Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.3-4 San José Dec. 2001
diet and fruit availability in a Costa Rican rain forest
Mariana Altrichter 1,4 , Eduardo Carrillo 2 , Joel Sáenz 1 and Todd K. Fuller 3
1 Programa Regional en Manejo de Vida Silvestre para Mesoamérica y el Caribe, Universidad Nacional, Apdo. 1350, Heredia, Costa Rica. E-mail: m_altrichter@yahoo.com; jsaenz@una.ac.cr
2 Centro Agronómico de Investigación y Enseñanza (CATIE). Unidad de Áreas Protegidas y Biodiversidad. Turrialba, Apdo. 7171. E-mail: ecarrill@catie.ac.cr
3 Department of Wildlife and Fisheries Conservation. University of Massachusetts at Amherst. Holdsworth Natural Resources Center, Amherst MA 01003. E-mail: tkfuller@forwild.umass.edu
4 Current Address: School of Renewable Natural Resources, University of Arizona, 104 BioSciences East, Tucson, AZ 85721.
Received 04-V-2000. Corrected 06-II-2001. Accepted 08-II-2001.
Abstract
We studied fruit availability, diet and habitat use by white-lipped peccary (Tayassu pecari) in Corcovado National Park, southwest Costa Rica, from July 1996 to April 1997. The results show that the availability of important fruits for the white-lipped peccary differs between habitats and climatic seasons. Fruit availability was highest in the primary forest than secondary and coastal forest. There was a period of shortage of fruits to ends of the wet season, during which the consumption of not seasonal resources like leaves and shafts increased. The important fruits during this period of shortage were Ficus sp and Licania operculipetala. The several types of forest were used according to the fruit availability, and it was a direct relation between the consumption and the fruit availability.
Key words: Tropical rain forest, Tayassu pecari, white-lipped peccary diet, habitat use, fruit availability, Corcovado National Park.
It is known that a high seasonality exists in tropical forests delineated mainly by rain patterns (Foster 1990, Leigh 1990). This seasonality determines changes in food availability (Boinski 1987), producing critical periods of food shortages that affect diet, mobility, habitat use and other biological aspects of the animal species found there. Nevertheless, implications of spatial- seasonal fluctuations in resources available to animal communities have only recently begun to be investigated (Terborgh 1986).
Food availability for frugivorous species depends on several factors, including animals nutritional requirements and abundance, distribution, diversity, phenology, and nutritive contentof the plant resources. The nutritive value of plant parts varies considerably. Fruits havelow protein content but higher soluble carbohydrate content, which provides more energy than leaves (Morton 1973), while leaves have the inverse characteristics (López 1999). According to the energy optimization model (MacArthur and Pianka 1966), it is more efficient for animals to eat other plant parts only when fruit resources become scarce, while fruit specialization can be expected when they are abundant (Clark 1982).The white-lipped peccary (Tayassu pecari Link, 1795) is mainly frugivorous, but its diet also includes stems, leaves, roots, flowers and animal matter (Chapman 1936, Kiltie 1981, Kiltie and Terborgh 1983, Barreto et al. 1997). It is unknown if the white-lipped peccarys diet responds to the seasonality present in tropical forests and/or the availability of food, as has been observed in other swine species such as T. tajacu (McCoy 1985) and Potamochoerus porcus (Breytembach and Skinner 1981).
It is important to understand how white-lipped peccary responds to spatial-seasonal shifts in fruit availability to determine: 1) critical periods of fruit scarcity, 2) important fruit species during such a period, and 3) to obtain information about habitat quality and the way that it is used. Through this knowledge, predictions can be made with regard to white-lipped peccary population responses to external pressures such as habitat loss. This information is absolutely necessary to determine conservation strategies for this little-known species (Robinson and Redford 1991). White-lipped peccary is one of the most hunted animals in the neotropics and its populations are endangered in the majority of its range (Emmons 1984, March 1990).Within this framework the study objectives were to determine if there is a spatial/seasonal variation in fruit availability in Corcovado National Park and to determine if the white-lipped peccarys diet is dependent upon those changes. Using an optimum foraging hypothesis, several predictions can be made about the white-lipped peccarys diet: a) when availability of fruit is low, their diet shifts to non-seasonal resources, b) when there is a high availability of fruit, they feed mainly on this resource.
