Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.1 San José Mar. 2001
Mariana Altrichter*1, Carlos Drews1, Eduardo Carrillo1 and Joel Sáenz 1
Recibido 27-IX-1999. Corregido 11-VII-2000. Aceptado 31-VII-2000.
Abstract
White-lipped peccaries are non-seasonal breeders in South America, but little is known about their reproduction in Central America. There are few studies about the sex ratio of this species in the field. We studied the reproduction and sex ratio of white-lipped peccaries during 200 hours of field observation of four radiomarked and two unmarked herds, from July 1996 to April 1997, in Corcovado National Park, Costa Rica. Sex ratio data of three additional, radiomarked herds observed in 1998 were also included. We recorded numbers of mountings, presence of newborns and numbers of nursing interactions. The peccaries showed a distinct reproductive seasonality, with one mating peak in February and another in July. The greatest number of newborns and the peak in nursing activity were observed during July and August, when fruit availability for the peccaries was high. The adult sex ratio was significantly female biased (1.4:1 - 1.8:1), also in contrast with South American populations.
Key words: Corcovado, Costa Rica, mating, nursing, reproduction, sex ratio, Tayassu pecari, white-lipped peccary.
The white-lipped peccary Tayassu pecari L. (1795) is one of the most threatened neotropical mammals and its status deteriorates due to habitat loss and hunting (Emmons 1984, Oliver 1990). Little is known about its population dynamics and behavior. White-lipped peccaries range from southern Mexico to northern Argentina, and prefer unaltered humid and semideciduous forests (Sowls 1997). They are social animals, living in herds from fifteen to several hundred individuals (Sowls 1997). This species has been characterized as mainly frugivorous in the Peruvian Amazon (Kiltie 1981, Bodmer 1989) and the rain forests of Costa Rica (Altrichter 1997). For an overview of peccary natural history see Mayer and Brandt (1982) and Sowls (1997).
There is little published information on reproduction in the white-lipped peccary. Anecdotal observations (Miller 1930, Leopold 1959, Sowls 1984) and research in the Peruvian Amazon (Gottdenker and Bodmer 1998), indicate that white-lipped peccaries breed throughout the year. Gottdenker and Bodmer (1998) suggest that this breeding pattern may result from the relatively constant rainfall and the resulting year long food availability. However, in many Central American rain forests a pronounced seasonality delineated by rainfall causes a variable fruit production during the year with periods of food scarcity (Janzen 1983, Boinski 1986, Altrichter 1997). Therefore, one might expect that peccaries inhabiting Central American rain forests breed so as to have litters during periods of high food abundance.
Studies of collared peccary (Tayassu tajacu, L. 1758) from Arizona and of Chacoan peccaries (Catagonus wagneri, R. 1930) from Paraguay suggest that adult sex ratios are close to even in these species (reviews in Mayer and Brandt 1982, Sowls 1984, 1997). An essentially 1:1 fetal sex ratio in white-lipped peccaries of the Peruvian Amazon (Gottdenker and Bodmer 1998) and an adult sex ratio of 1.8 females per male in one herd in the Brazilian Amazon (Fragoso 1994) is the only information available for this species. In this paper we address adult sex ratio and the seasonality of reproduction in white-lipped peccaries of a Costa Rican rain forest. This information is pertinent to the design of management and conservation strategies for this species.
Materials and Methods
Study area: We conducted this study in Corcovado National Park (Osa Peninsula), Costa Rica (8º26' to 8º39' N and 83º25' to 83º45' W). The park extension is about 46 774 ha (García 1997), most of which is very humid tropical forest according to Holdridges (1967) life zones classification. The dry season extends from December to April and the rainy season from May to November. October and November are months of maximum precipitation (>500 mm/month) and February and March are the driest months (<100 mm/month) (Vaughan 1981). Mean annual temperature is 26 ºC and the precipitation ranges from 3800 mm in lowland to 6500 mm in the mountains (Harthsorn 1983). The majority of the park is lowland, covered mainly with primary forest (Naranjo 1994).
Observations: We report data from nine peccary herds. We radiotracked seven peccaries, from four different herds and observed two additional herds without radiotransmitters, from July 1996 to April 1997. Three herds were radiomarked and their sex ratio recorded in 1998. Observations were made at close range (5-15 m) during maximum possible diurnal time by the first author and an assistant. The peccaries seemed habituated to our presence and allowed us to move around and within the herd without fleeing. Observability of the animals did not vary noticeably between seasons because the forest is evergreen and because herd cohesion remains roughly constant throughout the year (Campero 1999, Fragoso 1998). We recorded copulations, nursing interactions, percentage of newborns and sex composition of herds. Mean values are given in the text with their corresponding standard deviation.
