Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.48 n.1 San José Mar. 2000
Received 15-IV-1999. Corrected 28-X-1999. Accepted 10-XI-1999.
An updated list of the Odonata of Costa Rica is presented containing 268 species. Since the last published list for the country, 41 additional species have been reported. The country is the best studied in Central America. The most species-rich families are Libellulidae, Coenagrionidae, Gomphidae, and Aeshnidae, together comprising ~75% of the total fauna. Most species in the country are also found in South America, indicating a tendency for wide ranges rather than endemism. However, about a fifth of the species appear to be endemic to the Costa Rica-Panama region. Estimates of the range of the proportion of total world species occurring in Costa Rica lead to predictions of a range of 5600-9000 species of Odonata worldwide.
Key words Odonata, Costa Rica, checklist, aquatic entomology, diversity, tropics.
Members of the order Odonata are among the most familiar groups of insects, perhaps surpassed only by the butterflies (order Lepidoptera). These insects are commonly known as dragonflies and damselflies in English and libélulas or caballitos del diablo in Spanish. Although adults are colorful and easy to spot, especially the males, our knowledge of tropical Odonata is not much greater than that of most other insect groups in tropical regions. Studies on Neotropical Odonata are virtually restricted to taxonomic works, with very few complete faunal lists. Very little is known about the ecology and behavior of most species in this order of aquatic insects.
Studies on Costa Rican Odonata started as early as 1890 with the publication of P. P. Calverts Odonata chapter in the Biologia Centrali-Americana (Calvert 1892-1908). In that account, 293 species of Odonata were reported as occurring in Mexico and Central America, of which 101 were listed for Costa Rica. In the same work, Calvert estimated 163 species for Costa Rica, based on taxa recorded from adjacent countries but not from Costa Rica at that point. The most recent list of species of Costa Rican Odonata was provided by Paulson (1982), who also presented a list of synonyms that is useful when using old taxonomic works on Odonata.
The objective of this study is to contribute to the knowledge of the Costa Rican fauna by providing an updated checklist of species occurring in the country. In addition, we highlight major characteristics of the Odonata fauna of Costa Rica in contrast with that of other countries in America.
Materials and Methods
Data were obtained from the collections and databases of the authors and the Museo de Insectos at the Universidad de Costa Rica. In addition, information was obtained from collections made by S. Brooks, British Museum of Natural History, England (Brooks 1989); T. Donnelly, Binghamton, New York; S. Dunkle, Plano, Texas; G. Harp, State University, Arkansas; and R. Novelo-Gutiérrez, Instituto de Ecología A.C., Mexico.
Results
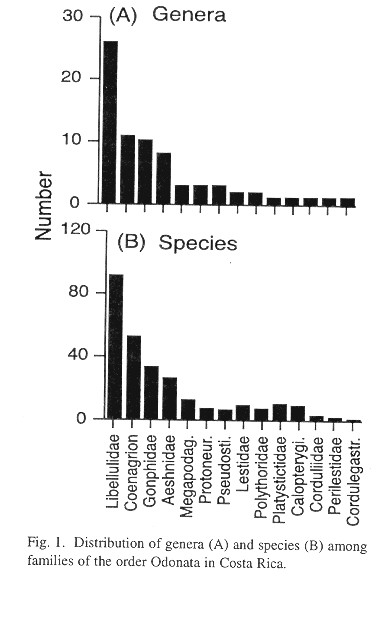
At present, 268 species of Odonata have been recorded from Costa Rica, distributed among 14 families and 73 genera (Table 1). Four families stand out as the most diverse, in number of both genera and species: Libellulidae, Coenagrionidae, Gomphidae, and Aeshnidae (Fig. 1). The four families combined comprise ~75% of the total Odonata fauna of Costa Rica.