Materials and methods
Study area: The study was conducted in Corcovado National Park (Corcovado), in the Osa Peninsula, in Southwest Costa Rica (8º26 and 8º39N by 83º25 and 83º45W). The total area of the park is approximately 46 774 ha and the study area covered approximately 3 000 ha. The majority of the park is found within the "very humid tropical forest" life zone according to the Holdridge system. The climate can be described as hot, rainy and very humid, with a dry season from December to April and a rainy season from May to November (Vaughan 1981). The months with the highest levels of precipitation are October and November (> 500 mm/month) and the driest months are February and March (< 100 mm/month). The mean annual temperature is 26 ºC and the precipitation varies from 3 800 mm in the low-lands to 6 500 mm annually in the highlands (Hartshorn 1983).
A large part of the park topography is comprised of plains but there is a mountainous sector that reaches 745 m, composed of steep slopes. The areas stream heads are located in this mountainous region. Phillips (1993) determined 32 categories of vegetation on the Osa Peninsula.
Methods: The study was conducted from July 1996 to April 1997. Because white-lipped peccaries had probably moved out of the study area, they were not observed during December, but data were taken on fruit availability during that month.
Fruit availability: The amount of fruit on the forest floor was used as the index of availability because land frugivores do not have access to fruits found in the canopy. Even though all fruit-bearing species were recorded, only those consumed by, or potentially consumed by, white- lipped peccary were used in the diet analysis. Potential species were those cited by other authors as being part of the peccaries diet (Kiltie 1981, Bodmer 1990, 1991, Bodmer et al. 1995, March 1995, Barreto et al. 1997) and those listed for the collared peccary (T. tajacu) found in humid forests (Torrealba 1993). Fruits were collected and conserved in 80 % alcohol solutions and later identified.
Atotal of 18 km of trails (transects), representative of different habitats, were covered every 10 to 12 days. Categories of one to four (from few to many) were assigned to the quantity of fruit found on the forest floor under the fruit-bearing trees of each species, as visible from the trail. These values of one to four were assigned gauging less than 4 fruits in 2 m2 as very few, and more than 12 fruits in 2 m2 as many. The average was taken of the availability values per habitat, per month. To ensure that the index was comparable between habitats, the average was divided by the total distance covered in each type of habitat and multiplied by 100, to avoid using fractional numbers, in the following way: availability index ID = (∑di /t*km)*100, where d = the value assigned from 1 to 4 to the i fruit bearing trees, t = the number of transects covered for each habitat, km = the total kilometers covered in each habitat, and 100 = a constant. Later, the monthly average was taken as ∑ID/n, where n = the number of trips made monthly, varying from two to three. The months when the secondary and primary habitats together had available fruit indexes equal or greater to the mean were grouped into "high availability months" and the remaining months were grouped into "low availability months".
Diet estimate: Direct observation and feces analysis were used to estimate diet. Seven white- lipped peccaries belonging to four different herds were marked with radio collars. By means of radio telemetry, the herds were located and followed, maintaining visual contact for the maximum time possible between the 0700 and 1700 hr. Direct observation was made of what peccaries ate using the sweep technique (Martin and Bateson 1986) every 5 min. with a minimum of four animals in sight. During the observations, the following feeding activities were recorded: a) fruit consumption, b) plant parts consumption (like leaves and stems), c) underground matter consumption and d) animal matter consumption. Additionally, each time a white-lipped peccary was seen eating plants or fruits, the species consumed was recorded.
To perform the feces analysis, a maximum of five feces per day were collected from a herd while it was being followed. The ten points technique (Korschgen 1987, McCoy et al. 1990) was used to obtain random samples from each excrement. Through an accumulated mean curve of the most common components (fiber and fruit), it was determined that 50 samples were adequate to analyze each excrement. Four categories were identified: a) fruits, b) plant parts, c) animal matter and d) unidentified matter.
To quantify elements found in the feces, the following formula was used: Pi = (T/S) * 100 (Romero and Mandujano 1995), where Pi = the percentage of the component i, T = the number of points touched by needles in component i, and S = the total number of points (50).