Fruit availability: The availability of fruit components of white-lipped peccary diet in Corcovado National Park was estimated concurrently (Altrichter 1997). An index was used that included number of trees fruiting and amounts of fruits on the floor, underneath the trees. The index expresses a monthly mean of fruit availability.
Mating and nursing: We recorded mating interactions ad libitum whenever peccaries were seen interacting, and made concomitant counts of all visible individuals. Nursing events were recorded during scan samples at 10 min intervals. We recorded number of copulations and nursing events observed daily and calculated a mean hourly rate every two weeks and monthly. Such hourly rates allow direct comparisons between days and other time periods, given that observation time differed between units. The rates, however, are not absolute values corresponding to overall interaction frequencies in the herd, because only a proportion of herd animals were visible at any given time. Errors resulting in omissions of copulations in our ad libitum records are probably systematic and unlikely to bias the patterns described because both observers and observation conditions were more or less constant throughout the study. Since observation time differed between days, we indicate frequency of interactions per hour of observation to facilitate comparisons.
Sex ratio: Body size was used to assign individuals to the adult class. Sex determination was possible because the adult males scrotum is conspicuous at close range. Since during 1996 and 1997 we determined the sex of every adult individual for only one herd, the sex composition of the other five herds was estimated using two indirect approaches. Firstly, sexes were recorded in groups of at least five adult animals. We assumed that the sex composition of visible groups over the course of several observations would be a random sample of herd composition. These groups were not apart from the herd but corresponded to those individuals directly visible to us. Secondly, we determined daily sex ratios of randomly selected adults for behavioral focal-samples on days with four or more focal animals (Altrichter 1997). In 1998 all adults from three herds were sexed repeatedly. Since herds split and merged in new combinations during 1998, sex ratios reported here for these three herds are considered statistically independent from the 1996 and 1997 data.
During the 10 months of study, herds were observed and their behavior recorded on 71 days. Herd sizes varied from 21 to 70 individuals. The number of animals counted during interactions was variable (range 3 - 21), but no obvious differences or patterns in this variation were found between months (Kruskal-Wallis ANOVA, p = 0.11). During December we were unable to find any peccary herd.
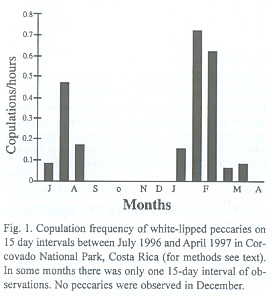
Copulations and nursing frequency: We observed 33 copulations. The frequency of copulations per hour of observation varied significantly between periods of 15 days during the study (Fig.1, Kruskal-Wallis ANOVA, p< 0.05). It was highest during the first 15 days of February, with a mean of 0.72 ± 0.17 copulations per hour. No copulations were observed during September, October, November, and April. The frequency of copulations was significantly higher in the dry season (0.45/h ± 0.16) than in the wet season (0.16/h ± 0.09) (Mann-Whitney U-test, p< 0.05).
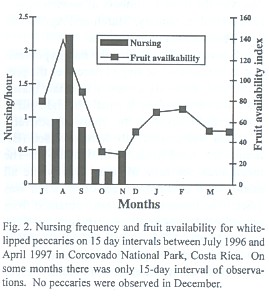
Litters conceived in February, the highest mating peak (Fig. 1) were born during months of high fruit availability, as indicated by peaks in nursing activity in August (Fig. 2). We observed 46 nursing events. Nursing frequency was greatest in the latter 15 days of August (2.22 events/h), and no nursing was observed from January to April (Fig. 2, Kruskal-Wallis ANOVA, p< 0.001). Some suckling youngs were as large as three quarters the size of the mother.
Presence of newborns: The highest numbers of newborns (one month old or less) were recorded in July and August (range 15.2 % - 31.6 % of the herd) and the lowest in February. In February, we observed only one newborn among all herds under observation. This newborn disappeared within three weeks of birth. Actually the lowest numbers of newborns were recorded in January, March and April, with zero observations of newborns.
