Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.50 n.3-4 San José Dec. 2002
Abstract
Experimental preservation of velvet worms (phylum Onychophora), a very rare but evolutionarily important group that has existed for more than 500 million years, showed that the absence of bucal parts, adhesive-expelling organs, gonopore, eyes, legs, claws, annulation and papillation in fossils may not represent absence in the living animals. In fossils, leg thickness and claw orientation can be unreliable. The experiments indicate that not only absence, but even presence of certain structures can simply be the result of tissue decomposition. Computer-aided photorealistic reconstructions of fossil onychophorans are presented. We recommend future researchers to conduct taphonomy experiments specially before analysing unusual fossils.
Key words
Taphonomy, Cambrian, Onychophora, evolution, model, fossil, photorealistic reconstruction.
Onychophoran worms are rare and most biologists have never seen them alive, but they are important because they have been considered "missing links" between annelids and arthropods as well as "living fossils" because their shape has not changed for 500 million years (Monge-Nájera 1995 , Hou and Bergström 1997 ). Nevertheless, only four of the 130 known species have been studied in some detail (Monge-Nájera 1994b , 1995 , 1999 ) and invertebrate textbooks are not only outdated but also fail to transmit our ignorance about the great mayority of species ( Monge-Nájera 2001 ).
Except for mandibles, onychophoran bodies are soft and unlikely to fossilize, but special conditions led to the fossilitation of severas specimens (Monge-Nájera and Hou 2000 ), mainly in Chengjiang (China) and Burgess Shale (Canada), where they were marine during the Cambrian (all known living species are terrestrial and their continental distribution has been studied paleobiogeographically, Monge-Nájera 1996 ).
A recent study indicated that Cambrian onychophoran communities did not differ as much as expected from a modern coastal tropical community. The proportion of the total invertebrate population represented by onychophorans and even ecological indices that mathematically measure biodiversity were similar in the Cambrian and present ( Monge-Nájera and Hou 2000 ). Evidence suggests that onychophorans have been evolving as a separate group by more than 500 million years and that the modem concept of a Cambrian "explosion" reflects an artifact of fossilization, in agreement with what was believed nearly 200 years ago (Monge-Nájera and Hou 2000 ).
Experimental taphonomy of extant species has been found useful to interpret certain fossils for almost two centurias (see Briggs and Kear 1994 , Orr et al. 1998 for recent studies). This paper complements Monge-Nájera and Hou's (2000) paleoecological study by reporting on experimental decay of these rare animals and presents computer-aided photorealistic reconstructions of extinct onychophorans.
Materials and methods
To test the effect of compression and decay on onychophoran bodies five Epiperipatus biolleyi Bouvier, 1902 from Coronado, San José, Costa Rica (collected December 1992 and March 1993) were killed with an overdose of ethyl acetate ( Monge-Nájera and Morera 1994 ) and placed in a 0.5 cm thick bed of marine mud (from the Punta Morales mudflat, Costa Rica, describes by Vargas 1998 a,b , 1996 ) under a weight of 17 kg. This compression was used to simulate burial under a layer of sediment, as some believe was the case when the fossilized animals died (Conway Morris 1985 , Hou et al. 1991 ). The pressure was enough to obtain a degree of flatening that in our opinion is similar to the one perceived in the fossils. Decay is very rapid in onychophorans, and for this reason three of the specimens were compressed 3.5 hr in mud and marine water and photographed. For comparison, the other two were left in mud with 70 % ethanol to prevent the action of decomposing microorganisms and photographed after 24 hr because no macroscopic indication of change had been noticed in that period.
A computer was used to produce photorealistic reconstructions of fossil onychophorans by applying standard functions of the software Photoshop 5.0 to photographic material of living onychophorans' skin texture, color and body shape.
Results
In experimentally compressed onychophorans the general body shaped was preserved (Fig. 1 ) but mouth, mandibles, adhesive-expelling organs (that look like tip-less legs) and gonopore became invisible or indicated only by a spot ( Fig. 1 ).