Habitat classification: Thirty-seven transects of 60 m by 10 m were established systematically and four habitat variables were measured: a) crown height, b) chest height diameter, c) number of heliconias (Heliconia spp.) and d) number of dwarf palms (Geonoma spp.). The two latter variables were measured in perpendicular lines crossing the transect every 5 m and selected as indicators of secondary and primary forests respectively. Three types of habitat were defined: a) primary forest, b) secondary forest, and c) coastal forest.
Habitat use: As a preliminary estimation of habitat use, each day that a herd was followed, the types of habitats they used were recorded without identifying how many hours were spent in each. A frequency of habitat use index was obtained, expressed as frequency of use per month divided by the number of days of observation in that month. This index varied from 0 to 1. For a given habitat, the use index is 1 when the animals were seen in this habitat in each one of the observations that were made during the month. Given that the effort necessary to locate the groups was approximately the same, the frequency of use is comparable among the different habitats.
Data analysis: To characterize the availability of fruit, the following were determined: a) number of species with fruit each time data were taken and per month, and b) Shannon diversity index H each time data were taken and per month as: H = Σ-pilnpi , where pi = n/N, n = the number of trees with fruit in each species and N = the total number of trees with fruit. Where possible, parametric tests were used, with data transformed previously to achieve variance normality and homogeneity if it became necessary.
To determine the relationship between diet, habitat use and fruit availability, the time dedicated to feeding was compared between months with high and low fruit availability, and between the wet and dry seasons using the Mann-Whitney test. An ANOVA was used to compare the habitat use index in both the wet and dry seasons among the three habitats. A Spearman correlation analysis was done between the monthly habitat use index and the monthly mean percentage of time dedicated to fruit consumption. Two regression analyses were conducted, using fruit availability data (one per month) as the independent variable, and the mean percentage of fruit in the feces collected in the same period (data transformed using arcsine) as the dependent variable for one analysis, and the mean percentage of time dedicated to fruit consumption (data transformed using arcsine) as the dependent variable for the other analysis.
Results
Fruit availability: Taking all the fruit found in the study area into consideration, the availability index was significantly higher in the wet season than in dry season (Mann-Whitney, Z = -2.6, p < 0.01), peaking in August and September. In dry season, the availability was low and constant. The availability of fruits important for white-lipped peccaries followed a different pattern to that mentioned for all fruits sampled.
Diversity and number of species with fruits in the wet and dry seasons: The diversity of fruit bearing species important to the white-lipped peccary was significantly higher in the dry season (mean H = 2.099) than in the wet season (mean H = 1.454) (Mann-Whitney, Z = -1.25, p < 0.05). At the same time the number of species with fruit was higher in the dry season (mean 11.6) than in the wet season (mean 8.4), but these differences were not significant (Z = -1.2, p = 0.21).
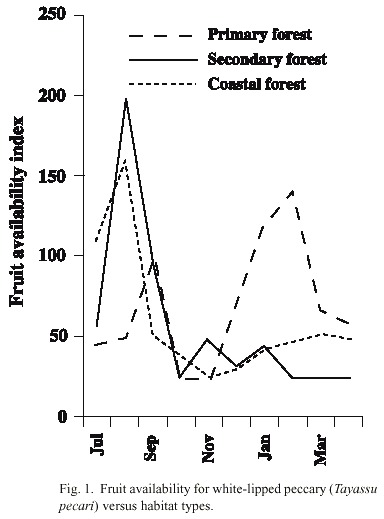
Comparison of fruit availability between habitats: Fruit-bearing species differed between habitats. In the coastal habitat the most abundant species in the wet season was Licania operculipetala, which is very rare in other habitats. Other abundant species were Quararibea asterolepis and figs (Ficus spp.) In the secondary forest areas, the most abundant fruit in the wet season were Spondias spp. and figs, meanwhile in the dry season was Inga spp. Zapote (Pouteria spp.) were most abundant in the wet season in primary forest habitats, while Brosimum spp., Anacardium excelsum, Virola spp., and Compsoneura sprucei were most abundant in the dry season. The availability of fruit was slightly higher in the primary forest habitat (mean 73) than in the secondary (mean 64) and coastal forest habitats (mean 63), but these differences were not significant (F 2,69 = 0.13, p = 0.87) (Fig. 1).
