Acrostichum L. is a member of the Pteridaceae family and Parkerioideae subfamily (Smith et al., 2006; PPG I, 2016); it belongs to the Ceratopteridoid clade and is sister to Ceratopteris, and represent the only aquatic genera of Pteridaceae (Schuettpelz & Pryer, 2007). Acrostichum aureum L. is pantropical, A. danaeifolium Langsd. & Fisch. neotropical and A. speciosum Willd. paleotropical. The genus is typically found in brackish or saline habitats near coasts. It can form dense stands following logging of mangroves and in freshwater swamps (Mickel & Smith, 2004), but it also can grow farther in land wet soil (León, 1990).
In Mexico only A. aureum and A. danaeifolium are found, which can be easily distinguished among them by the puberulent lower surfaces of the pinnae in A. danaeifolium, and glabrous in A. aureum. Also, the paraphyses are distinctive, being capitate with a dark and circular-lobed apex in A. aureum, and pale and non-capitate in A. danaeifolium (Adams & Tomlinson, 1979). Another difference is that in A. aureum, the leaves are fertile only in the five distal pinna pairs and terminal pinna, whereas in A. danaeifolium the fertile leaves are soriferous throughout their length. In general, A. danaeifolium is much more common than A. aureum (Mickel & Smith, 2004).
The first study of Acrostichum gametophyte was made by Schumann (1915). He studied and described the A. aureum gametophyte and sporophyte development, and compared its sporophyte with other acrostichoid species of Leptochilus Kaulf., Stenosemia C. Presl and Stenochlaena J. Sm., and he described the asymmetrical antheridia of Acrostichum as a peculiarity of this genus.
Nayar and Kazmi (1964) studied the gametophyte and young leaves of A. aureum. Gametophyte development is similar to the one reported by Stokey and Atkinson (1952) for A. speciosum. In A. aureum and A. danaeifolium, the prothallial plate expands laterally by the increased activity of some of the lateral intercalary cells of the ribbon thallus, and develops into a broad spatulate plate in which a meristem is differentiated on the side facing the posterior end of the prothallus, contrary to the condition in A. speciosum (Nayar & Kazmi, 1964).
Lloyd and Gregg (1975) described the gametophyte development of A. danaeifolium as similar to the reports made by Stokey and Atkinson (1952) and Nayar and Kazmi (1964). Eakle (1975) evaluated spore germination of A. aureum in experimental environmental conditions, and found that a photoperiodic quantitative long-day phenomenon occurs, with a length between 8 (dark) and 16 (light) hours per day. Lloyd (1980) described the development of the gametophyte of A. aureum coinciding with other authors on its morphology, and assessed sexual ontogeny. On the other hand, the juvenile leaves of the sporophyte are generally spatulate and nearly entire. The single vein supplying the lamina is forked once or twice they are dichotomizing two or three times. All the youngest leaves possess a simple lamina with a nearly entire margin, though herbaceous, the lamina is thick, lack of hairs (Stokey & Atkinson, 1952).
Kshirsagar and Mehta (1979) studied the “in vitro” life history of the fern A. aureum, made by factors that influence sex expression, density of gametophytes, pH, and sucrose in the gametophytes, and examined the formation of normal and apogamous sporophytes. They found that the gametophytes were potentially bisexual, but a neutral or alkaline pH favors the expression of archegonia. Growth responses of A. aureum suggest it can be classified as a true halophyte, whereas those of A. danaeifolium suggest it is a semi-halophyte (Li & Ong, 1997a, 1997b, 1998; Sun, Li, & Ong, 1999).
The aim of this work was to study the spore morphology, spore germination, gametophyte development, and young sporophytes morphology of A. aureum and A. danaeifolium, to obtain characters for systematic comparison, and contribute to the knowledge of the ontogenetic development of the sexual phase.
Material and methods
Study site and spore collection: Collections were made at Sontecomapan lagoon, Catemaco, Veracruz, Mexico, in a mangrove swamp at sea level, 18.59° N, 95.08 W. A total of 10-20 fertile pinnae of A. aureum (FNG293A and ACV01) were collected on 14 May 2014, dry season, and A. danaeifolium (FNG293B) were collected 22 June 2013, rainy season; vouchers were deposited at the UAMIZ (Universidad Autónoma Metropolitana, Iztapalapa Herbarium). Fertile pinnae were kept in paper bags envelopes and dried for eight days at room temperature to favor sporangia opening and subsequent spore liberation. The material was then sieved with a metallic mesh (with pores 0.074 mm in diameter) to eliminate traces of sporangia.