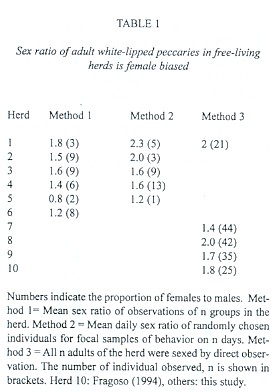
Sex ratio: We rejected the null hypothesis that in herds of white-lipped peccaries the probability of observing a male or a female biased sex ratio among adults is equal. In eight out of nine herds observed in this study the sex ratio of adults was consistently female biased (Binomial test, n = 9 herds, p< 0.05, Table 1). The mean adult sex ratio of four herds where all adults were sexed by direct observation was 1.8:1. The mean adult sex ratio obtained from pooled observations of peccary groups from six herds was 1.7:1, corresponding to 63% ± 2.4 females (n = 31 group observations). The mean daily adult sex ratio of randomly selected individuals from six herds was 1.4:1, corresponding to 59% ± 3.7 females (n = 34 days with four or more focal animals). The units contributing to the means of the latter two indirect methods are not statistically independent, because the same herds and, on some occasions probably the same individuals, were sampled for sex ratio data repeatedly.
Discussion
Mating seasonality: In contrast to the non-seasonal patterns of breeding observed for white-lipped and collared peccaries in the Peruvian Amazon (Gottdenker and Bodmer 1998), T. pecari in Corcovado National Park is a seasonal breeder with two peaks of mating events. Mating interactions occurred mainly in February and March and to a lesser extent in July and August. Indeed, the greatest abundance of newborns observed from June to August results from the February and March matings, in accordance to a gestation of about five months (Roots 1966, Sowls 1997). This pattern was also confirmed by the nursing activity, which peaked in late August. In addition, during the year previous to this study (April 1995 to March 1996) in one herd we observed a few newborns each month, with peaks during the months previously mentioned (unpublished data). This indicates that fertile matings may occur throughout the year. Apparently, however, not many pregnancies result from the mating events of July and August in the lowland rainforest of southern Costa Rica.
In other latitudes where there are differences in resource availability between dry and wet season (Texas: Bissonette 1982, Arizona: Day 1985, French Guyana: Henry 1994), collared peccaries give birth during the rainy season when food availability is highest. The same pattern has been observed in the Chaco region for Chacoan peccary (Sowls 1997). In Corcovado National Park, the relationship between availability of fruits consumed by peccaries and rainfall patterns is not simple. Fruit availability for peccaries peaks in July - September and January - March (Altrichter 1997). Therefore, litters conceived in the highest peak of mating (February) are born during months of high fruit availability. This time of peak nursing activity is highly demanding of nutrients. If females would get pregnant from July and August matings, litters would be born during months of low availability (November and December). As we were unable to find any herds in December, we do not know whether births took place during that month. No young were observed in January, however. Absence of young in January could also result from high juvenile mortality due to food scarcity at the end of the year (Altrichter 1997), or deaths in late November and December when peccaries travel outside the Corcovado national park boundaries and are more exposed to hunting (Altrichter 1999, unpublished).
Sex ratio: Published data thus far suggested that sex ratios at birth and during adulthood in peccaries are close to 1:1 (review in Sowls 1997). In Chacoan peccaries the adult sex ratio of collected specimens and of 12 adults from five herds was essentially 1:1 (Mayer and Brandt 1982). Pooled data from five herds of collared peccaries in Arizona yielded a sex ratio of 1.0 female to 0.65 males for all age classes combined (Mayer and Brandt 1982, calculated from data in Neal 1959 and Schweinsburg 1971). The sex ratios approach 1:1 among older age cohorts, however (Sowls 1974). In the Arizona population of collared peccaries the sex ratio of large samples of collected specimens was close to 1:1 (Knipe 1957, Sowls 1966, Mayer and Brandt 1982). In another study, average sex ratio of 11 herds of collared peccaries was not different from 1:1 (Bissonette 1982). One herd of seven collared peccaries from the Amazon basin was composed by four adult females and three adult males (Fragoso 1994).
Nonetheless, reports of fetuses and newborn collared peccaries suggest a slight female biased sex ratio during early development, although data in each study were not significantly different from 1:1 (Sowls 1966, 1984, Low 1970, Hellgren et al. 1995, Gottdenker and Bodmer 1998). In eight separate samples from Arizona and Texas (Sowls 1966, 1984, Low 1970) females accounted for 53%-66% of the animals. Only two studies report 47% and 42.1% females among fetuses (Hellgren et al. 1995, Gottdenker and Bodmer 1998, respectively). Sowls (1984, p. 82) suggests that sex ratio is biased in favor of females at birth and shifts gradually toward males in older age cohorts of hunted animals.
