Introduction
Rhodoliths are incrusting, non-articulate aggregates of calcareous algae that usually occur in shallow waters, providing hard substrate for other organisms (Foster et al., 2007; Amado-Filho et al., 2012). These algae retain calcium carbonate, and provide important information on paleoclimatic and paleo-environmental conditions in a particular area (Amado-Filho & Pereira-Filho, 2012; Aguirre, Braga, & Bassi, 2017). Rhodolith banks are recorded from several regions in the world, mainly in the Gulf of California, in the Caribbean, along the Atlantic coast of Canada, in Great Britain, Norway, Japan, and Australia (Foster, 2001; Amado-Filho & Pereira-Filho, 2012). In Brazil, they may represent the largest known depositional bank of CaCO3 worldwide (Amado-Filho et al., 2012).
Rhodoliths represent an important tridimensional, biogenic environment (Nelson, 2009; Otero-Ferrer et al., 2018), being known as “bioengineers” or “habitat modifiers” (Bruno & Bertness, 2001). They provide shelter and food for a large diversity of benthic invertebrates (Riul, Lacouth, Pagliosa, Christoffersen, & Horta, 2009; Amado-Filho & Pereira-Filho, 2012). Bosence (1979) indicated that the internal morphology of some types of rhodoliths, which do not have interconnected inner branches, favor the colonization by polychaetes and mollusks.Thus, the conservation of rhodoliths guarantees the habitat of a large diversity of the marine fauna.
Previous work across the world has recurrently highlighted that this habitat holds a large diversity and quantities of marine invertebrates (e.g. Bosence, 1979; Giménez-Casalduero, Rodríguez-Ruiz, Vivas, & Ramos-Esplá, 2001; Hinojosa-Arango & Riosmena-Rodríguez, 2004; Metri, 2006; Prata, Costa, Manso, Crispim, & Christoffersen, 2017; Otero-Ferrer et al., 2018), revealing the importance of these habitats as refuge sites for the young stages of mollusks and several commercial fishing stocks (Riosmena-Rodríguez et al., 2017). Rhodoliths may thus be considered hotspots of biodiversity. Although relatively resilient against environmental oscillations ( Riosmena-Rodríguez, Nelson, & Aguirre, 2017), rhodolith banks are extremely vulnerable to human activities, such as the extraction of commercial species associated with the rhodoliths, petroleum surveys and leakages, platform constructions, traffic of vessels (Horta et al., 2015), fishing activities, effluent discharges (Horta et al., 2016) and tourism. Besides, in the sphere of global ecological changes, global warming and ocean acidification can also affect rhodolith seabeds (Riosmena-Rodríguez et al., 2017). The impacts of these activities may contribute significantly to their survival but also to the loss of the associated diversity. Europe has implemented conservation measures that place the rhodolith habitat as a priority in the list of habitats requiring conservation, restoration and monitoring (OSPAR - Convention for the Protection of the Marine Environment of the North-East Atlantic) (Nelson, 2009; Instituto Brasileiro de Petróleo, Gás e Biocombustíveis [IBP], 2014). Rhodoliths have been included in a network of protection areas known as “Natura 2000” (Riosmena-Rodríguez et al., 2017). Despite these recognitions of the importance of rhodoliths and their associated species, in tropical zones such as the Brazilian coastline, conservation measures for rhodolith banks are still scarce (Horta et al., 2016).