Mandibles were visible only in an individual that died with the mouth extruded. The eyes were always invisible. The leg tips and claws were lost in some cases. When visible, the claws sometimes turned toward the front or toward the rear of the animal, losing their natural position. Some legs look thicker or even thinner than they were in life and when appressed against the body became practically invisible in some cases (Fig. 1 ).
Sand grains and organic remains from the mud may give a false impression of organs (Fig. 1 ). The digestive tract was never visible (Fig. 1 ). The only visible difference between specimens that decayed naturally and those that were placed in alcohol was that when bacterial decomposition was allowed, annulation and papillation disappeared from severas body parts and portions of skin were detached, giving some antennae a slightly branched appearance (Fig. 1 ).
The above results served as a basis, together with the senior author's direct experience with living onychophorans, to produce the photorealistic reconstructions of Cambrian onychophorans that appear in Fig. 2 .
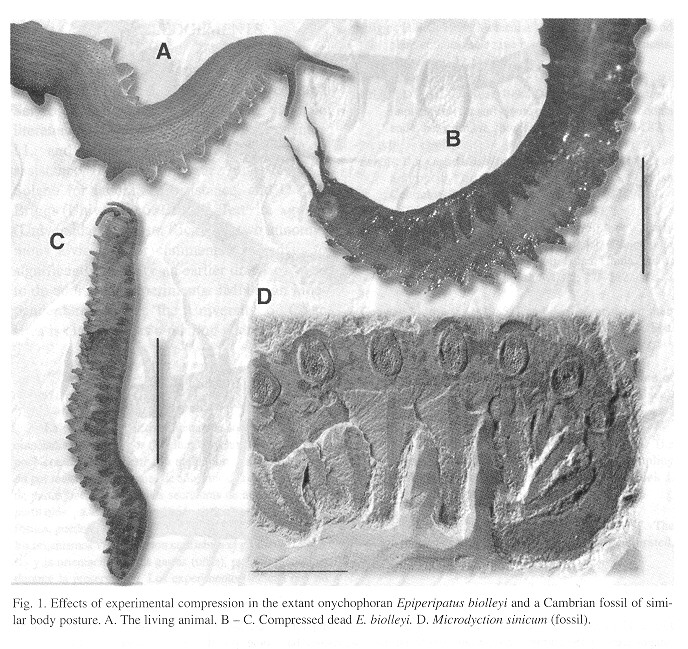
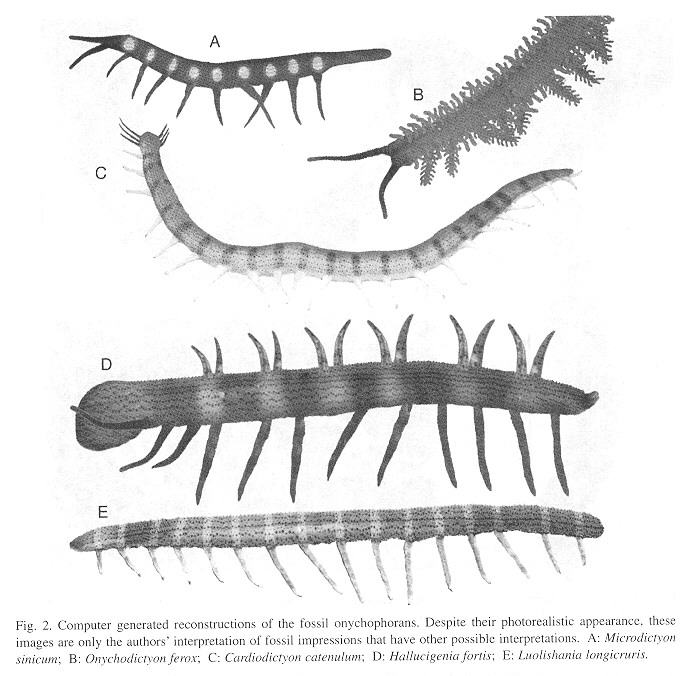
Discussion
The experimental compression results suggest that in fossil onychophorans (both Cambrian and Carboniferous) the general shape can be reconstructed but that the absence of bucal parts, adhesive-expelling organs, gonopore, eyes, annulation, papillation, leg tips, claws and even whole legs, may not represent absence in the living animals. In the past, ways of feeding alien to onychophorans have been proposed because mandibles were not found (e.g. Robison 1985 , Chen et al. 1995 ) but our results do not support those hypotheses.