Sowing: Spores were sown in four Petri dishes (5 cm in diameter), containing agar, previously enriched with sterilized Thompson media (Klekowski, 1969). The spores were spread on the surface of solidified medium with a thin brush, with an average density of 40 for A. aureum and 36 spores/cm2 for A. danaeifolium. To avoid contamination and dehydration, the dishes were kept within transparent plastic bags in growth chamber under a 12 h light/dark photoperiod (Lumistell ICP-19). To determine photoblastism, one of the dishes was kept in the dark.
Electron microscopy: Dry spores were spread with a brush and mounted on aluminum stubs using double-sided tape, in accordance with Bozzola and Russell (1998), and were coated with gold using a Denton Vacuum Desk II sputter coated. Besides, gametophytes were fixed in 0.8 % FAA-sucrose for 24 h; then, they were washed, dehydrated in a graded ethanol series, and critical-point dried by means of a Leica CPD 030 critical point dryer. After drying they were mounted in a similar manner as the spores. Photomicrographs of spores, prothalli, and the young sporophytes were made with a Jeol JSM5310-LV scanning electron microscope. Digital images were obtained in TIFF format using the Orion-Joel digital system.
Results
Spore morphology: Trilete, tetrahedral-globose to nearly globose, radially symmetrical, in proximal view. Spores of both species in proximal view, measured an average of 19(23)28 µm for A. danaeifolium (Figure 1A), and 19(22)25 µm for A. aureum (Figure 1B). They are homosporous, hyaline with a weakly brownish color, non-chlorophyllous, with the laesura short. Differences between the spores of the two species are found in the exine. In A. aureum, the exine is slightly rougher with the surface papillate to tuberculate, with rodlets attached to the tubercles in proximal and distal view. Whereas the exine of A. danaeifolium is rugose, with strands or rods mostly associated with coarsely papillate structure, papillate surface in proximal and distal view.
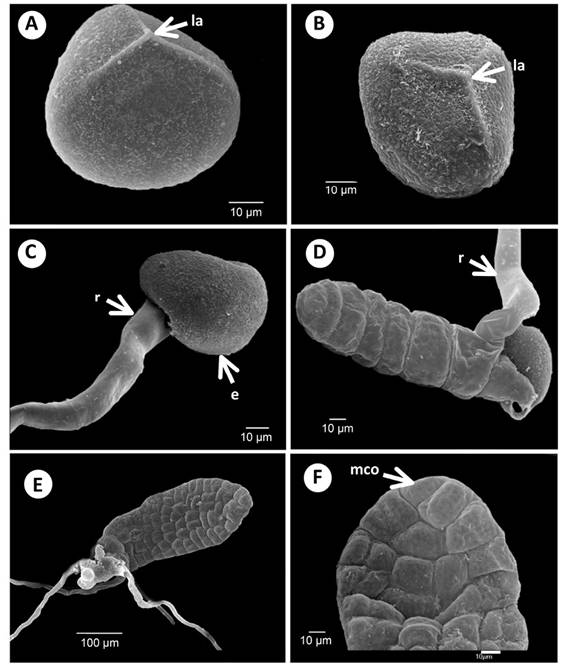
Figure 1 Spore morphology, early germination, and young gametophytes of Acrostichum: A) Spore proximal view of A. danaeifolium. B) Spore proximal view of A. aureum. C) Beginning germination of A. danaeifolium, 18 days. D) Filament gametophyte of A. danaeifolium, 18 days. E) Bidimensional gametophyte of A. danaeifolium, 22 days. F) Close up of meristematic cell of A. danaeifolium, 24 days. la. Laesura. mco. Meristematic cell obconic. e. Exine. r. Rhizoid.
Germination and filamentous phase: In accordance with Mendoza-Ruiz (2001), the criterion used in this study to determine spore germination is the formation of the first rhizoid and/or prothallial cells or both simultaneously. Under our culture conditions, germination took place on the 5-6 days after sowing. The germination percentage was high (90 - 95 %) for A. danaeifolium, but lower (70 %) for A. aureum. After 100 days, none of the spores grown in darkness germinated.