Polychaetes have an important role in trophic chains. They may occupy distinct niches and have a high diversity of species and distinct levels of tolerance to different types of pollution and environmental impacts. Polychaetes are thus considered good bioindicators of human-caused perturbations (Reish, 1979; Bellan, 1980; Samuelson, 2001; Berlandi, Figueiredo, & Paiva, 2012). On the other hand, polychaetes represent up to about 70% of the benthic macrofaunal biomass of shelf environments. Consequently, they may reflect the general condition of the macrofauna (Knox, 1977; Paiva, 1993). Several studies use the assemblages of polychaetes to evaluate the state of ecosystems (Giangrande, Licciano, & Musco, 2005), mainly in soft-bottom environments, but few in “hard-bottom” habitats (e.g. Bellan, 1980; Giangrande, Delos, Musco, Licciano, & Pierri, 2004; Giangrande et al., 2005; Hinojosa-Arango & Riosmena-Rodríguez, 2004), including rhodoliths (e.g. Giménez-Casalduero et al., 2001; Berlandi et al., 2012). Such studies have revealed a high diversity of polychaetes inhabiting rhodolith seabeds, in particular specimens belonging to the families Eunicidae, Nereididae and Syllidae, but also some uncommon species (e.g. Amphicteis gunneri (M. Sars, 1835), Pherusa scutigera (Ehlers, 1887) and Phyllodoce schmardaei Day, 1963) (Giménez-Casalduero et al., 2001; Neves, 2011; Costa, 2016).
The present study analyzed the structure of the polychaete assemblage associated with rhodoliths in the reef environment at Seixas Beach, João Pessoa, Paraíba, in Northeast Brazil. The Seixas Beach is subjected to a high touristic pressure, mainly in the dry season, which has increased gradually over time (G1-Paraíba, 2016). Indeed, in the year 2015, the surrounding city of João Pessoa received around 1 210 000 tourists (G1-Paraíba, 2016). The beach also has coral reef areas, in depths between 3.0 to 6.0 m that are highly used as recreative areas. These activities result in the presence of numerous vessels (with a capacity of 100 people/vessel during the dry season, Melo, Lins, & Eloy, 2014) and diving activities within those depths (Melo, Crispim, Lima, & Nishida, 2006). We thus plan to evaluate comparatively the composition of the polychaete assemblages in rhodoliths at two depths (1.5 and 4.0 m), and per month, that correspond to the two seasons of the year (January, September and November-dry season, and March, May and July-rainy season), to study the effect of pressure exerted by tourism. We hypothesized that increased tourism pressure occurs at the deepest (4.0 m) than in shallowest area (1.5 m), due to the presence of vessels and due to their use as a recreational diving area, and during the months of the dry season, which coincides with an increase in touristic activities.
Materials and methods
Study area and sampling: The study was conducted at Seixas Beach (07º09’ S and 34º47’ W), located on the coast of the State of Paraiba, Northeast Brazil. This region has an equatorial climate with a dry summer (Alvares, Stape, Sentelhas, Gonçalves, & Sparovek, 2013). The rainy season lasts from March to August, with a total cumulative annual precipitation varying from 900 to 1 800 mm (Lima & Heckendorff, 1985). The annual temperature range is low, with temperatures varying between 24 and 27 ºC. The coastal reefs at Seixas Beach are located approximately 700 m from the shoreline, in the Municipality of João Pessoa (Melo, Crispim, Viana, & Lins, 2008). Depth in the reef areas varies from 0.5 to 1.5 m, during low tide, and from 3.0 to 6.0 m at high tides (Melo, Lins, & Eloy, 2014). The latter depths become exposed to the turbulence provoked by vessels known as catamarans, which are largely used to transport tourists, and diving activities in the region in recent years.
Sampling took place during low tide in 2015, with a bimonthly periodicity (i.e., January, March, May, July, September and November), and at two depths, 1.5 m (07º09’13” S e 34º47’21” W) and 4.0 m (07º09’13” S and 34º47’10” W). As mentioned above, the depth 4 m is potentially more affected by tourism pressure than the area at 1.5 m. An initial visual prospection also confirmed this trend. One quadrat of 15 x 15 cm was placed in each depth at each sampling date and all rhodoliths present within the quadrat were collected (usually four to five rhodoliths), in a total of 12 samples. Samples were placed in bags and taken to the laboratory, where they were processed. First the calcareous algae were identified and then the rhodoliths were broken using a grinder, to remove the associated fauna. All polychaetes were removed, stored in 4 % buffered formalin solution and washed in the laboratory. The specimens were then sorted and preserved in 70 % ethanol, and subsequently identified to the highest possible taxonomic resolution and counted. Polychaetes were identified based on the specialized literature, e.g. Day (1967a, Day, 1967b), Nonato & Luna (1970), Fauchald (1977, Fauchald, 1992), Uebelacker & Johnson (1984), Amaral & Nonato (1994), Blake, Hilbig, & Scott (1995, 1996, 1997), Camargo & Lana (1995), Capa (2003), Santos & Lana (2003), Viéitez et al. (2004), Barroso & Paiva (2007), De Assis, Samiguel, & Christoffersen (2007), Costa, De Assis, & Christoffersen (2008) and Böggemann (2009).