List of species of the order Odonata from Costa Rica.
| Suborder Zygoptera | G. gracilis (Burmeister, 1839) |
| Polythoridae | G. laticeps Williamson, 1923 |
| Cora chirripa Calvert, 1907 | G. membranalis Karsch, 1891 |
| C. marina Selys, 1868 | Mexicana Selys, 1868 |
| C. notoxantha Ris, 1918 | G. nervosa Rambur, 1842 |
| C. obscura Ris, 1918 | G. tibiata Karsch, 1891 |
| C. semiopaca Selys, 1878 | Neuraeschna maya Belle, 1989 b |
| C. skinneri Calvert, 1907 | Remartinia luteipennis (Burmeister, 1839) |
| Miocora peraltica Calvert, 1917 | Staurophlebia reticulata (Burmeister, 1839) |
| Triacanthagyna caribbea Williamson, 1923 | |
| Calopterygidae | T. ditzleri Williamson, 1923 |
| Hetaerina caja (Drury, 1773) | T. satyrus (Martin, 1909) |
| H. capitalis Selys, 1873 | T. septima (Selys, 1857) |
| H. cruentata (Rambur, 1842) | |
| H. fuscoguttata Selys, 1878 | Gomphidae |
| H. majuscula Selys, 1853 | Agriogomphus tumens (Calvert, 1905) |
| H. miniata Selys, 1879 | Aphylla obscura (Kirby, 1899) |
| H. occisa Hagen in Selys, 1853 | A. protracta (Hagen in Selys, 1859) d |
| H. sempronia Hagen in Selys, 1853 | Archaeogomphus furcatus Williamson, 1923 |
| H. titia (Drury, 1773) | Desmogomphus paucinervis (Selys, 1873) |
| Epigomphus armatus Ris,1918 | |
| Lestidae | E. camelus Calvert, 1905 |
| Archilestes grandis (Rambur, 1842) | E. corniculatus Belle, 1989 a |
| A. latialatus Donnelly, 1981 | E. echeverrii Brooks, 1989 a |
| A. neblina Garrison, 1982 a | E. houghtoni Brooks, 1989 a |
| Lestes alacer Hagen, 1861 | E. quadracies Calvert, 1903 |
| L. forficula Rambur, 1842 | E. subobtusus Selys, 1878 |
| L. henshawi Calvert, 1907 | E. subsimilis Calvert, 1920 |
| L. sigma Calvert, 1901 | E. tumefactus Calvert, 1903 |
| L. tenuatus Rambur, 1842 | E. verticicornis Calvert, 1908 |
| L. tikalus Kormondy, 1959 | Erpetogomphus bothrops Garrison, 1994 bd |
| E. constrictor Ris, 1918 c | |
| Perilestidae | E. elaphe Garrison, 1994 bd |
| Perissolestes magdalenae (Williamson and Williamson, 1924) c | E. eutainia Calvert, 1905 c |
| P. remotus (Williamson and Williamson, 1924) | E. schausi Calvert, 1919 c |
| E. tristani Calvert, 1912 | |
| Megapodagrionidae | Perigomphus pallidistylus (Belle, 1972) |
| Heteragrion albifrons Ris, 1918 | Phyllocycla volsella (Calvert, 1905) |
| H. atrolineatum Donnelly, 1992 b | Phyllogomphoides appendiculatus (Kirby, 1899) |
| H. erythrogastrum Selys, 1886 | P. bifasciatus (Hagen in Selys, 1878) |
| H. majus Selys, 1886 | P. burgosi Brooks, 1989 a |
| H. mitratum Williamson, 1919 c | P. pugnifer Donnelly, 1979 |
| Philogenia carrillica Calvert, 1907 | P. suasus (Selys, 1859) |
| P. championi Calvert, 1901 | Progomphus anomalus Belle, 1973 c |
| P. expansa Calvert, 1924 | P. clendoni Calvert, 1905 |
| P. lankesteri Calvert, 1924 | P. longistigma Ris, 1918 |
| P. peacocki Brooks, 1989 a | P. mexicanus Belle, 1973 |
| P. terraba Calvert, 1907 | P. pygmaeus Selys, 1873 |
| Thaumatoneura inopinata McLachlan, 1897 | Cordulegastridae |
| Cordulegaster godmani McLachlan, 1878 | |
| Pseudostigmatidae | |
