Introduction
Most of the research on polychaetes (annelids) collected in southern regions refers to subantarctic species from the Patagonian shelf, the Strait of Magellan and Antarctica collected during international exploratory cruises (Blake, 1983; Bremec & Elías, 1999; Gambi & Mariani, 1999; Hartman, 1953, 1966; Hartmann-Schroeder, 1983; Hartmann-Schroeder & Hartmann, 1962; Lana & Bremec, 1994; Mariani, Gambi, Lorenti, & Mazzella, 1996; Montiel, Gerdes, Hilbig, & Arntz, 2005, Montiel, Gerdes, & Arntz, 2005; Montiel, Quiroga, & Gerdes, 2011; Orensanz, 1974, 1990; Rozbaczylo, Ríos, & Mutschke, 1997; Sanfilippo, 1994; Uschakov, 1962; Wesenberg-Lund, 1962; Bremec, Elías, & Gambi, 2000; Bremec, Souto, & Genzano, 2010; Elías, Bremec, Lana, & Orensanz, 2003). In particular at Burdwood Bank (BB), a few locations were sampled in the last decades during “Walther Herwig” (1978, stations 595 and 596; Hartmann-Schroeder, 1983), “Shinkai Maru” (1978-1979, stations 133, 135 and 138; Bremec et al., 2010) and “Polarstern” (2002, stations 145 and 150; Montiel et al., 2003) cruises. The plateau represents one of the “stepping stones” linking the southern tip of South America with the Antarctic Peninsula (Thomson, 2004; Arntz, Lovrich, & Thatje, 2005; Griffiths, Linse, & Barnes, 2008) and, in the case of polychaetes, faunal relationships between the Magellan region and the Antarctic continent were not established. Apart from a single taxon, orbiniid species found in the Southern Ocean are effectively isolated from South American species (Blake, 2017). It was suggested that comparable studies are needed to investigate affinities between both neighboring ecosystems, separated only by the Drake Passage and the Antarctic Convergence (Cañete, Leighton, & Aguilera, 1999; Montiel, Gerdes, Hilbig et al., 2005; Montiel, Gerdes et al., 2005).
BB is a segment of the North Scotia Ridge in the SW Atlantic Ocean, located between 54°-55° S and 56°-62° W. This undersea plateau is located about 200 km south from Malvinas Islands (Falkland Islands) and about 150 km east from Staten Island, Tierra del Fuego Province. The rocky slopes of the bank rise from more than 4 000 m depth in the Yaghan Basin (Drake Passage) to form a wide plateau with depths that vary between 50 m and 200 m, where the bottom abruptly breaks into a wall reaching 1 100 m to more than 3 000 m depth. The direct influence of the Antarctic Circumpolar Current and persistent westerlies blowing at 50° S make the region an extremely dynamic environment of subantarctic water, fairly homogeneous vertically in the plateau (without a thermocline), ranging seasonally from 4 to 9 ºC and with a mean salinity of 34 (Piola & Gordon, 1989; Guerrero, Baldoni, & Benavides, 1999). BB is surrounded by the Malvinas Current, a branch of the Circumpolar Antarctic Current that flows from the Drake Passage, which is one of the most important and nutrient rich currents of the sea (Piola & Gordon, 1989; Lutz et al., 2010). Although the existence of BB has been known since 1842 (Findlay, 1867), the benthic realm has been only scarcely studied.
The first open-sea (non-coastal) Marine Protected Area in Argentina, named “Namuncurá I” (NMPA, National Law 26875, Argentina), was created in 2013 at Burdwood Bank, Argentine Sea, and comprises nearly 28 000 km2 circumscribed by the 200 m isobath. It comprises three different management areas according to the protection level required: the central “core” (strict protection, only control and monitoring activities), surrounded by a “buffer” area (authorized activities, e.g. scientific research) and an external “transition” area (productive and extractive activities contemplated in the Management Plan). Beyond the transition area, only the southern shelf-break is protected since December 2018, when the “Namuncurá II” MPA was created (National Law 27490, Argentina). After recent research cruises, Schejter et al. (2016) preliminary recorded nearly 250 species of epibenthic organisms, and particular studies on Peracarida (Doti et al., 2014; Chiesa, Urteaga, Martínez, Doti, & Roccatagliata, 2015), Cnidaria (Cairns, 2012), Porifera (Schejter, Bertolino, & Calcinai, 2017) and Asteroidea (Fraysse, Calcagno, & Pérez, 2018) were developed.
The present objectives are to provide the inventory of polychaetes collected at the core (98 m depth), buffer (128 m depth) and transition (133-189 m depth) areas of NMPA and BB slope (220-798 m depth), where intensive sampling with trawls was performed to characterize the faunal assemblages and to explore affinities of the polychaete fauna between BB and other Magellanic areas.