Furthermore, in fossils, leg thickness and claw orientation may be unreliable and the branched antennae, interpreted as "grasping organs" in Aysheaia (review in Gould 1989 ) may also be post-mortem artifacts. The long papillae of Onychodictyon (Hou and Bergström 1995 ) could similarly be artifacts: more fossils are required to conclude that they were real.
Several reasons have been proposed to explain why longitudinal bands thought to represent the digestive tract vary their position in fossil onychophorans (Chen et al. 1995 ) but experience with dissection of extant species shows that this is normal for many internal organs because there are no mesenteries to hold them in a fixed position (J. Monge-Nájera personal observation).
Unidentified bands in fossil legs have been mentioned by Chen et al. (1995) . We believe that they may represent the normal fluid-filled cavity of living onychophoran legs (Bouvier 1905 ). Neither these bands nor indication of the digestive tract were visible in the experimentally compressed specimens: apparently they must be formed as part of fossilization rather than during decay.
For centuries, science has been limited to drawings for the reconstruction of - extinct species. Computer aided reconstructions became available at the end of the 20th century and have been extensively used to reconstruct dinosaurs in educational material but not in the primary scientific literature, despite the more satisfactory realism possible with computers. This may represent cultural inertia but another possible reason is that in contrast with photorealistic images, drawings are inmediately perceived as mere interpretations. lf this is correct, we expect our use of photorealistic reconstructions to be controversias. However, we consider photorealism a superior method for the reconstruction of the organisms' appearance, which also is the goal of drawings. Photorealism appears valid to us as long as it is clearly stated that the images are reconstructions, as we have done here.
These experiments indicate that not only absence, but even presence of certain structures that may lead to the proposal of comprehensive evolutionary models such as the Cambrian "explosion, disparity and decimation" model (Gould 1989 ), can simply be the result of tissue decomposition. We recommend future researchers to conduct taphonomy experiments specially before analysing unusual fossils.
Acknowledgements
We thank José A. Vargas Z. for assistance and support, Bernal Morera, Harlan Dean and Sergio Salazar Vallejo for providing valuable literature and information, Zaidett Barrientos LL. and Fernando Barrientos LL. for field assistance, Karina Rodríguez and Juan C. Solano for laboratory assistance, and D.E.G. Briggs (University of Bristol), Teresita Aguilar (Universidad de Costa Rica) and two annomymous reviewers for comments that led us to significantly improve an earlier draft and even to do additional experiments. JMN is an independent researcher: the University of Costa Rica is cited only as a mailing address.
Resumen
La preservación experimental de los gusanos "aterciopelados" u onicóforos (Phylum Onychophora), un grupo poco común pero evolutivamente importante que ha existido por más de 500 millones de años, mostró que la ausencia de partes bucales, glándulas secretoras de adhesivo, gonoporo, ojos, patas, garras, anulación cuticular y papilas en los fósiles, puede no representar la ausencia de tales órganos en los organismos vivos. No son contables el grueso de las patas y la orientación de las garras (uñas), pues se modifican durante la preservación. Los experimentos indican que no sólo la ausencia, sino también la presencia de ciertas estructuras pueden ser simplemente resultado de la descomposición de los tejidos. Se presentan reconstrucciones fotorrealistas computarizadas de onicóforos fósiles. Para futuras investigaciones, recomendarnos conducir experimentos tafonónúcos previos al análisis del material preservado, especialmente antes de analizar fósiles poco comunes.