| Mecistogaster linearis (Fabricius, 1776) | Corduliidae |
| M. modesta Selys, 1860 | Neocordulia batesi (Selys, 1871) |
| M. ornata Rambur, 1842 | N. campana May and Knopf, 1988 b |
| Megaloprepus caerulatus (Drury, 1782) | N. griphus May, 1991 a |
| Pseudostigma aberrans Selys, 1860 | |
| P. accedens Selys, 1860 c | Libellulidae |
| Anatya normalis Calvert, 1899 | |
| Platystictidae | Brachymesia furcata (Hagen, 1861) |
| Palaemnema baltodanoi Brooks, 1989 a | B. herbida (Gundlach, 1889) |
| P. chiriquita Calvert, 1931 | Brechmorhoga nubecula (Rambur, 1842) |
| P. collaris Donnelly, 1992 b | B. pertinax (Hagen, 1861) |
| P. dentata Donnelly, 1992 b | B. praecox (Hagen, 1861) |
| P. distadens Calvert, 1931 | B. rapax Calvert, 1898 |
| P. gigantula Calvert, 1931 | B. vivax Calvert, 1906 |
| P. melanota Ris, 1918 | Cannaphila insularis Kirby, 1889 |
| P. nathalia Selys, 1886 | C. mortoni Donnelly, 1992 b |
| P. paulirica Calvert, 1931 | C. vibex (Hagen, 1861) |
| P. reventazoni Calvert, 1931 | Dythemis multipunctata Kirby, 1894 |
| D. sterilis Hagen, 1861 | |
| Protoneuridae | Elasmothemis cannacrioides (Calvert, 1906) |
| Neoneura amelia Calvert, 1903 | Erythemis attala (Selys, 1857) |
| N. esthera Williamson, 1917 | E. haematogastra (Burmeister, 1839) |
| Protoneura amatoria Calvert, 1907 | E. mithroides (Brauer, 1900) |
| P. aurantiaca Selys, 1886 | E. peruviana (Rambur, 1842) |
| P. sulfurata Donnelly, 1989 a | E. plebeja (Burmeister, 1839) |
| Psaironeura remissa (Calvert, 1903) | E. simplicicollis (Say, 1839) |
| P. selvatica Esquivel, 1993 a | E. vesiculosa (Fabricius, 1775) |
| Erythrodiplax abjecta (Rambur, 1842) | |
| Coenagrionidae | E. andagoya Borror, 1942 |
| Acanthagrion inexpectum Leonard, 1977 | E. berenice (Drury, 1770) c |
| A. speculum Garrison, 1985 ad | E. castanea (Burmeister, 1839) |
| A. trilobatum Leonard, 1977 | E. famula (Erichson, 1848) |
| Anisagrion allopterum Selys, 1876 | E. fervida (Erichson, 1848) |
| A. kennedyi Leonard, 1937 | E. funerea (Hagen, 1861) |
| Argia adamsi Calvert, 1902 | E. fusca (Rambur, 1842) |
| A. anceps Garrison, 1996 ad | E. kimminsi Borror, 1942 |
| A. chelata Calvert, 1902 | E. kimminsi Borror, 1942 |
| A. cupraurea Calvert, 1902 | E. umbrata (Linnaeus, 1758) |
| A. cuprea (Hagen, 1861) c | Idiataphe amazonica (Kirby, 1889) c |
| A. difficilis Selys, 1865 | I. cubensis (Scudder, 1866) |
| A. eliptica Selys, 1865 c | Libellula croceipennis Selys, 1868 |
| A. extranea (Hagen, 1861) | L. foliata (Kirby, 1889) |
| A. fissa Selys, 1865 | L. herculea Karsch, 1889 |
| A. frequentula Calvert, 1907 | L. mariae Garrison, 1992 a |
| A. gaumeri Calvert, 1907 c | Macrothemis aurimaculata Donnelly, 1984 b |
| A. indicatrix Calvert, 1902 | M. delia Ris, 1913 |
| A. insipida Hagen in Selys, 1865 | M. extensa Ris, 1913 |
| A. johannella Calvert, 1907 | M. hemichlora (Burmeister, 1839) |
| A. medullaris Hagen in Selys, 1865 | M. imitans Karsch, 1890 |
| A. oculata Hagen in Selys, 1865 | M. inacuta Calvert, 1898 |
| A. oenea Hagen in Selys, 1865 | M. inequiunguis Calvert, 1895 |