Seasonal comparison of fruit availability: Fruit availability varied significantly during the study period in the primary forest habitat (F 9,14 = 3.08, p < 0.05). There were two peaks in availability, one in the wet season in September and another in the dry season from December to February. In the secondary forest habitat there was also significant seasonal variation. July and August had the highest availability (F 9,14 = 3.47, p < 0.05). In the coastal habitat, fruit availability was highest in July - August and low from September to April, although this variation was not significant (F 9,12 = 2.6, p = 0.06) (Fig. 1). Fruit availability in the primary habitat was significantly higher in the dry season (mean 104, n = 12) than in the wet season (mean 46, n = 12) (Mann- Whitney, Z = -2.91, p < 0.01). In the coastal habitat, fruit availability was significantly higher in the wet season (mean 103) than in the dry season (mean 27) (Z = -2.27, p < 0.05). In the secondary forest habitat, there was no significant difference in fruit availability between seasons (Z = -0.89, p < 0.37) (Fig. 1). By observing fruit availability in both, the primary and secondary habitats together, it can be determined that July, October, November and April were the months with low fruit availability, and that August, September, January, February and March were those with high vailability of fruit.
Diet: Through direct observation it was found that white-lipped peccaries dedicated 62.5 % of their time to fruit consumption, 29.0 % to underground consumption, 8.4 % to plant part consumption, and 0.1 % to animal matter consumption, during the 10 months of study. The elements found in the feces were composed of 61.6 % fruit, 37.5 % plant parts, 0.4 % animal matter and 0.5 % unidentified matter.
Comparison of time dedicated to feeding between months with high and low fruit availability: In both the primary and secondary habitats, the time dedicated to fruit consumption was significantly higher in the months with high fruit availability than in those with low fruit availability (Z = -8.54, p < 0.001 and Z = -2.83, p < 0.05 respectively). Instead, the time dedicated to underground feeding was significantly less in the months with high fruit availbility than in those with low fruit availability, in both primary and secondary habitats (Z = -6.56, p < 0.001 and Z = - 3.15, p < 0.05 respectively). In the primary habitat, the time dedicated to consumption of plant parts was significantly less in the months with high fruit availability than in the months with low fruit availability (Z = -6.54, p < 0.001). In the secondary habitat, the time dedicated to the consumption of plant parts was not significantly different between months with high and low fruit availability (Z = 0.36, p = 0.71).
Comparison of time dedicated to feeding between the dry and wet seasons: In the primary forest habitat, the time dedicated to fruit consumption was significantly higher in the dry season (mean 90 %, n = 88) than in the wet season (mean 24 %, n = 373) (Mann-Whitney, Z = -13.78, p < 0.001). The time dedicated to plant consumption was significantly higher in the wet season (mean 11.2 %) than in the dry season (mean 3 %) (Z = -3.21, p < 0.01). The time dedicated to underground feeding was significantly higher in the wet season (mean 65 %) than in the dry season (mean 7 %) (Z = -13.22, p < 0.001).
In the secondary forest habitat, the time dedicated to fruit consumption was significantly higher in the dry season (mean 64 %, n = 91) than in the wet season (mean 28 %, n = 96) (Z = -5.18, p < 0.001). The time dedicated to plant consumption was higher in the dry season (mean 28 %) than in the wet season (mean 12%) (Z = -1.91, p < 0.05), and underground consumption was higher in the wet season (mean 60 %) than in the dry season (mean 9 %) (Z = -8.19, p < 0.001).
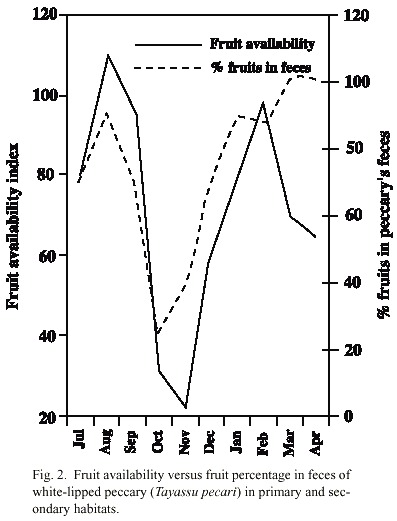
Relationship between fruit consumption and fruit availability in primary and secondary habitats: There was a positive significant regression between the fruit availability and the pecentage of fruits found in the feces (F 1,8 = 5.93, p < 0.05). The minimum points of availability in October and November coincided with the minimum percentages of fruit in the feces (Fig. 2). The relationship between fruit availability and time spent eating fruits was positive but not significant (F 1,8 = 2.02, p = 0.19).