In both species, the basal cell second division produces a brownish rhizoid that emerges through the laesura, and forms the first initial filament cell (Figure 1C-D). The cell wall plane of the second division is perpendicular to the cell wall plane of the first division. The preceding type of germination is defined as the Vittaria-type.
The filament initial cell then divides in the same plane to form a filament of 9-13 cells, in A. aureum (29 - 40 days), and A. danaeifolium (18 days) (Figure 1D). The filament barrel-shaped have cells with numerous chloroplasts, and develop 2 or 3 hyaline and non-chlorophyllous rhizoids, which are longer than the filament. The basal cell remains in the spore wall through the subsequent development of the vegetative and adult phases of the gametophyte. This phase is very brief, because one day later, the filamentous gametophytes begin to differentiate into laminar gametophytes.
Laminar phase: According to our observations, prothallial development for A. danaeifolium and A. aureum correspond to the Ceratopteris-type (Figure 1E-F and Figure 2A-C). Formation of a prothallial plate is initiated by longitudinal divisions in the anterior cells (often including the terminal cell) of the germ filament. The prothallial plate is spatulate with 55 cells in A. danaeifolium (24 - 28 days) and near 100 cells in A. aureum (33 - 40 days), a pluricellular meristem is established in this non-meristic plate (Figure 2B).
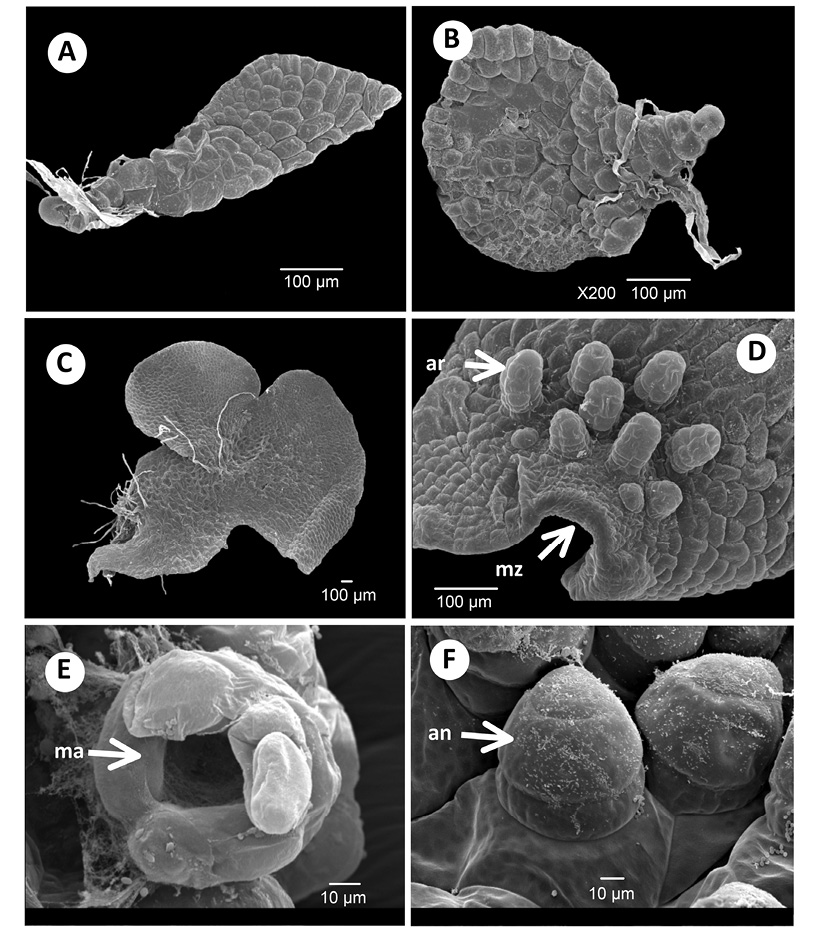
Figure 2 Young gametophytes and gametangia of Acrostichum: A) Bidimensional gametophyte of A. aureum, 29 days. B) Bidimensional gametophyte of A. aureum, 40 days. C) Bidimensional gametophyte of A. aureum, 70 days. D) Archegonia of A. danaeifolium, 90 days. E) Archegonia mouth of A. danaeifolium, 181 days. F) Antheridia of A. danaeifolium, 185 days. an. Antheridia. ar. Archegonia. ma. Mouth of archegonia. mz. Meristematic zone.