Data Analyses: The following diversity indices were determined from the polychaete abundance matrix with the DIVERSE function in the PRIMER-PERMANOVA software: Species number (S); Shannon-Wiener diversity index (H′, loge individual-1); and Pielou’s evenness index (J). In addition, we checked the influence of tourism pressure on diversity index values, total abundance and polychaete assemblage composition, following an experimental design that does not use replication, and includes the factors “depth” (two fixed levels: 1.5 m and 4.0 m) and “month” (six fixed levels: March, May, July, September, November and January). Exploratory analyses showed that data were not parametric (i.e., Shapiro-Wilk test, p < 0.05; Levene test, p < 0.05). Therefore, all analyses were performed with the 2-factor PERMANOVA procedure, which allows testing the influence of two factors in a crossed design for multivariate or univariate data (Anderson, Gorley, & Clarke, 2008). We applied the PERMANOVA to test differences between depth and month for the assemblage total abundance, diversity indices, and species composition. Values of the assemblage abundance and diversity indices were converted into an Euclidean Distance similarity matrix. For the assemblage composition, the abundance per species matrix was converted into a Bray-Curtis similarity matrix, with a dummy variable to compensate for zero occurrences (Clarke, Gorley, Somerfield, & Warwick, 2014). The tested data were further explored using Principal Coordinates Analyses (PCoA), to clarify patterns of change. All analyses were performed with the PRIMER-PERMANOVA software (Clarke et al., 2014). In addition, Spearman correlation coefficients were calculated between the total abundance per depth and precipitation, using the library ‘Hmisc’, implemented in the R software (R Core Team, 2018).
Results
A total of 733 individuals, organized into 21 polychaete families, 36 genera, and 49 species were collected during 2015 at Seixas Beach (Table 1). A total of 445 individuals came from the station at the depth of 1.5 m, while another 288 specimens were captured at 4.0 m. This difference between sites was caused largely by the higher diversity and abundance obtained at 1.5 m in January. When grouped by season of the year, a total of 333 individuals were present in the dry season, against 440 for the rainy season. However, we did not find differences when comparing the total abundance for the two depths in the six months (2-way PERMANOVA, p > 0.01, Table 2). Also, the variation of the abundance and precipitation along the year did not show a clear correlation for each of the tested depths (Spearman, p > 0.2). The abundance peaked in January (dry season) at 1.5 m (182 individuals), while for 4.0 m the highest abundance was observed in March (rainy season, 109 individuals, Fig. 1).