The sex ratio of adult white-lipped peccaries in our study was significantly female-biased. It ranged between 1.4:1 and 1.8:1, depending on the methodology used. The mean sex ratio of four herds in which all adults were sexed through direct observation was 1.8:1, equal to the sex ratio of 25 adults of one herd of white-lipped peccaries in the Amazon basin reported by Fragoso (1994). Sex and age composition of individual collared peccary herds reflect the population demography as a whole and do not result from behavioral subdivisions, such as bachelor herd or harems in other ungulates (Sowls 1984). We assume that this inference applies to white-lipped peccaries as well, for we have not commonly observed single males, any bachelor herds or obvious harems in our study population. The factor determining this female-bias is unknown. Differential exposure to hunting could account at least partly for a higher mortality in male T. pecari. Hunting of peccaries persists in the periphery and within the national park boundary (Altrichter 1999, unpublished). We have observed that adult males tend to stay behind the herd, facing a threatening situation, while females and young escape.
Differential male mortality, however, seems common in mammals, including humans (review in Trivers 1985, p.301-314), a trend which results in female biased adult sex ratios. Strong differential male mortality is expected in species where variation in male reproductive success is large (e.g. Trivers 1985). We would thus predict that male-male competition over females is strong in white-lipped peccaries and that a polygynous mating system prevails.
In conclusion, comparative studies of the socioecology of peccaries are necessary to understand the differences in this group of neotropical ungulates. Breeding seasonality of white-lipped peccaries in a Central American lowland rainforest was found to be associated with changes in fruit availability. The association between high availability of food and birth peaks is common to various populations of peccaries in seasonal environments. Peccaries from the Peruvian Amazon, however, are not seasonal breeders. Similarly, the female biased adult sex ratio of white-lipped peccaries in the Costa Rican rainforest contrasts with the even sex ratios reported for collared and Chacoan peccaries. Understanding the habitat differences within the distribution range of peccaries is likely to shed light on the differences in their reproductive patterns.
Acknowledgments
We thank Omar Laquis for his invaluable help in the field. Ricardo Ferrari and two anonymous referees reviewed and considerably improved earlier versions of this manuscript. This research was supported by Wildlife Conservation Society, World Wide Fund (WWF) and the Regional Wildlife Management Program of the National University of Costa Rica. We are grateful to the Ministry of the Environment and Energy of Costa Rica, which authorized our research in Corcovado National Park.
Resumen
Los "chanchos cariblancos" Tayassu pecari no son reproductores estacionales en Sur América. En Centro América se conoce muy poco sobre la reproducción de esta especie en la naturaleza. Estudiamos reproducción y la proporción de sexos durante 200 horas de observación de cuatro manadas marcadas con radiocollares y de dos manadas sin marcar, desde julio de 1996 hasta abril de 1997 en el Parque Nacional Corcovado, Costa Rica, agregando datos de proporción sexual de tres manadas observadas en 1998. Registramos el número de cópulas y de amamantamientos, así como la presencia de recién nacidos en las manadas. Observamos una marcada estacionalidad en la reproducción, con un pico en el número de cópulas en febrero y otro en julio. La mayor cantidad de recién nacidos y el pico de actividad de amamantamiento fueron en julio y agosto, cuando la disponibilidad de alimento era alta. Entre adultos predominaron numéricamente las hembras (1.4:1 - 1.8:1), lo cual difiere de lo conocido sobre las poblaciones suramericanas.
References
Altrichter, M. 1997. Estrategia de alimentación y comportamiento del chancho cariblanco Tayassu pecari en un bosque húmedo tropical de Costa Rica. M. Sc. thesis, National University of Costa Rica, Heredia, Costa Rica. [ Links ]
Bissonette, J. A. 1982. Ecology and social behavior of the collared peccary in Big Bend National Park, Texas. Nat. Park Serv. Sci. Monogr. 16, Washington, D.C. 85 p. [ Links ]
Bodmer, R. E. 1989. Frugivory in amazon ungulates. Ph. D. thesis. University of Michigan, Michigan. [ Links ]
Boinski, S. H. 1986. The ecology of squirrel monkeys in Costa Rica. Ph. D. thesis, University of Texas, Austin, Texas. [ Links ]
Campero, H. 1999. Variación y estructura genética dentro y entre grupos de chanchos de monte Tayassu pecari en el Parque Nacional Corcovado, Costa Rica. M. Sc. thesis, National University of Costa Rica, Heredia, Costa Rica.
Day, G. I. 1985. Javelina, research and management in Arizona. Arizona Game and Fish Department. Phoenix, Arizona. 122 p.