| A. pocomana Calvert, 1907 | M. musiva Calvert, 1898 |
| A. popoluca Calvert, 1902 | M. pseudimitans Calvert, 1898 |
| A. pulla Hagen in Selys, 1865 | Miathyria marcella (Selys, 1857) |
| A. rogersi Calvert, 1902 | M. simplex (Rambur, 1842) |
| A. talamanca Calvert, 1907 | Micrathyria aequalis (Hagen, 1861) |
| A. terira Calvert, 1907 | M. atra (Martin, 1897) |
| A. tezpi Calvert, 1902 | M. catenata Calvert, 1909 |
| A. translata Hagen in Selys, 1865 | M. dictynna Ris, 1919 |
| A. ulmeca Calvert, 1902 | M. didyma (Selys, 1857) |
| A. underwoodi Calvert, 1907 | M. hagenii Kirby, 1890 |
| A. variabilis Selys, 1865 | M. laevigata Calvert, 1909 |
| Chrysobasis lucifer Donnelly, 1967 | M. mengeri Ris, 1919 |
| Enallagma civile (Hagen, 1861) | M. ocellata Martin, 1897 |
| E. novaehispaniae Calvert, 1907 | M. pseudeximia Westfall, 1992 bd |
| Ischnura capreolus Hagen, 1861 | M. schumanni Calvert, 1906 |
| I. hastata (Say, 1839) | M. tibialis Kirby, 1897 c |
| I. ramburii (Selys, 1850) | Nephepeltia chalconota Ris, 1919 c |
| Leptobasis vacillans Hagen in Selys, 1877 | N. phryne (Perty, 1834) |
| Metaleptobasis bovilla Calvert, 1907 | Oligoclada umbricola Borror, 1931 c |
| M. westfalli Cumming, 1954 | Orthemis biolleyi Calvert, 1906 |
| Nehalennia minuta (Selys, 1857) | O. cultriformis Calvert, 1899 |
| Neoerythromma cultellatum (Selys, 1876) | O. discolor (Burmeister, 1839) c |
| Telebasis aurea May, 1992 a | O. ferruginea (Fabricius, 1775) |
| T. corallina (Selys, 1876) | O. levis Calvert, 1906 |
| T. digiticollis Calvert, 1902 | Paltothemis lineatipes Karsch, 1890 |
| T. filiola (Perty, 1834) | Pantala flavescens (Fabricius, 1798) |
| T. garleppi Ris, 1918 | P. hymenaea (Say, 1839) |
| T. griffinii (Martin, 1896) | Perithemis domitia (Drury, 1773) |
| T. isthmica Calvert, 1902 | P. electra Ris, 1930 |
| T. salva (Hagen, 1861) | P. mooma Kirby, 1889 |
| Pseudoleon superbus (Hagen, 1861) | |
| Suborder Anisoptera | Rhodopygia hinei Calvert, 1907 |
| Aeshnidae | Sympetrum illotum (Hagen, 1861) |
| Aeshna cornigera Brauer, 1865 | S. nigrocreatum Calvert, 1920 |
| A. jalapensis Williamson, 1908 | Tauriphila argo (Hagen, 1869) |
| A. psilus Calvert, 1947 | T. australis (Hagen, 1867) |
| A. williamsoniana Calvert, 1905 c | T. azteca Calvert, 1906 |
| Anax amazili (Burmeister, 1839) | Tholymis citrina Hagen, 1867 |
| A. concolor Brauer, 1865 | Tramea abdominalis (Rambur, 1842) |
| Coryphaeschna adnexa (Hagen, 1861) | T. binotata (Rambur, 1842) |
| C. amazonica De Marmels, 1989 bd | T. calverti Muttkowski, 1910 |
| C. apeora Paulson, 1994 a | T. insularis Hagen, 1861 c |
| C. diapyra Paulson, 1994 a | T. onusta Hagen, 1861 |
| C. viriditas Calvert, 1952 | Uracis fastigiata (Burmeister, 1839) |
| Gynacantha auricularis Martin, 1909 c | U. imbuta (Burmeister, 1839) |
| G. caudata Karsch, 1891 | U. turrialba Ris, 1919 |
Key to changes from Paulson (1982): a- newly described from Costa Rica, b- newly described from elsewhere, originally or subsequently recorded from Costa Rica, c- newly recorded from Costa Rica, d- reidentified, previously listed under a different name.