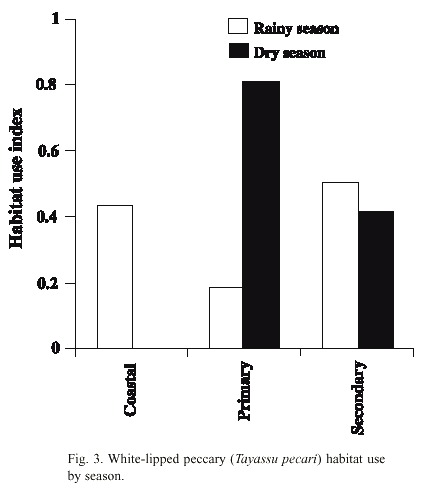
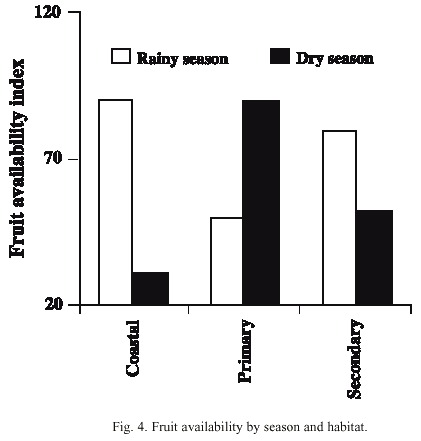
Comparison between habitat use in the wet and dry seasons and fruit availability: There was a notable difference in habitat use in the dry season. In almost 80 % of the cases when they were observed during this season, the animals used the primary forest habitat, where there was the highest availability of fruits (Figs. 3, 4). The coastal habitat, which had the lowest availability of fruits, was not used (F 2,9 = 15.92, p < 0.001). In the wet season, the secondary and coastal habitats were used more than the primary habitat. Fruit availability in this season was higher in the secondary and coastal habitats than in the primary habitat (F 2,12 = 8.43, p < 0.01) (Figs. 3, 4). In general, the peccaries used the secondary forest habitat more regularly than the other habitats throughout the study period (Fig. 3).
Relationship between habitat use and fruit consumption: There was a positive significant correlation between primary forest habitat use and the percentage of time dedicated to fruit consumption in this habitat (r sp = 0.73, p < 0.05). This correlation was also significant for the coastal habitat (rsp = 0.71, p < 0.05). For the secondary forest habitat the correlation was not significant (rsp = 0.10, p = 0.80).
Discussion
Boinski (1987) conducted a phenological study on fruit species in Corcovado and found that the highest number of species bear fruit at the beginning of the wet season and the lowest at the end of the wet season, from October to December. The same seasonal variation was found in a tropical humid forest in Peru (Terborgh 1992) and in Barro Colorado, Panama (Croati 1975). When we considered the total fructification in Corcovado, the same seasonal differences were found, with the lowest number recorded during the dry season. However, analyzing only the species important to peccaries, the seasonal variation in fruit availability differed between habitats. In the coastal and secondary habitats, fruit availability followed the same general pattern, while in the primary forest habitat there was a higher availability of fruit in the dry season. In general, in the wet season there were fewer fruit-bearing species important to the peccaries, but these had higher fruit production (i.e. Ficus spp., Spondias spp., L. operculipetala, Pouteria spp.). In the dry season, there was a higher number of fruit species, but overall they had less fruits (i.e. Brosimum spp., Inga spp., A. excelsum). The period when fruits were scarce was reduced to the end of the wet season between October and December. In addition to having lower fruit production in these months (Boinski 1987), it is possible that the availability of fruit on the forest floor diminishes due to the high rates of precipitation during these months and the runoff that sweeps fruit from the soil.