Emmons, L. H. 1984. Geographic variation in densities and diversities of non-flying mammals in Amazonia. Biotropica 16: 210-222. [ Links ]
Fragoso, J. M. 1994. Large mammals and the community of an Amazonian rain forest. Ph. D. thesis, University of Florida, Florida. [ Links ]
Fragoso, J. M. 1998. Home range and movement patterns of white-lipped peccary (Tayassu pecari) herds in the Northern Brazilian Amazon. Biotropica 30: 458-469. [ Links ]
García, R. V. 1997. Biologia de la conservación y áreas silvestres protegidas: situación actual y perspectivas en Costa Rica. Instituto Nacional de Biodiversidad. Heredia, Costa Rica. 64 p
Gottdenker, N. & R. E. Bodmer. 1998. Reproduction and productivity of white-lipped and collared peccaries in the Peruvian Amazon. J. Zool. Lond. 245: 423-430. [ Links ]
Hartshorn, G. S. 1983. Plants. p. 118-157. In D. H. Janzen (ed.). Costa Rican natural history. The University of Chicago, Chicago, Illinois. [ Links ]
Hellgren, E. C., D. R. Synatzke, P. W. Oldenberg & F. S. Guthery. 1995. Demography of a collared peccary population in South Texas. J. Wildl. Mgmt. 59: 153-163.
Henry, O. 1994. Saisons de reproduction chez trois Rongeurs et un Artiodactyle en Guyane Française, en fonction des facteurs du milieu et de lalimentation. Mammalia 58: 183-200.
Holdridge, L. 1967. Life Zone Ecology. Tropical Science Center, San José, Costa Rica. 206 p. [ Links ]
Janzen, D. H. 1983. Costa Rican natural history. The University of Chicago, Chicago, Illinois. 816 p. [ Links ]
Kiltie, R. A. 1981. Stomach contents of rain forest peccaries (Tayassu tajacu and T. pecari). Biotropica 13: 235-236. [ Links ]
Knipe, T. 1957. The javelina in Arizona. Wildl. Bull. No 2. Ariz. Game and Fish Department. 96 p. [ Links ]
Leopold, A. S. 1959. Wildlife of Mexico: the game birds and mammals. University of California Press, Los Angeles, California. 568 p. [ Links ]
Low, W. A. 1970. The influence of aridity on reproduction in the collared peccary Dicotyles tajacu (Linn.) in Texas. Ph. D. thesis, University of British Columbia, Vancouver.
Mayer, J. J.& P. N. Brandt. 1982. Identity, distribution and natural history of the peccaries, Tayassuidae, p. 433-455. In Mares, M. A.& H. H. Genoways (eds.). Mammalian biology in South America. Special Publication Series Pymatuning Laboratory of Ecology. University of Pittsburgh, Pennsylvania.
Miller, F. N. 1930. Notes on some mammals of southern Matto Grosso, Brazil. J. Mamm. 11:18. [ Links ]
Naranjo, E. P. 1994. Abundancia, uso de hábitat y hábitos de alimentación del tapit Tapirus bairdii En un bosque tropical húmedo en Costa Rica. M. Sc. thesis, National University of Costa Rica, Heredia, Costa Rica.
Neal, B. J. 1959. A contribution on the life history of the collared peccary in Arizona. Am. Midl. Nat. 61: 177-190. [ Links ]
Roots, C. G. 1966. Notes on the breeding of the white-lipped peccaries at Dudley Zoo. Int. Zoo Ybk. 6: 198-625. [ Links ]
Oliver, W. L. R. 1990. Pigs and peccaries group report. Species Newsletter of IUCN/SSC No. 13-14: 80-82. [ Links ]
Schweinsburg, R. E. 1971. The home range, movement and herd integrity of the collared peccary in Southern Arizona. J. Wildl. Mgmt. 35: 433-460. [ Links ]
Sowls, L. K. 1966. Reproduction in the collared peccary (Tayassu tajacu). p 155-172 In I. W. Rowlands (ed.). Comparative biology of reproduction in mammals. Zool. Soc. London.
Sowls, L. K. 1974. Social behavior of the collared peccary, Dicotyles tajacu L. p. 144-165. In V. Geist & F. Walther (eds.). The behaviour of ungulates and its relation to management. IUCN. Morges, Switzerland.
Sowls, L. K. 1984. The Peccaries. The University of Arizona, Tucson, Arizona. 251 p. [ Links ]
Sowls, L. K. 1997. Javelinas and other peccaries: their biology, management and use. Texas A&M University, College Station, Texas. 324 p [ Links ]
Trivers, R. 1985. Social Evolution. Benjamin Cummings, Menlo Park, California. 462 p. [ Links ]
Vaughan, C. 1981. Parque Nacional Corcovado. Plan de manejo y desarrollo. Universidad Nacional. San José, Costa Rica. 364 p. [ Links ]
1 Programa Regional en Manejo de Vida Silvestre, Universidad Nacional, Apartado 1350-3000 Heredia, Costa Rica. Fax: (506) 237-7036; maltrich@una.ac.cr
* Corresponding author