Table 1 List of species of polychaetes found at two depths in Seixas Beach throughout 2015 (x = presence)
| Family | Species | January | March | May | July | September | November | ||||||
| 1.5 m | 4.0 m | 1.5 m | 4.0 m | 1.5 m | 4.0 m | 1.5 m | 4.0 m | 1.5 m | 4.0 m | 1.5 m | 4.0 m | ||
| Ampharetidae | Amphicteis gunneri (M. Sars, 1835) | X | |||||||||||
| Amphinomidae | Eurythoe complanata (Pallas, 1766) | X | X | X | X | X | X | X | X | X | |||
| Capitellidae | Capitella capitata (Fabricius, 1780) | X | X | ||||||||||
| Neopseudocapitella brasiliensis Rullier & Amoureux, 1979 | X | X | X | X | X | X | |||||||
| Chrysopetalidae | Bhawania obscura (Grube, 1868) | X | X | ||||||||||
| Cirratulidae | Cirratulus africanus Gravier, 1906 | X | |||||||||||
| Cirriformia capensis (Schmarda, 1861) | X | ||||||||||||
| Dodecaceria capensis Day, 1961 | X | X | X | ||||||||||
| Timarete punctata (Grube, 1859) | X | X | X | X | |||||||||
| Dorvilleidae | Dorvillea angolana (Augener, 1918) | X | X | X | |||||||||
| Eunicidae | Eunice atlantica Kinberg, 1865 | X | |||||||||||
| Eunice biannulata Moore, 1904 | X | X | X | X | X | X | X | X | X | X | |||
| Eunice filamentosa Grube & Örsted in Grube, 1856 | X | X | X | ||||||||||
| Eunice guanica (Treadwell, 1921) | X | X | X | X | |||||||||
| Eunice imogena (Monro, 1924) | X | ||||||||||||
| Eunice wasinensisFauchald, 1992 | X | X | X | X | X | X | X | X | X | X | X | ||
| Lysidice ninetta Audouin & H Milne Edwards, 1833 | X | X | X | X | X | X | X | X | X | ||||
| Lysidice unicornis (Grube, 1840) | X | X | X | X | X | ||||||||
| Marphysa angelensis Fauchald, 1970 | X | ||||||||||||
| Marphysa regalis Verrill, 1900 | X | X | X | X | X | ||||||||
| Marphysa stylobranchiata Moore, 1909 | X | X | X | X | X | ||||||||
| Palola brasiliensis Zanol, Paiva & Attolini, 2000 | X | ||||||||||||
| Flabelligeridae | Pherusa scutigera (Ehlers, 1887) | X | |||||||||||
| Hesionidae | Hesione splendida Lamarck, 1818 | X | X | X | |||||||||
| Oxydromus pugettensis (Johnson, 1901) | X | X | X | X | X | X | X | X | X | X | |||
| Syllidia amaralae Rizzo & Salazar-Vallejo, 2014 | X | ||||||||||||
| Lumbrineridae | Lumbrineris inflata Moore, 1911 | X | X | X | X | X | X | ||||||
| Lumbrineris latreilli Audouin & Milne Edwards, 1834 | X | ||||||||||||
| Lysarete brasiliensis Kinberg, 1865 | X | ||||||||||||
| Maldanidae | Nicomache lanaiDe Assis, Samiguel & Christoffersen, 2007 | X | X | ||||||||||
| Nereididae | Ceratonereis (Ceratonereis) singularis Treadwell, 1929 | X | X | X | X | X | X | X | |||||
| Nereis riisei Grube, 1857 | X | X | X | X | X | X | X | X | X | ||||
| Pseudonereis gallapagensis Kinberg, 1865 | X | X | X | X | |||||||||
| Oenonidae | Arabella iricolor (Montagu, 1804) | X | X | X | |||||||||
| Drilonereis falcata Moore, 1911 | X | X | |||||||||||
| Orbiniidae | Naineris dendritica (Kinberg, 1866) | X | X | ||||||||||