The young prothallus of both species is distinctly lopsided, and the asymmetry often persists until maturity. The meristem is established laterally, more towards the basal end of the prothallial plate than towards the apex. The asymmetry of the young prothallus is only transitory, soon the meristematic region becomes apical, and a symmetrical cordate thallus is formed (Figure 2C).
Adult gametophytes: Thirty days after sowing, the adult gametophyte in both species of Acrostichum is cordiform-spathulate or cordiform-reniform, and glabrous. It has broad wings, a thin cushion, and a deep notch. The wings are clearly demarcated (Figure 2C). Gametophytes reach maturity in approximately 90 days in A. danaeifolium and 76 - 196 days in A. aureum. Gametophytes of both species are bisexual with antheridia and archegonia on the same gametophyte (Figure 2D-F). Sex organs are of the common leptosporangiate type. Gametangia appeared only in the abaxial surface of the gametophyte with some archegonia near the notch (Figure 2D), and many antheridia distributed mainly towards the base, mixed between rhizoids.
Archegonia appeared first in A. aureum (76 days) and later in A. danaeifolium (90 days), they may be either protandrous or protogynous. The neck of the mature archegonia is short and curved away from the notched end of the gametophyte, and it is composed of four rows each with four cells long, and slightly curved towards the apical region (notch) of the gametophyte (Figure 2D). The mouth consists of four cells. Archegonia have an egg cell, a ventral-canal cell, and a neck-canal cell, which are surrounded by the layer of cells of the cover (Figure 2E).
Antheridia are hemispheric to subglobose and develop on the abaxial surface of the cushion and wings of cordiform-spatulate gametophytes of A. aureum (196 days) and A. danaeifolium (135 days) (Figure 2F and 3F). Antheridia consist of a basal cell, an annular cell, and an undivided asymmetrical opercular cell (Figure 3A). Antheridial dehiscence was not observed. Also, cordiform-spathulate gametophytes with abundant marginal and superficial antheridia were observed after 185 days of growth in A. danaeifolium.
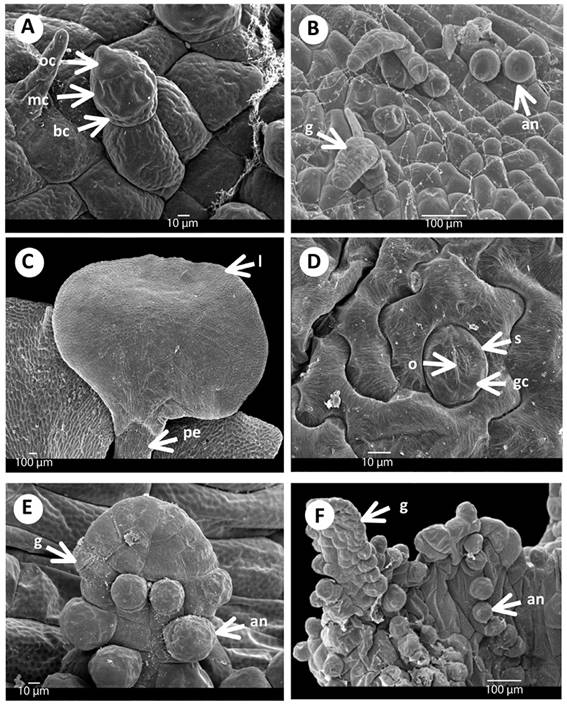
Figure 3 Gametangia and first leaf of Acrostichum: A) Antheridia of A. danaeifolium with asymmetrical opercular cell, 185 days. B) Gametophyte of A. aureum with antheridia and gemmae, 196 days. C) First leaf of A. aureum, 250 days. D) Anomocytic stomata of A. danaeifolium, 216 days. E) Gemmae on the gametophyte with antheridia of A. danaeifolium,185 days. F) Gemmae with antheridia of A. aureum, 185 days. an. Antheridia. bc. Basal cell. g. Gemmae. gc. Guard cell. l. Lamina. mc. Medial cell. o. Ostiole. oc. Opercular asymmetrical cell. pe. Petiole. s. Stomata.