Paulson (1982) listed 227 species of Odonata from Costa Rica. The present list contains 268 species, 41 more than Paulson recorded. The difference in the number of species is the result of the naming of previously undescribed species (25, of which 5 had been recorded under another name) and the recording of additional species (21) from the country. Three species were recorded as questionable in Paulsons list: Hetaerina infecta (old record not confirmed), Acanthagrion quadratum (misidentified, actually A. speculum), and Anax junius (old record not confirmed). Only one species has been deleted from the list, Perithemis thais (now considered an undescribed species). Finally, the names of 20 of the species on Paulsons (1982) list have changed (Table 2) because of synonymies or misidentifications.
The proportion of undescribed to described species in a region should decrease as the region becomes better known. Paulson (1982) listed 23 undescribed species that occurred in Costa Rica, representing 1.0% of the known fauna. Subsequently, 11 of these species have been described, one as a subspecies, and 13 species not known to Paulson have been described as well. At present, there are still at least 11 Costa Rican species awaiting description (i.e., one Miocora, four Palaemnema, two Argia, one Leptobasis, one Gynacantha, one Micrathyria, and one Perithemis), representing 0.4% of the known fauna.
The broad distributional patterns of the Costa Rican fauna were analyzed (Table 3). A surprisingly large proportion (about one-fifth) appear to be endemic either to Costa Rica (13.1%) or the Costa Rica-Panama region (6.7%). Another one-fourth (25.7%) of the species are endemic to Middle America region (Mexico to Panama). Almost half of the species clearly have South American affinities, either reaching their northern limit in Costa Rica (5.6%) or widespread in Middle and South America (40.2%), although their area of origin is only speculative. Only a small proportion of the fauna clearly comes from the north, reaching its southern limit in Costa Rica/Panama (6.3%), or is widespread in North, Middle, and South America (4.5%). A single species, Tramea insularis, is largely restricted to the West Indies and may be only a vagrant in Costa Rica.
Origin of the Costa Rican Odonata fauna (in percentage, total 268 species).
| | | |
| Middle and South America | | |
| Primarily Middle America | | |
| Costa Rica only | | |
| Costa Rica-Panama only | | |
| Primarily South America | | |
| North and Middle America | | |
| | | |
| | |
Discussion
The list of species presented here represents a significant advance in comparison with previous accounts; however, our list is by no means a final one. Some regions of the country remain poorly explored, for example the southern parts of both the Cordillera de Talamanca and the Caribbean lowlands. Several families remain poorly collected, especially those that inhabit small forested streams. In addition, several genera (e.g., Argia) need to be reviewed to clarify the number of species they include. The number of Odonata species that occur in Costa Rica is not easy to predict, but we suggest that the present list includes at least 80% of the fauna, giving a potential total of ~340 species.