Terborgh (1992) mentions 12 key species of trees that fruit during the months when fruits are scarce in the Amazon. These play an important role in the ecosystem because the frugivores feed on them during the critical months. Wild figs, Ficus spp., are more important for monkeys, and palm seeds could be more important to the peccaries. Nevertheless, palms were not very important in the diet of the peccaries in Corcovado. Given that palm seed consumption requires great effort since they are so hard (Kiltie 1981), it is possible that while the availability of other fruits is high, the peccaries prefer seeds that are less tough. During the critical months, the peccaries fed mainly on wild figs and L. operculipetala. These two species should, therefore, be considered the key species for the white-lipped peccary in this type of forest. The removal of these plant species from the ecosystem could result in a sharp decline in its capacity to support white-lipped peccary populations. However, it must be noted that fruit availability can vary annually according to climate conditions, and there may be years in which fruit availability is much lower and palm seeds become an important resource.
There was a differential use of habitats related to the availability of fruits. In the wet season, the lowest availability of fruit was found in the primary forest habitat, which was the least used of the three. In the dry season, the peccaries used more the primary forest, where there was the highest fruit availability. Peccaries didnt use the coastal habitat in the dry season. In this season, the coastal habitat had the lowest fruit availability of the three habitats. There were differences not only in frequency of habitat use but also in resource use within each habitat. Throughout the entire study period, consumption of plant parts, specially stems of Heliconia spp., was high in the secondary habitat. In the coastal habitat, peccaries fed almost exclusively on fruits of L. operculipetala and plant parts from the Araceae family. Breytembach and Skinner (1981) discovered a variation in the habitat use of the Potamochoerus porcus that they also attributed to food availability in each habitat. Nevertheless, other factors must also be taken into consideration.
Physical aspects of the habitats that could affect their use are water availability, shade, shelter, mud holes, etc. These factors must enter the equation if a complete understanding of the relationship between habitat quality and use is to be achieved.
When fruit availability is low, white-lipped peccary showed no preference between using the secondary or primary forest to feed, while in the months where there was a high availability of fruit, they fed on fruit mainly from the primary forest habitat and plant parts from the secondary forest habitat. During months with highest fruit abundance, a preference for certain fruits was noted, such as L. operculipetala in August and September and Brosimum spp. from January to March. In October and November when fruits were scarce, plant part consumption was very high, even surpassing fruit consumption. In these months, wild fig consumption was higher than in any other time during the study, although they were always available. These fruits have low food value (Fleming 1986), and they are eaten almost solely in response to the scarcity of higher quality foods.
This selective behavior when confronted with an abundance of fruit, and the change in diet in response to scarcity, support one of the predictions of the optimum forage hypothesis (MacArthur and Pianka 1966). Variation in diet related to fruit availability was observed in other swine species like T. tajacu in dry tropical forest (McCoy et al. 1990) and in other wild pigs in South Africa (Breytembach and Skinner 1981). In the latter study, the authors identified this behavior as opportunistic. Nevertheless, in this study, a number of signs indicated selective behavior on several levels: 1) in a given season the peccaries used one type of habitat more than others, 2) although they had a wide variety of species from which to choose, they showed preference for specific species, and 3) they did not eat just any part of these species, but the exocarp of some, the seeds of others, and new leaves of others. Each of these selective steps would improve food quality, which indicates that opportunistic behavior only comes into play when fruits are scarce.
Tree-climbing frugivores do not have the option of changing their diet to foliage when fruits are scarce because leaf and fruit production are synchronized (Terborgh 1992). Nevertheless, leaves and stems from herbaceous species, within the reach of the peccaries, constitute an available resource independent of the tree species. This is why the change from frugivore to folivore behavior in the critical months appears to be an adaptive option that in part would help to avoid nutritive stress. McCoy et al. (1990) found that T. t ajacu ate more fiber and increased its range in
the critical months. The great distances that white-lipped peccaries covered during the wet season, and their leaving the study area in December could be related to low fruit availability. This suggests that peccaries have at least two possible responses to fruit scarcity: 1) change the diet and feed on a non-seasonal resource such as stems and leaves, and 2) increase the mobility in order to find more fruit.