| Naineris setosa (Verrill, 1900) | X | ||||||||||||
| Phyllodocidae | Phyllodoce malmgreni Gravier, 1900 | X | |||||||||||
| Phyllodoce schmardaei Day, 1963 | X | ||||||||||||
| Pilargidae | Synelmis sotoi Salazar-Vallejo, 2003 | X | |||||||||||
| Polynoidae | Lepidonotus spiculus (Treadwell, 1906) | X | X | ||||||||||
| Lepidonotus squamatus (Linnaeus, 1758) | X | X | X | X | |||||||||
| Sabellariidae | Phragmatopoma caudata Krøyer in Mörch, 1863 | X | |||||||||||
| Sabellidae | Branchiomma nigromaculatum (Baird, 1865) | X | |||||||||||
| Jasmineira caudata Langerhans, 1880 | X | ||||||||||||
| Syllidae | Syllis guidae Nogueira & Yunda-Guarin, 2008 | X | X | X | X | X | X | ||||||
| Syllis prolifera Krohn, 1852 | X | ||||||||||||
| Terebellidae | Terebella plagiostoma Schmarda, 1861 | X | X | X | X | X | X | ||||||
| Terebella pterochaeta Schmarda, 1861 | X | ||||||||||||
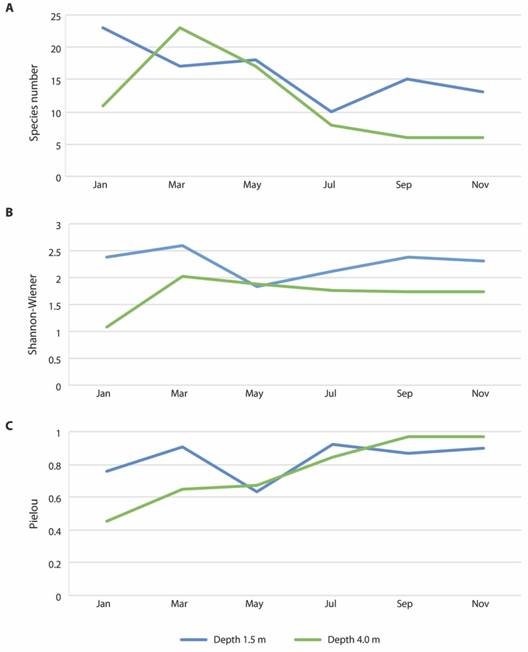
Fig. 1 Polychaete abundance at depths of 1.5 and 4.0 m, and monthly cumulative precipitation (station CEDRES) along 2015. January, September and November: dry season; March, May and July: rainy season (Source: Agência Executiva de Gestão das Águas do Estado da Paraíba [AESA/PB], 2018).
Table 2 Results of PERMANOVA for the total abundance of polychaetes, testing the factors Depth (1.5 and 4.0 m) and Month
| Source | df | Sum of Squares | Mean Square | Pseudo-F | P(perm) | Unique perms |
| Depth | 1 | 2054.10 | 2054.10 | 1.56 | 0.26 | 975 |
| Month | 5 | 22981.00 | 4596.30 | 3.50 | 0.11 | 997 |
| Residual | 5 | 6563.40 | 1312.70 | |||
| Total | 11 | 31599.00 |
Regarding the polychaete assemblage composition we found differences between months (2-way PERMANOVA, factor Month: Pseudo-F = 2.21, p-perm = 0.02, Table 3). Samples from January and May from both depths and from March from in 4.0 m alone clustered, despite being from different seasons (Fig. 2). No differences were found per depth (2-way PERMANOVA, p > 0.01). The most abundant species throughout the year was Eunice wasinensis, with a total of 280 individuals (38.2 % of the total abundance). Other common species that appeared in all sampling dates were Eurythoe complanata, Eunice biannulata, E. wasinensis, Lysidice ninetta, Oxydromus pugettensis and Ceratonereis (Ceratonereis) singularis (Tab. 1). Eunice wasinensis was the most abundant species in January (61 individuals) and May (45), at the depth of 1.5 m. In May, Terebella plagiostoma (29 individuals) and E. guanica (18) were also abundant. On the other hand, at the depth of 4.0 m, E. wasinensis was the most abundant species in January, March, and May (53, 58 and 41 individuals, respectively).
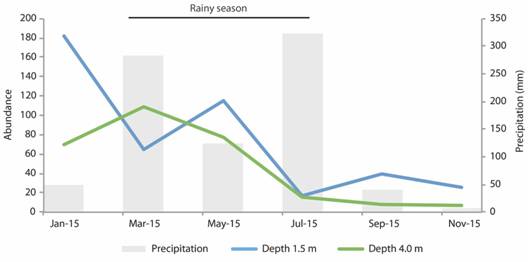
Fig. 2 Principal Coordinates Analysis (PCoA) of polychaete species composition with indication of the tested months within each season, and depths, in the rhodoliths beds of Seixas Beach.