Sporophytes: Despite the addition of droplets of sterile water to the cultures to facilitate antherozoid movement, in both species fertilization occurred after 250 days. Young sporophytes became visible on the prothalli in A. aureum at 250 days and in A. danaeifolium after 216 days, from spores sowing. These are glabrous and dichotomously branched with their first leaves depressed obovate, and with open dichotomous venation (Figure 3C). The petiole is long terete, and leaf epidermal cells are distinctly wavy and the stomata are of the anomocytic-type, with two guard cells that have a length of approximately 30 µm, and the stomata are surrounded by epidermal undulate cells wall (Figure 3D).
Vegetative propagation: These gemmae become visible in A. danaeifolium at 185 days and A. aureum at 196 days (Figure 3B and 3E-F). The prothalli produced gemmae which, when shed, develop into new prothalli. The gemmae are pluricellular, dumbbell-shaped, densely chlorophyllous structures. In fact, a single prothallial cell may give arise to many gemmifers each of which generates one or more gemmae. The presented results are compared with other authors in Table 1.
Table 1: Morphological comparison of the characteristics of the species of the genus Acrostichum and the genus Ceratopteris
| Character/taxa | A. aureum | A. danaeifolium | A. speciosum* | Ceratopteris* |
|---|---|---|---|---|
| Origin | Pantropical | Neotropical (American) | Paleotropical (Australasian) | Tropics |
| Habitat | Brackish and saline mangroves and swamps | Mangroves, brackish, saline or freshwater swamps, edges of hot springs | Mangroves, brackish and freshwater marshes | Aquatic and fresh or occasionally brackish |
| Sterile pinnae | Glabrous | Puberulent | Glabrous | Glabrous, succulent |
| Sporangia | Acrostichoid | Acrostichoid | Acrostichoid | 1-4 rows along the rib |
| Spores: size and laesura | Trilete, tetrahedral-globose 19-25 µm | Trilete, tetrahedral-globose 19-28 µm | Trilete, tetrahedral-globose | Trilete, parallel crested 70-160 µm |
| Type of germination | Vittaria | Vittaria | Vittaria | Vittaria |
| Type of prothallial development | Ceratopteris | Ceratopteris | Ceratopteris | Ceratopteris |
| Young prothalli | Lopsided | Lopsided | Lopsided | Lopsided |
| Adult form gametophyte | Cordiform-spathulate | Cordiform-spathulate and glabrous | Cordiform-elongate | Cordiform |
| Antheridia | Asymmetrical | Asymmetrical | Asymmetrical | Undivided cap cell |
| Sporophyte (days) | 250 | 216 | Not date | Not date |
| Vegetative propagation (days) | 196 | 185 | Not date | Not date |
* Stokey and Atkinson (1952); Tryon and Tryon (1982); Tryon and Lugardon (1990).
Discussion
Researchers such as Schumann (1915), Stokey and Atkinson (1952), Nayar and Kazmi (1964), Nayar and Kaur (1969 and 1971), Lloyd and Gregg (1975), Kshirsagar and Mehta (1979), Lloyd (1980), Lloyd and Buckley (1986) described the morphology of the gametophyte of A. aureum, A. danaeifolium, and A. speciosum, and our observations of gametophyte development data showed that they are similar, the difference being that the morphology is clearer with the scanning electron microscope.
The difference between the two species under study lies in the ornamentation of the exine, and this was described before by Erdtman (1971), Nayar and Kaur (1971), Lloyd and Gregg (1975), García de López (1978), Adams and Tomlinson (1979), Tryon and Tryon (1982), Kubitzki (1990), Tryon and Lugardon (1992), Moran, (1995), Mickel and Smith (2004) our data support the descriptions of these authors.
In most homosporous ferns, spore germination produces a primary rhizoid, followed by a germinal multicellular filament subsequent prothallial development based on the plane of cell division, and in the direction of the growth of the rhizoid and prothallial cell, this pattern of development was observed in A. aureum and A. danaeifolium. Early in germination, there is not significant morphological variation in day (5-7 / 6-7 days germination after sowing), these data are consistent with what was mentioned by Stokey and Atkinson (1952) and Nayar and Kazmi (1964).