Acknowledgments
We thank Omar Laquis for his invaluable help in the field. Miguel Rodríguez and anonymous reviewers helped to improve this manuscript. This research was partially supported by the Wildlife Conservation Society, World Wide Fund, Regional Wildlife Management Program of the National University of Costa Rica and the Cooperation Agency of Spain (Agencia de Cooperación Española).
Resumen
Actualmente se reconoce que existe una alta estacionalidad en la producción de frutos en los bosques tropicales, que produce períodos críticos de escasez que afectan, entre otros, la dieta, movilidad y el uso de hábitat de las especies animales. No se conoce la forma en que el chancho cariblanco (Tayassu pecari) responde a esta variación. Nosotros estudiamos la dieta y el uso del hábitat del chancho cariblanco y la variación en la disponibilidad de frutos en el Parque Nacional Corcovado, al suroeste de Costa Rica, desde julio de 1996 hasta abril de 1997. Los resultados muestran que la disponibilidad de frutos importantes en la dieta del chancho cariblanco difiere entre hábitats y entre estaciones climáticas. Fue más alta en el bosque primario que en el bosque secundario y el bosque costero. Hubo un período de escasez de frutos a finales de la estación húmeda, durante el cual aumentó el consumo de recursos no estacionales como hojas y tallos. Los frutos importantes durante este periodo de escasez fueron Ficus spp. y Licania perculipetala. Los diferentes tipos de bosque fueron usados de acuerdo a la disponibilidad de frutos, y se encontró una relación directa entre el consumo y la disponibilidad de frutos.
References
Barreto, G.R., O.E. Hernández & J. Ojasti. 1997. Diet of peccaries (Tayassu tajacu and T. pecari) in a dry forest of Venezuela. J. Zool. Lond. 1: 241-256. [ Links ]
Bodmer, R.E. 1990. Responses of ungulates to seasonal inundations in the Amazon floodplain. J. Trop. Ecol. 6: 191-201. [ Links ]
Bodmer, R.E. 1991. Strategies of seed dispersal and seed predation in Amazonian ungulates. Biotropica 23: 255-261. [ Links ]
Bodmer, R.E., L.K. Sowls & A.B. Taber. 1995. Economic importance and human utilization of peccaries. In W.L. Oliver & R. Gland (eds.). Status survey and conservation action plan: Pigs, peccaries, and hippos. IUCN/SSSC Pigs and Peccaries Specialist Group and IUCN/SSSC Hippo Specialist Group, Switzerland [ Links ]Boinski, S. 1987. Habitat use by squirrel monkeys (Saimiri oerstedi) in Costa Rica. Folia Primatol. 49: 151- 167. [ Links ]
Breytembach, G.J. & J.D. Skinner. 1981. Diet feeding and habitat utilization by bushpigs (Potamochoerus porcus) Linnaeus. S. Afr. J. Wildl. Res. 12: 1-7. [ Links ]
Chapman, F.M. 1936. White lipped peccary. Natur. Hist. 38: 408-412. [ Links ]
Clark, D.A. 1982. Foraging behavior of a vertebrate omnivore (Rattus rattus): Mealstructure, sampling and diet breadth. Ecology 63: 763-772. [ Links ]
Croati, T.B. 1975. Phenological behavior of habitat and habitat classes on Barro Colorado Island (Panama Canal Zone). Biotropica 7: 270-277. [ Links ]
Emmons, L.H. 1984. Geographic variation in densities and diversities on non-flying mammals in Amazonian. Biotropica 16: 210-222. [ Links ]
Fleming, T.H. 1986. Opportunism versus specialization: The evolution of feeding strategies in frugivorous bats, p. 105-108. In A. Estrada & T. Fleming (eds.). Frugivores and seed dispersal. Dr. W. Junk, Netherlands, Boston. [ Links ]
Foster, R.B. 1990. Ciclo estacional de caída de frutos en la isla de Barro Colorado, p. 219-233. In E.G. Leigh, A.S. Rand & D.M. Windsor (eds.). Ecología de un bosque tropical: Ciclos estacionales y cambios a largo plazo. Smithsonian Tropical Research Institute, Panama. [ Links ]