References
Alongi, D.M. 1989. Ecology of tropical soft-bottom benthos: a review with emphasis on emerging concepts. Rev. Biol. Trop. 37: 85- 1 00. [ Links ]
Bergström, J. 1986. Metazoan evolution - a new model. Zool. Seripta 15: 189-200. [ Links ]
Bouvier, E.L. 1905. Monographie des Onychophores. Ann. Sei. Nat. (Zool.) (9)2: 1-383. [ Links ]
Briggs, D.E.G. 1999. Molecular taphonomy of animal and plant cuticles: selective preservation and diagenesis. Phil. Trans. R. Soc. Lond. B 354: 7-17. [ Links ]
Briggs, D.E. & A.J. Kear. 1994. Decay of Branchiostoma: implications for soft-tissue preservation in conodonts and other primitive chordates. Lethaia 26: 275-287. [ Links ]
Briggs, D.E.G. & H.B. Whittington. 1985. Modes of life of arthropods from the Burguess Shale, British Columbia. Trans. Rey. Soc. Edinburg 76: 149-160. [ Links ]
Chen, J., G. Zhou & L. Ramsköld. 1995. A new Early Cambrian onychophoran-like animal, Paucipodia gen. nov., from the Chengjiang fauna, China. Trans. Rev. Soc. Edinburgh Earth Sci. 85: 275-282. [ Links ]
Conway Morris, S.C. 1985. Cambrian Lagerstätten: their distribution and significance. Phil. Trans. R. Soc. Lond. B 311: 49-65. [ Links ]
Conway Morris, S.C. 1986. The community structure of the middle Cambrian phyllopod bed (Burgess Shale). Palaeontology 29: 423-467. [ Links ]
Fortey, R.A., D.E.G. Briggs & M.A. Wills. 1996. The Cambrian evolutionary 'explosion': decoupling cladogenesis from morphological disparity. Biol. J. Linn. Soc. London 57: 13-33. [ Links ]
Fortey, R.A., D.E.G. Briggs & M.A. Wills. 1997. The Cambrian evolutionary 'explosion' recalibrated. BioEssays 19: 429-434. [ Links ]
Gould, S.J. 1989. Wonderful life. The Burgess Shale and the nature of history. W.W. Norton, New York. 347 p. [ Links ]
Hou, X. & J. Bergström. 1994. Palaeoscolecid worms may be nematomorphs rather than annelids. Lethaia 27: 11-17. [ Links ]
Hou, X. & J. Bergström. 1995. Cambrian lobopodians ancestors of extant onychophorans? Zool. J. Linn. Soc. London 114: 3-19. [ Links ]
Hou, X. & J. Bergström. 1997. Arthropods from the lower Cambrian Chengjiang fauna, southwest China. Scandinavian University Press, Oslo, Norway. 116 p. [ Links ]
Hou, X., L. Ramskbld & J. Bergström. 1991. Composition and preservation of the Chengjiang fauna - a Lower Cambrian soft-bodied biota. Zool. Scripta 20: 395-411.
Hou, X., J. Bergström & P. Ahlberg. 1995. Anomalocaris and other large animals in the Lower Cambrian Chengjiang fauna of southwest China. GFF 117: 163-183. [ Links ]
Jin, Y, X. Hou & H. Wang. 1993. Lower Cambrian pediculate lingulids from Yunnan, China. J. Paleontol. 67: 788-798. [ Links ]
Manton, S.M. 1953. Locomotory habits and the evolution of the larger arthropodan groups. Symposia of the Society for Experimental Biology, Evolution 7: 339376. [ Links ]
Monge-Nájera, J. 1994a. Ecological Biogeography in the Phylum Onychophora. Biogeographica 70: 111-123. [ Links ]
Monge-Nájera, J. 1994b. Reproductive trends, habitat type and body characteristics in velvet worms (Onychophora). Rev. Biol. Trop. 42: 611-622. [ Links ]
Monge-Nájera, J. 1995. Phylogeny, biogeography and reproductive trends in the Onychophora. Zool. J. Linn. Soc. (London) 114: 21-60. [ Links ]