Table 3 Results of PERMANOVA for the species composition of polychaetes, testing the factors Depth (1.5 and 4.0 m) and Month
| Source | df | Sum of Squares | Mean Square | Pseudo-F | P(perm) | Unique perms |
| Depth | 1 | 2465.40 | 2465.40 | 1.41 | 0.27 | 967 |
| Month | 5 | 19281.00 | 3856.10 | 2.21 | 0.02 | 998 |
| Residual | 5 | 8711.80 | 1742.40 | |||
| Total | 11 | 30458.00 |
Regarding diversity, the values of all indices showed a similar variation trend at both depths, and there was no clear pattern in relation to monthly variations (Fig. 3). Still, there was a tendency for higher values of all diversity indices to occur at the depth of 1.5 m, particularly in January. Yet, differences only occurred for depth in the Shannon-Wiener values (PERMANOVA - Depth: Pseudo-F = 9.49, p = 0.02, Table 4), which were generally higher at the 1.5 m depth (Fig. 3). For the other indices and seasons, there were no significant differences (p > 0.01, Table 5). Regarding Pielou’s evenness, values tended to increase along the year (Fig. 3).
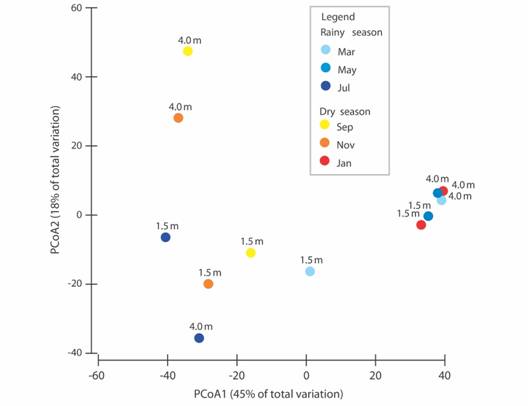
Fig. 3 Variation of diversity indices values for the polychaete assemblage associated to rhodolith beds at Seixas Beach, during 2015 at depths of 1.5 and 4.0 m.
Table 4 Results of PERMANOVA of Shannon-Wiener diversity of polychaetes, testing the factors Depth (1.5 and 4.0 m) and Month
| Source | df | Sum of Squares | Mean Square | Pseudo-F | P(perm) | Unique perms |
| Depth | 1 | 0.94 | 0.94 | 9.49 | 0.02 | 981 |
| Month | 5 | 0.38 | 7.62E-2 | 0.77 | 0.59 | 998 |
| Residual | 5 | 0.49 | 9.87E-2 | |||
| Total | 11 | 1.81 |
Table 5 Results of PERMANOVA of Pielou diversity of polychaetes, testing the factors Depth (1.5 and 4.0 m) and Month
| Source | df | Sum of Squares | Mean Square | Pseudo-F | P(perm) | Unique perms |
| Depth | 1 | 0.02 | 0.02 | 1.06 | 0.36 | 962 |
| Month | 5 | 0.20 | 0.04 | 2.56 | 0.18 | 997 |
| Residual | 5 | 0.08 | 0.02 | |||
| Total | 11 | 0.29 |
DISCUSSION
Our study showed that Eunicidae was the dominant family in the sampled rhodolith beds, E. wasinensis being the most abundant species throughout the study period, mainly at the deeper depth. Batista (2004) found a similar pattern when studying the polychaete fauna of rhodoliths in Cabo Branco Beach (close to the Seixas Beach): 68 % of the total abundance corresponded to the Eunicidae. This eunicid dominance, a taxon composed mostly of detritivores (Jumars, Dorgan, & Lindsay, 2015), has been associated with degraded habitats, a consequence of the presence of a large quantity of decomposing organic detritus from the reef substrate (Santa-Isabel, Leão, & Peso-Aguiar, 2000). However, other studies have also observed the mutualism between eunicid polychaetes and reef habitats, acting as reef aggregating agents (Roberts, 2005). So the dominance of eunicids in our study may be a reflection of such mutualism, and not necessarily the result of a degraded habitat. The presence of the genus Syllis spp. in the Seixas Beach, particularly in the depth 1.5 m, supports this generalization, since Syllis “sensu lato” has been described as sensitive or non-tolerant to organic pollution (Bellan, 1980; Surugiu, 2005). In temperate regions, such as in the Western Mediterranean (Iberian Peninsula), eunicids are also among the most represented polychaete families (Giménez-Casalduero et al., 2001). On the other hand, a study done along the littoral of Spain (along the coast of Catalonia) indicated the prevalence of Syllidae (with circa 31 % of the total) and Serpulidae (approximately 12 % of the total) (Martín, 1987; Berlandi et al., 2012).
