference of genes is an important process and widely distributed within the evolutionary scenario of prokaryote organisms (Nikoh & Nakabachi, 2009; Jayaprakashvel, Bhrathi, Muthezhilan, & Hussain, 2017).
In addition to chromosomes, prokaryotes possess mobile genetic elements, such as genomic islands, plasmids, transposons, insertion sequences or bacteriophages, which allow them to induce structural and physiological changes, as well as the acquisition or loss of genomic regions. Moreover, the fact that a great number of pathogenic and symbiotic determinants are located in mobile genetic elements allows a source of permanent variation to be generated within these organisms. In addition, some authors have suggested that the acquisition and incorporation of plasmids into bacteria could constitute a process key to the adaption of these microorganisms to new ecological niches and to their development as symbionts or pathogens (Vivian, Murillo, & Jackson, 2001; Suzuki et al., 2015; Jayaprakashvel et al., 2017). Genetic variability plays a very important role by generating the conditions that allow the evolution of new types of interactions among organisms, thus HGT between different species could represent a powerful mechanism through which the final result of the interaction between a pathogen or symbiont and its host could be altered permanently (Hentschel et al., 2000; Suzuki et al., 2015; von Wintersdorff et al., 2016; Porse, Schou, Munck, Ellabaan, & Sommer, 2018).
Not only is HGT responsible for speciation and subspeciation in bacteria; it also constitutes an important mechanism in eukaryote organisms. There are sufficient information related to the role of conjugation processes in the transference of genetic information from bacteria to eukaryote cells. Such eukaryote cells include yeasts, filamentous fungi, and plant cells (De la Cruz & Davies, 2000; Rankin et al., 2011; Suzuki et al., 2015). For example, the mechanism through which Agrobacterium tumefacienstransfers genes from the bacterium to plant cells is well known: it occurs through the action of the T-DNA segment present in the Ti plasmid (Suzuki et al., 2015). Stable natural transgenic plants of sweet potato containing Agrobacterium T-DNA sequences with their foreign genes expressed at detectable levels in different tissues were reported by Kyndt et al. (2015). Likewise, the work ofDiao, Freeling, & Lisch (2006), provides evidence of HGT through the transposons of superior plants. In this regard, some bacteria, retroviruses, and DNA viruses constantly integrate different kinds of genetic elements into the chromosomes of animal and plant cells through mechanisms such as conjugation and transformation (De la Cruz & Davies, 2000, Oliver et al., 2006; Klasson, Kambris, Cook, Walter, & Sinkins, 2009; Nikoh & Nakabachi, 2009; Suzuki et al., 2015). Moreover, the mechanism by which eukaryotes acquire genes from distantly related organisms remains obscure (Suzuki et al., 2015).
Although, in general terms, it has been accepted that some kind of “chemical stimuli” (very likely a phytohormone) from the insect is involved in the induction and morphogenesis of galls (McCalla et al., 1961; Miles, 1968; Rohfritsch & Shorthouse, 1982; Hori, 1992; Leitch, 1994; Ananthakrishnan, 1998; Raman, 2011; Yamaguchi et al., 2012; Connor et al., 2012; Erb, Meldau & Howe, 2012; Giron et al., 2013, Tooker & Helms, 2014; Bailey et al., 2015; Oates et al., 2016, Giron et al., 2016), up to now it has not been possible to determine with certainty whether insects could synthesize phytohormones. However, Yamaguchi et al. (2012) found abnormally high concentrations of a type of zeatine in the glands of the “sawfly” Pontania sp. (Hymenoptera, suborder Symphyta) which, according to these researchers’ criteria could be strong evidence that this insect can synthesize cytokinins as well as IAA. Likewise, Shih, Lin, Huang, Sung, and Yang (2018) found evidence that gall induction could be related to the secretion of phytohormones like cytokinin and auxin, as well as Brassinosteroids (steroids hormones), from the inductor insect. In a similar direction, Bartlett & Connor (2014) showed evidence consistent with the hypothesis that exogenous cytokinins, in combination with IAA from the gall-inducing insect, lead to gall induction. Additionally, Brütting et al. (2018) demonstrated, using 15N-isotope labeling, the transference of the cytokinin N6-isopentenyladenine (IP) from the free-living herbivore and non-galling insect Tupiocoris notatus to Nicotiana attenuata plants via their oral secretions.