Monge-Nájera, J. 1996. Jurassic-Pliocene biogeography: testing a model with velvet worm (Onychophora) vicariance. Rev. Biol. Trop. 44: 159-175. [ Links ]
Monge-Nájera, J. 1999. Onychophora, pp. 23-35. In J. Llorente, E. González, A. García & N. Papavero (eds.). Biodiversidad, taxonomía y biogeografía de artrópodos de México: hacia una síntesis de su conocimiento, vol. II. Universidad Nacional Autónoma de México, México, DF. [ Links ]
Monge-Nájera, J. 2001. Walking fossils. Fauna 3: in press. [ Links ]
Monge-Nájera, J. & B. Morera. 1994. Morphological and physiological characteristics of two species of Epiperipatus from Costa Rica (Onychophora: Peripatidae). Rev. Biol. Trop. 42: 181-188. [ Links ]
Monge-Nájera, J. & J.P. Alfaro. 1995. Geographic variation of habitats in Costa Rican velvet worms (Onychophora Peripatidae). Biogeographica 71: 97-108. [ Links ]
Monge-Nájera, J. & W. Lourengo. 1995. Biogeographic implications of evolutionary trends in onychophorans and scorpions. Biogeographica 71: 179185. [ Links ]
Monge-Nájera, J. & X. Hou. 2000. Disparity, decimation and the Cambrian "explosion": comparison of early Cambrian and Present faunal communities with emphasis on velvet worms (Onychophora) Rev. Biol. Trop. 48: 333-351. [ Links ]
Monge-Nájera, J., Z. Barrientos & E Aguilar. 1993. Behavior of Epiperipatus biolleyi (Onychophora: Peripatidae) under laboratory conditions. Rev. Biol. Trop. 41: 689-696. [ Links ]
Orr, P.J., D.E.G. Briggs & S.L. Kearns. 1998. Cambrian Burgess Shale animals replicated in clay minerals. Science 281: 1173-1175. [ Links ]
Rhebergen, E & S.K. Donovan. 1994. A Lower Palaeozoic 'onychophoran' reinterpreted as a pelmatozoan (stalked echinoderin) column. Atlantic Geol. 30: 19-23. [ Links ]
Rigby, J.K. & X. Hou. 1995. Lower Cambrian demosponges and hexactinellid sponges from Yunnan, China. J. Paleontol. 69: 1009-1019. [ Links ]
Robison, R.A. 1985. Affinites of Aysheaia (Onychophora), with description of a new Cambrian species. J. Paleontol. 59: 226-235. [ Links ]
Tait, N.N., D.A. Briscoe & D.M. Rowell. 1995. Onychophora - ancient and modern radiations. Mem. Ass. Australas. Paleontols. 18: 21-30. [ Links ]
Vargas, J.A. 1987. Community structure of macrobenthos and the results of macropredator exclusion on a tropical intertidal mud fiat. Rev. Biol. Trop. 36 (2A): 287-308. [ Links ]
Vargas, J.A. 1988a. The benthic community of an intertidal mud fiat in the Gulf of Nicoya, Costa Rica. Description of the community. Rev. Biol. Trop. 35: 299-316. [ Links ]
Vargas, J.A. 1988b. A survey of the meiofauna of an Eastern Tropical Pacific mud fiat. Rev. Biol. Trop. 36 (2B): 541-544. [ Links ]
Vargas, J.A. 1996. Ecological dynamics of a tropical intertidal mudflat community, pp. 355-371. In K.E Nordstrom & C.T. Roman (eds.). Estuarine Shores: Evolution, Environments and Human Alterations. Wiley, New York. [ Links ]
Whittington, H.B. 1978. The lobopod animal Aysheaia pedunculata Walcott, Middle Cambrian, Burgess Shale, British Columbia. Phil. Trans. Roy. Soc., London B. 284: 165-197. [ Links ]
1 . Biología Tropical, Universidad de Costa Rica, San José, Costa Rica. Fax (506)2075550; rbt@biologia.ucr.ac.cr
2 . Yunnan Research Centre for Chengjiang Biota, Yunnan University, Kunming 650091, Yunnan Province, People's Republic of China; xghou@ynu.edu.cn