Hartshorn, G.S. 1983. Plants, p. 118-157. In D.H. Janzen (ed.). Costa Rican natural history. University of Chicago, Chicago. [ Links ]
Kiltie, R.A. 1981. Stomach contents of rain forest peccaries (Tayassu tajacu and T. pecari). Biotropica 13: 234-236. [ Links ]
Kiltie, R.A. & J. Terborgh. 1983. Observations on the behavior of rain forest Peccaries in Peru: Why do white- lipped peccaries form herds? Z. Tierpsychol. 62: 241-255. [ Links ]
Korschgen, L.J. 1987. Procedimientos para el análisis de los hábitos alimentarios, p.119-133 In R.R. Tarres (ed.). Manual de técnicas de gestión de vida silvestre. The Wildlife Society, Maryland. [ Links ]
Leigh, E.G. 1990. Introducción: La selección natural y los ciclos del bosque, p. 175-178. In E.G. Leigh A.S. Rand & D.M. Windsor (eds.). Ecología de un bosque tropical: Ciclos estacionales y cambios a largo plazo. Smithsonian Tropical Research Institute, Panamá. [ Links ]
López, M.T. 1999. Aspectos nutricionales de la dieta del chancho de monte (Tayassu pecari) en el Parque Nacional Corcovado, Costa Rica. Tesis de Maestría Universidad Nacional, Heredia, Costa Rica. [ Links ]
MacArthur, R.H. & E.R. Pianka 1966. On the optimal use of a patchy environment. Amer. Natur. 100: 603-609. [ Links ]
March, I.J. 1990. Evaluación de hábitat y situación actual del pecarí de labios blancos (Tayassu pecari) en México. Tesis de Maestría, Universidad Nacional, Heredia, Costa Rica. 250 p. [ Links ]
March, I.J. 1995. The white-lipped peccary (Tayassu pecari), p. 7-13. In W.L. Oliver & R. Gland (eds.). Status survey and conservation action plan: Pigs, peccaries, and hippos. IUCN/SSC Pigs and Peccaries Specialist Group and the IUCN/SSSC Hippo Specialist Group, Switzerland. [ Links ]
Martin, P. & P. Bateson. 1986. Measuring behaviour: An introductory guide. Cambridge University, Cambridge. [ Links ]
McCoy, M. 1985. Seasonal movement, home range, activity and diet of collared peccaries in Costa Rican dry forest. Master Thesis, Humboldt State University. 109 p. [ Links ]
McCoy, M.B., C. Vaughan, M.A. Rodríguez & D. Kitchen. 1990. Seasonal movement, home range, activity and diet of collared peccaries (Tayassu tajacu) in Costa Rican dry forest. Vida Silv. Neotrop. 2: 6-20. [ Links ]
Morton, E.S. 1973. On the evolutionary advantages and disadvantages of fruit-eating in tropical birds. Amer. Natur. 107: 8-22. [ Links ]
Phillips, P.R. 1993. Key to vegetation types for the Osa Peninsula, Costa Rica. Center for Space Research, University of Texas, Austin, Texas. [ Links ]
Robinson, J.G. & K.H. Redford. 1991. Neotropical wildlife use and conservation. University of Chicago, Chicago. 520 p. [ Links ]
Romero, L.E. & S. Mandujano 1995. Hábitos alimentarios del pecarí de collar (Pecari tajacu) en un bosque tropical caducifolio de Jalisco, México. Acta Zool. Mex. 64: 1-20. [ Links ]Terborgh, J. 1986. Community aspects of frugivory in tropical forest, p. 371-384. In A. Estrada & T. Fleming (eds.). Frugivores and seed dispersal. Junk, Netherlands. [ Links ]
Terborgh, J. 1992. Diversity and the tropical rain forest. Scientific American Library, New York. [ Links ]
Torrealba, I.M. 1993. Ecología de los grupos de saíno (Tayassu tajacu) y daños que ocasionan en los cultivos vecinos a la estación biológica La Selva, Costa Rica. Tesis de Maestría, Universidad Nacional, Heredia, Costa Rica. [ Links ]
Vaughan, C. 1981. Parque Nacional Corcovado: Plan de manejo y desarrollo. Universidad Nacional, Heredia, Costa Rica. 362 p. [ Links ]