On the other hand, the possibility of a molecular induction mechanism in insect-induced plant galls that involves the transference of genetic elements has neither been considered nor explored extensively. Cornell (1983) suggested the possibility that the gall-inducing insect could insert some genetic elements, mutualistic viroid, or virus into the plant genome, which would regulate and control the process of gall formation. However, this author did not offer any evidence that could support this statement. The molecular basis of the induction of plant galls by insects is still unknown (Stone & Schönrogge, 2003; Raman, 2011; Oates et al., 2016; Bailey et al., 2015; Giron et al., 2016). Moreover, the physiological nature of the stimuli given by the inducing insect and the influence of its own genomic constitution, as well as the reaction generated by the plant, are questions that remain completely open.
Stone & Schönrogge (2003) mentioned three great problems or challenges in identifying the molecules responsible for the process of gall formation. First is the difficulty of establishing an appropriate assay for the plant tissues involved in the process of induction. Second, the possible inducing molecules used by insects could be chemically similar to those normally present in the plant. Third, since it is expected that the signals coming from the insect generate a cascade of responses in the plant, it would be very difficult to separate the first morphogenetic impact originated by the inductor from the secondary responses generated by the plant.
In the particular case of gall-inducing insects belonging to Cecidomyiidae, it has been reported that either the egg or the ovipositing female could generate the initial stimulus and that the larva, by secreting substances that promote the growth of plant tissue under its action, could cause the formation of the gall (Hori, 1992).
Regarding the family Cynipidae (Hymenoptera), different studies have associated both auxins and cytokinins with the processes of gall induction and morphogenesis. Moreover, the morphogenesis and induction of these structures have been correlated with the activity of oviposition of the female, secretions of the insect egg, and the activity and secretion of chemical substances from the larva (Miles, 1968; Hori, 1992, Shorthouse & Rohfritsch, 1992; Raman, 2011). As with the galls formed by insects from the family Cecidomyiidae, the mechanism of morphogenesis of galls formed by cynipids cannot be explained simply by the action of plant phytohormones. Nevertheless, Boysen-Jensen (1952) and Miles (1968) support the hypothesis of chemical induction, arguing that the larva moves instinctively and secretes regulatory substances in the proper locations of the “attacked” tissue at specific times, thereby generating a suitable environment that favors the development of the gall.
“Omics” is an informal term that refers to fields of study in biology ending in -omics, such as genomics, proteomics, or metabolomics, among others. Emerging work conducted with new omics technologies is expanding our understanding of some relevant aspects relating to plant gall induction and morphogenesis. In a recent paper regarding the identification of the galling effector repertoires of the Hessian fly, it was shown that around 7 % of its genome encodes putative effector proteins, which include the secreted salivary gland protein (SSGP)-71, a known member of an arthropod protein family (Zhao et al., 2015). Moreover, these authors showed that although SSGP-71 lacks sequence homology with other proteins, its structure resembles both ubiquitin E3 ligases from plants and E3-ligase-mimicking effectors from plant pathogenic bacteria. Protein analyses indicate that the mature SSGP-71 protein contains a cyclin-like F box domain near the N-terminus and a series of leucine-rich repeats (LRRs). F box domains are frequently associated with LRRs, and both domains mediate protein-protein interactions, according to Ho, Tsai, and Chien (2006). These types of proteins are associated with the transfer of ubiquitin to target proteins destined for degradation in the proteasome. In addition, they play essential roles in phytohormonal signaling, plant development, and plant immunity. Zhao et al. (2015) also proposed that SSGP-71 proteins are a novel class of F-box-LRR mimics that enable the insect to hijack the plant proteasome in order to directly produce nutritive tissue and additionally defeat basal plant immunity. These authors further propose that their results prove that these effectors are the agents responsible for arthropod-induced plant gall formation. Likewise, Shih et al. (2018) demonstrated, by using transcriptome analysis, the modification of normal plant tissue to form galls. Moreover, they indicated that the manipulation of genes related to gall