Filament phase which is usually 6-8 cells but sometimes 10-12 cell long, the filamentous phase are 6-12 prothallic cells with barrel form and chloroplast (Stokey & Atkinson, 1952) and correspond to Vittaria-type germination that is, the basal cell (spore cell) divides to form a rhizoid cell. The cell wall plane is parallel to the equatorial plane of the spore. The second cell division forms the first filament initial cell, and the cell wall plane is at a right angle to that of the division, or parallel to the polar axis of the spore (Stokey & Atkinson, 1952). The prothalli of Acrostichum aureum and A. danaeifolium resemble that of A. speciosum (Stokey & Atkinson, 1952) in morphology and development, but there are minor differences especially with regard to the development of a prothallial plate.
In A. aureum and A. danaeifolium prothallial development is of the Ceratopteris-type; in this type of development, the meristem established laterally in young gametophytes is asymmetric (lopsided), for a shorter period during its development; all species in this study shared this character.
Sex organs are of the common leptosporangiate type in species of study, adult gametophytes are cordiform and glabrous. The asymmetrical antheridia, we consider it as a synapomorphy for Acrostichum (Stokey & Atkinson, 1952; Nayar & Kazmi, 1964; Nayar & Kaur, 1969, 1971; Lloyd & Gregg, 1975; Lloyd, 1980; Lloyd & Buckley, 1986). This asymmetry was described by Schumann (1915) for A. aureum; nevertheless, the opening of the cap cell was not seen in this investigation. Antheridial dehiscence occurs through a pore in the opercular cell (Nayar & Kazmi, 1964).
Archegonia are morphologically standardized and have little significant differences in numbers of neck cells and length (Stokey & Atkinson, 1952; Nayar & Kazmi, 1964; Nayar & Kaur, 1969, 1971).
In both species the sporophyte developed after the 250 days sowing, and they have on its abaxial epidermis stomata of anomocytic type that lack of subsidiary cells as described Van Cotthem (1984).
The development and structure of the gametophytes show some similar characteristics between the sister group Acrostichum and Ceratopteris: 1) Spore germination is of the Vittaria-type; 2) Prothallial developments is of the Ceratopteris-type; 3) Young prothalli are markedly lopsided; 4) Gametangia are the common leptosporangiate type.
However Acrostichum and Ceratopteris showed different features: 1) Acrostichum is typically found in brackish or saline habits near coasts, true halophyte or semi-halophyte, while Ceratopterisinhabits fresh or occasionally brackish wetlands and rarely in terrestrial situations on poorly drained soils worldwide of tropics and subtropics (Dettmann & Clifford, 1991); 2) Spores in Acrostichumand Ceratopteris are trilete, tetrahedral-globose to nearly globose, but they differ in ornamentation, 3) Antheridia in Acrostichum have an asymmetric opercular cell whereas Ceratopteris showed undivided cap cell.
The morphological similarities found in the study of the sexual phase of A. danaeifolium and A. aureum are important data for taxonomic purposes at a generic level, and if we add the knowledge of the sporophyte phase of the genus Acrostichum and Ceratopteris, the data supports the molecular phylogeny results, which place them as sister groups. All recent molecular data supportCeratopteris as a member of the Pteridaceae s.l. (Gastony & Rollo, 1995; 1998; Hasebe et al., 1995, Pryer, Smith, & Skog, 1995; Smith et al., 2006). Five primary clades of Pteridaceae have been resolved by molecular phylogenetic analyses (Prado, Del Nero Rodrígues, Salatino, & Salatino, 2007; Schuettpelz, Schneider, Huiet, Windham, & Pryer, 2007; Schuettpelz & Pryer, 2007). In all of these analyses, Ceratopteris is the sister group of Acrostichum (Mickel & Smith, 2004; PPG I, 2016).
The knowledge of the vegetative and reproductive morphology of gametophytes and characteristics such as spore morphology, spore germination pattern, gametophyte form, presence or absence of hairs, photosynthetic gametophytes or not, position, shape and number of antheridia and archegonia cells, number of cells of the antheridium wall and number of archegonia neck cells and gametophytes gemmae production, are diagnostic characters that will be important in future to apply in the knowledge of the phylogeny of ferns based on morphology and rbcL sequences (Pryer et al., 1995).












 uBio
uBio 