With 268 species known to occur, the Odonata fauna of Costa Rica is surely the best known of all Central American countries. Panama and Guatemala, with over 200 species each (D. Paulson unpublished information), are even less well explored than Costa Rica. Other well-studied Neotropical countries that support richer faunas are much larger than Costa Rica. For example, Brazil has over 650 species (D. Paulson unpublished information), Venezuela 455 (De Marmels 1990), and Mexico 330 (González-Soriano & Novelo-Gutiérrez 1996), but Costa Rica is only about 0.7%, 7%, and 3% of the size of those three countries, respectively, and has a more limited range of environments. Thus Costa Rica has a rich dragonfly fauna for its size.
Analysis of general distribution patterns of the Odonata species recorded in Costa Rica confirms the tropical origin of its fauna. Most species have wide distributions in Middle and South America (Table 3). Although 13.1% of the fauna is endemic to Costa Rica, this is likely the result of poor sampling efforts in adjacent countries rather than such a high degree of endemism. Studies of other taxa indicate that most tropical insects are widely distributed and that high levels of endemism or patchy distributions are probably a sampling artifact (e.g., Richards 1978, Janzen 1986, Gaston et al. 1996).
The most recent estimation of the total number of Odonata species was of 5600 (Bridges 1994). However, many more species remain to be discovered in poorly collected regions, such as most of Central America. Following the procedure used by Gaston et al. (1996) for Hymenoptera, we use the present list to speculate about the total number of species of Odonata worldwide. The lower limit of such an estimate will be the 5600 known species, of which Costa Rica has 4.8%. However, studies of other animal and plant groups indicated that Costa Rica has from 3-10% of the global species richness of most groups (Gaston et al. 1996). In that context, if we assume that Costa Rica has between 3% and 4.8% of the total Odonata fauna, then the potential total number of species in the order is in the range of 5 600 - 9 000. Our estimate is in general agreement with a previous one that suggested the world Odonata fauna to be composed of <10 000 species (Tennessen 1997).
In conclusion, the order Odonata in Costa Rica, although better studied than in other Central American countries, still needs a lot of attention. The distribution of most species is imperfectly known, and nothing is known about the ecology and behavior of virtually all species. More significantly at this time of rapidly vanishing natural habitats, we have no estimate of what percent of the species are protected within the boundaries of national parks and other protected areas. The larvae could be important indicators in monitoring water quality in rivers and lakes, but only half of the species in the country have their larval morphology described (Ramírez 1997), and only the general habitat of most of them is known.
Resumen
Se presenta una lista actualizada de los Odonata de Costa Rica, la cual contiene 268 especies. A pesar de ello, se espera que algunas especies más se agreguen en el futuro. En comparación con trabajos anteriores, el número de especies para Costa Rica se incrementó en 41, siendo el país mejor estudiado de Centro América. Las familias con mayor diversidad de especies son: Libellulidae, Coenagrionidae, Gomphidae, y Aeshnidae, que en conjunto representan ~75% del total de la fauna de Odonata en Costa Rica. La mayoría de las especies del país también se encuentran en Sur América, lo que indica una inclinación hacia ámbitos de distribución amplios, mas que altos grados de endemismo. Sin embargo, el 18% de las especies es aparentemente endémico de Costa Rica y Panamá. Con base en los números aquí presentados, se esperaría que la fauna mundial de Odonata se encuentre dentro del ámbito de 5600 a 9000 especies.
Acknowledgements
We are grateful to the Museo de Insectos at Universidad de Costa Rica, S. Brooks, T. Donnelly, S. Dunkle, G. Harp, and R. Novelo-Gutiérrez for sharing their data on Costa Rican Odonata. A. Ramírez was supported by the National Science Foundation (grant DEB-95-28434) during the writing of this paper.