formation might be induced by auxin, cytokinin, and even steroid hormones (Brassinosteroids) secreted by gallers of Hemiptera, Lepidoptera, and Diptera. Similarly, other transcriptomic and genomic studies provide evidence leading to altered gene expression in galled plant tissues. These altered genes and effector proteins could be involved in several aspects of gall insect biology, including feeding, metabolic alterations, suppression of defense responses, and developmental manipulation of the host plant tissue (Rawat, Neeraja, Nair, & Bentur, 2012; Hearn, 2013). Even more interesting, Hearn (2013) also determined that genes expressed in gall wasp genomes encode plant-cell-wall-degrading enzymes that could originate from plant pathogenic bacteria. Pawłowski, Staszak, Karolewski, and Giertych (2017), using a proteomic approach to compare the galls induced by three oak gall species, Cynips quercusfolii, Cynips longiventris, and Neuroterus quercusbaccarum, with non-gall plant tissue in the host plant Quercus robur, described several proteins that could potentially be related to plant gall formation. On the other hand, for non-insect galls, a transcriptomic approach by Olszak et al. (2018) showed evidence that galls induced by Plasmodiophora brassicae in Arabidopsis reprogram critical steps of the host cell cycle. That distortion leads to initial cell hyperplasia, which increases the number of cells, followed by overgrowth of cells colonized by the pathogen. The authors showed that P. brassicae infection stimulates the formation of the E2Fa/RBR1 complex and upregulation of MYB3R1, MYB3R4, and A- and B‐type cyclin expression. Those cell cycle factors were previously described as important regulators of the G2‐M cell cycle checkpoint.
An interesting survey in nematode galls (Meloidogyne incognita), using high throughput sequencing for small non-coding RNAs, identified siRNA clusters that were differentially expressed in infected roots of Arabidopsis thaliana. Those siRNAs were overrepresented in infected tissue, with a size 23 - 24 nt, corresponding to heterochromatic siRNAs (hc-siRNAs), which are known to regulate the expression of transposons and probably genes at the transcriptional level, by an RNA-directed DNA methylation (RdDM) pathway that induces the silencing of transposable elements (Medina et al., 2018).
Insect-induced plant galls and phytochemistry
An interesting aspect of some plant galls is the particular or even radical phytochemistry between these structures and normal plant tissues. Research conducted on galls of different species of plants have revealed that the composition and concentration of chemical substances in these structures can differ from those of other plant tissues and organs (Tooker & de Moraes, 2008; Saltzmann et al., 2008; Giron & Huguet, 2011; Huang et al., 2015; Oates et al., 2015; Hall, Carrol, & Kitching, 2017; Kot, Jakubczyk, Karaś, & Złotek, 2017). Tissues near the outside of the gall frequently accumulate high levels of tannins and other chemical compounds related to the process of defense of the gall and, in consequence, of the insect (Ananthakrishnan, 1998; Li et al., 2017; Chen et al., 2018; Nogueira et al., 2018). A study byVereecke et al. (1997) revealed that the chemical composition of ethanol and aqueous extracts of galls produced in the leaves ofNicotiana tabacum differs drastically from that of non-infected plant tissue extracts. It has been reported that the concentrations of some carbohydrates such as hemicellulose, xylose, and arabinose increase during gall development in the treeZelcowa (Yeo, Chae, So, Lee, & Sakurai, 1997). Other authors have also reported differences in the concentrations of certain secondary compounds as well as certain types of phytohormones in plant gall tissue (Kraus & Spiteller, 1997; Pinkwart, Diettrich, & Luckner, 1998). Kot et al. (2017), Li et al. (2017) and Hall et al. (2017) demonstrated that galls induced by cynipid species and the wasp Leptocybe invasa (Hymenoptera: Eulophidae), respectively, contain high levels of phenolic compounds compared with control tissues. Moreover, increased production of waxes in the gall induced by the insect Baccharopelma spp. (Hemiptera: Psyllidae) in leaves of Baccharis spicata (Lam) Baill has been related to a protective function against desiccation by Agudelo et al. (2018).
Several authors have reported high concentrations of certain nutritive substances in gall tissues; some of those substances include sugars, proteins, phosphates, lipids, and nitrogen compounds (Tooker et al., 2008; Giron & Huguet, 2011; Huang et al., 2015; Li et al., 2017). In contrast, some researchers have described that galls can present low levels of certain chemical compounds related to the processes of plant defense, such as some phenolic compounds (Price et al., 1986; Agudelo et al., 2018).