The number of species of polychaetes found at Seixas was generally higher than that reported from other tropical areas. These numbers range between nine and 19 families, comprising usually less than 27 species (Batista 2004; Metri, 2006; Neves, 2011). The exception is represented by the paper of Riul (2007), in which at least 34 different families were found. These differences may be attributed, in part, to sampling procedures (e.g. periodicity), and to morphological differences in the algal composition (Berlandi et al., 2012). In addition, rhodoliths may be colonized by Bryozoa, Hydrozoa, Porifera and others taxa, which may, in turn, compete with polychaetes for the available space (Berlandi et al., 2012). Still, the generally higher number of species in this study suggests that, despite the gradual increase of tourism pressure at Seixas Beach, their rhodolith beds still present high polychaete diversity, associated with a good preservation of the beds.
Regarding comparisons of the present data, our initial hypothesis was that the 4.0 m depth would be more subjected to tourism pressure, due to the presence of vessels, anchoring on the coral reefs and diving activities, and therefore would have lower diversity and abundance. In addition, we speculated that the dry season would also be more subjected to disturbance, since tourism pressure is more intensified during this season (Melo et al., 2014). Our results, however, were only partially congruent with these hypotheses. There were no differences in the total abundance, species richness, and Pielou values of the assemblages of polychaetes, when comparing depths and months. For the species composition there were significant differences per month, but these did not affect the whole dry season, since the species composition was more similar for the months from January (dry), March and May (rainy). For the Shannon-Wiener index alone, values were significantly higher in the shallower depth, 1.5 m, which is compelling with our initial hypothesis. The Pielou index was lower in the depth of 4.0 m than in 1.5 m for January and March, suggesting less even assemblages, mainly due to the dominance of E. wasinensis in the deeper region. During the following months, Pielou evenness increases, suggesting a homogenous distribution of abundance among species. However, abundance was also extremely low. As mentioned, part of the tourism pressure on the beach is due to catamaran traffic and anchoring, whose influence is likely to be more intense at the depth of 4.0 m (Melo, Lins, & Eloy, 2014; Melo, Crispim, Lima, & Nishida, 2006), and may therefore be contributing to differences between depths. Yet these differences were only found for a single descriptor and were not found between seasons, suggesting that the higher tourism pressure expected during the dry season does not affect the polychaete assemblage. The relatively high evenness throughout the whole study also suggests that abundance is regularly distributed among different taxa along the year.
Overall, based on these results, we are not able to conclude that there is a potential negative effect of tourism on the polychaete assemblage associated to the rhodolith beds at the Seixas Beach.However, our results refer to a single beach (without spatial replication) and this cannot be extrapolated for a wider geographical range. More information (comparisons of different beaches with different tourism pressures) and other formal tourism indicator measurements are needed in order to reach more solid conclusions on the effects of tourism. Their impact may be quite high in Brazil, but this effect is generally not being monitored. Also, wider and long-term monitoring programs are needed throughout the Brazilian coast to identify diversity patterns and what may be affecting these in order to better preserve the rhodolith beds (Horta et al., 2016). The present study nevertheless contributes to increase the knowledge of the polychaete diversity associated with rhodolith beds in tropical areas, and highlights the need for more knowledge and studies on the impacts of local stressors on these assemblages.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