References
Bridges, C.A. 1994. Catalogue of the family-group, genus-group and species-group names of the Odonata of the World. Champaign, IL, privately published. [ Links ]
Brooks, S.J. 1989. Odonata collected from Guanacaste National Park, Costa Rica, July 1988. Not. Odonatol. 3: 49-52. [ Links ]
Calvert, P. P. 1892-1908. Odonata. p. 17-420. In F.D. Godwin. (ed.). Biologia Centrali-Americana. Porter and Dulau, London. [ Links ]
Carvalho, A.L. 1992. Revalidation of the genus Remartinia Navás, 1911, with the description of a new species and a key to the genera of Neotropical Aeshnidae (Anisoptera). Odonatologica 21: 289-298. [ Links ]
De Marmels, J. 1984. The genus Nehalennia Selys, its species and their phylogenetic relationships (Zygoptera: Coenagrionidae). Odonatologica 13: 501-527. [ Links ]
De Marmels, J. 1987. Ischnura (Anomalagrion) cruzi sp. n., eine neue Kleinlibelle aus Kolumbien (Odonata: Coenagrionidae). Mitteil. Ent. Gesell. Basel N.F. 37: 1-6. [ Links ]
De Marmels, J. 1990. An updated checklist of Odonata of Venezuela. Odonatologica 19: 333-345. [ Links ]
Garrison, R.W. 1986. The genus Aphylla in Mexico and Central America, with a description of a new species, Aphylla angustifolia (Odonata: Gomphidae). Ann. Ent. Soc. Am. 79: 938-944. [ Links ]
Garrison, R.W. 1990. A synopsis of the genus Hetaerina with descriptions of four new species (Odonata: Calopterygidae). Trans. Am. Ent. Soc. 116: 175-259. [ Links ]
Garrison, R.W. 1991. A synonymic list of the New World Odonata. Argia 3: 1-30. [ Links ]
Garrison, R.W. 1994. A revision of the New World genus Erpetogomphus Hagen in Selys (Odonata: Gomphidae). Tijd. v. Ent. 137: 173-269. [ Links ]
Gaston, K.J., I.D. Gauld & P. Hanson. 1996. The size and composition of the hymenopteran fauna of Costa Rica. J. Biogeogr. 23: 105-113. [ Links ]
González-Soriano, E. & R. Novelo-Gutiérrez. 1996. Odonata. p. 147-167. In J. Llorente-Bousquets, A.N. García-Aldrete & E. González-Soriano. (eds.). Biodiversidad, taxonomía, y biogeografía de artrópodos de México: Hacia una síntesis de su conocimiento. Universidad Nacional Autónoma de México, Mexico, D.F. [ Links ]
Janzen, D.H. 1986. Biogeography of an unexceptional place: what determines the saturniid and sphingid moth faunas of Santa Rosa National Park, Costa Rica, and what does it mean to conservation biology? Brenesia 25/26: 51-87. [ Links ]
May, M.L. 1991. A review of the genus Neocordulia, with a description of Mesocordulia subgen. nov. and of Neocordulia griphus spec. nov. from Central America, and a note on Lauromacromia (Odonata: Corduliidae). Folia Entomol. Mex. 82: 17-67. [ Links ]
Paulson, D.R. 1982. Odonata. p. 249-277. In S.H. Hurlbert & A. Villalobos- Figueroa. (eds.). Aquatic Biota of Mexico, Central America, and the West Indies. San Diego State University, San Diego, California. [ Links ]
Paulson, D.R. 1994. Two new species of Coryphaeschna from Middle America, and a discussion of the red species of the genus. Odonatologica 23: 379-398. [ Links ]
Ramírez, A. 1997. Lista de las especies de Odonata de Costa Rica que cuentan con su náyade descrita. Rev. Biol. Trop. 44/45: 225-232. [ Links ]
Richards, O.W. 1978. The social wasps of the Americas excluding the Vespinae. British Museum (Natural History), London. [ Links ]
Tennessen, K.J. 1997. The rate of species descriptions in Odonata. Ent. News 108: 122-126. [ Links ]
Westfall, M.J. 1988. Elasmothemis gen. nov., a new genus related to Dythemis (Anisoptera: Libellulidae). Odonatologica 17: 419-428. [ Links ]
Westfall, M.J. 1992. Notes on Micrathyria, with descriptions of M. pseudeximia sp. n., M. occipita sp. n., M. dunklei sp. n. and M. divergens sp. n. (Anisoptera: Libellulidae). Odonatologica 21: 203-218. [ Links ]