Due to the fact that many galls present high quantities of certain nutrients and low levels of other chemical substances that are damaging to insects, a hypothesis has been proposed related to the galler being able to manipulate the development of its host plant by generating a tissue with a higher nutrient value (nutritional hypothesis). Nevertheless, several studies conducted with the goal of proving this hypothesis revealed that the concentrations of certain chemical compounds considered as defensive in plants are higher in the gall tissues, which is contrary to the above-mentioned hypothesis and suggests the need for a reconsideration of the same (Nyman & Julkunen, 2000).
Taking into consideration studies such as the those carried out by Nyman & Julkunen (2000), Tooker & De Moraes (2008), Tooker et al. (2008), Giron & Huguet (2011), Huang et al. (2015), Oates et al. (2015), Li et al. (2017), Kot et al. (2017), Chen et al. (2018), and Agudelo et al. (2018), comparing the chemical composition of galls with that of normal plant tissue, the conclusion would be that gall-inducing insects could control some the chemical properties of these structures.
Conclusions and perspectives
Although the chemical induction hypothesis has been accepted with some discretion and questioning as the general mechanism of plant gall induction, there are, so far, no related studies on a putative induction mechanism involving exogenous genetic elements in the process of insect gall formation. Moreover, little has been speculated in relation to this topic. A possibility exists that the control of induction and morphogenesis of insect galls could be under strict genetic control, possibly mediated by the insertion of mobile genetic elements into the genome of plant gall cells. Likewise, that process could be mediated by means of an endosymbiotic bacteria from the insect. Thus, due to the demonstrated ability of the inductor to manipulate the process of morphogenesis in insect galls, the galling insect should be able to control the regulation and expression of those exogenous insertion sequences at different levels. Consequently, under this hypothetical scenario, the insertion sequences would function as mediators of the molecular interaction between animal and plant systems. Genes contained in these possible insertion sequences could be those related to the control of the host cellular machinery and analogous phytohormones genes to those present in the host plant, among others. Virtually no work has been conducted in this direction, probably because of insufficient knowledge and the complexity of insect-plant-gall system relationships.
On the other hand, if a relatively simpler plant gall induced by Agrobacterium species involves a complex interaction between the inductor organism and its host plant, which is mediated by the insertion of genetic elements into the genome of the host cells, why is it not then assumed that a similar or even more complex mechanism exists for the induction of more complex plant galls, which could also be induced by the delivery of genetic elements from the cecidogenic organism? We could also rephrase the question as follows: is cellular self-proliferation an essential requirement or condition for the genetic transformation of plant cells, as occurs in the case of “crown galls” induced by Agrobacterium?
It is essential to conduct studies to understand, at the molecular level, the mechanism of induction and morphogenesis of plant galls induced by insects, exploring the presence of any possible symbiotic organism and some kind of external genetic element to the plant gall cells, associated with any of the symbionts. With this goal in mind, an appropriate gall induction model system should be chosen. The choice of an insect-plant gall system to be used as an experimental model should take into account gall diversity and morphologic complexity in order to include, in the same plant species, prosoplasmic and kataplasmic gall types. The next step would be to compare the similarities and differences at the molecular level, among different kinds of galls and how these could affect the extraordinary morphology and diversity observed in nature. Due to the diversity of shapes, colors, and complex structures displayed by insect galls, these systems could constitute ideal models to study how form and structure are determined at the molecular level in biological systems, more specifically, taking as a parameter plant morphology.
Due to their physiological and biochemical particularities, the identification of chemical substances or even specific genes in plant galls that could be of interest or have practical applications, according to the genetic transformation hypothesis postulated in this article, could result in these tissues becoming real germplasm sources, which may have a great impact on conservation policies and offer a promising background for the development of applied biotechnologies. Considering all the above information, it is clear that plant galls represent an important germplasm sink and an otherwise promissory gene bank that should be explored, used, and preserved as an authentic treasure of our biodiversity.












 uBio
uBio 