Materials and methods
The NMPA and BB slope (S) were sampled during three research cruises onboard the GC-189 “Pr. García” (December 2015) and RV “Puerto Deseado” (March-April 2016; April-May 2017). Samples were taken in 30 stations (Fig. 1, Table 1) with bottom trawls of 10 mm mesh size. Faunal comparison with other Magellan areas was made using presence-absence data (due to the different sampling tools employed) from neighboring areas (N), Staten Island and coastal waters of Tierra del Fuego (TDF), and the Patagonian shelf (PS) (Table 2). Samples from N were collected during the “Puerto Deseado” 2017 expedition. Samples collected during “GC-189 Pr. García” 2015 and used for this regional comparison were collected with bottom trawls of 2mm mesh size (Güller, Abelando, Urcola, & Zelaya, 2015). Other additional polychaete data come from “Shinkai Maru” cruises IV, V, X and XI, collected with Picard dredge from soft bottoms and sieved with 1mm mesh screen (Bremec et al., 2010). Specimens collected during “Puerto Deseado” 2016-2017 and “GC-189 Pr. García” 2015 cruises were fixed in 4 % formaldehyde, preserved in alcohol 70 % and identified at the lowest taxonomic level possible. Taxonomic determinations were made based on Hartman, 1953, 1966; Wesenberg-Lund, 1962; Blake, 1983; Lana & Bremec, 1994; Bremec & Elías, 1999; Boggemann & Orenzanz, 2007; Barnich, Orensanz, & Fiege, 2012. Taxonomy and distribution were matched against the Register of Antarctic Marine Species (Clarke & Johnston, 2003), Bremec & Giberto (2008), Orensanz, Diez, Ferrando, & Trovant (2012), World Register of Marine Species (Horton et al., 2018) and Ocean biogeographic information system [OBIS] (2018) to ensure that synonymies or misspellings were removed, as well as to compare the known distribution to that recorded in this study.
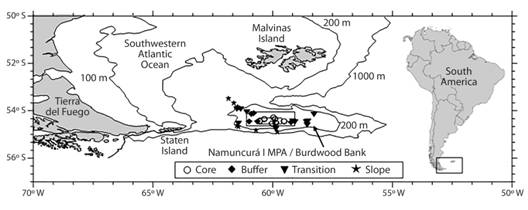
Fig. 1 Location of the sampling sites at Burdwood Bank in the different areas of Namuncurá Marine Protected Area and slope.
TABLE 1 Geographical position, depth and cruise information of the stations sampled at Burdwood Bank Slope (BBS), Transition (MPA-T), Buffer (MPA-B) and Core (MPA-C) areas during cruises “Puerto Deseado 2016” (BBB 16), “Puerto Deseado 2017” (PD BB 17) and “GC Pr. García 2015” (GC 15)
| Site | Cruise | Date (d/m/y) | Station | Haul | Sub-area | Lat. °S | Long. °W | Depth (m) |
| A | BBB16 | 13/04/2016 | 18 | 266 | BBS | -54.8209833 | -60.7035833 | 607 |
| B | BBB16 | 13/04/2016 | 21 | 239 | BBS | -54.8869667 | -59.815 | 785 |
| C | BBB16 | 13/04/2016 | 23 | 226 | MPA-T | -54.7596 | -59.8689167 | 182 |
| D | BBB16 | 29/03/2016 | 26 | 27 | MPA-T | -54.4158 | -58.5151667 | 137 |
| E | BBB16 | 28/03/2016 | 27 | 11 | MPA-T | -54.16775 | -58.2726167 | 100 |
| F | BBB16 | 30/03/2016 | 28 | 52 | MPA-B | -54.4593833 | -59.2201667 | 128 |
| G | BBB16 | 10/04/2016 | 30 | 184 | MPA-C | -54.2884833 | -59.9507833 | 96 |
| H | BBB16 | 10/04/2016 | 31 | 197 | MPA-C | -54.4993667 | -59.8588667 | 109 |
| I | BBB16 | 30/03/2016 | 32 | 77 | MPA-C | -54.5433167 | -60.0213167 | 98 |
| J | BBB16 | 8/04/2016 | 33 | 159 | MPA-B | -54.4295333 | -60.6477167 | 101 |
| K | BBB16 | 7/04/2016 | 34 | 146 | MPA-B | -54.4543 | -60.9803333 | 100 |
| L | BBB16 | 31/03/2016 | 35 | 89 | MPA-T | -54.5319833 | -61.4385167 | 125 |
| M | BBB16 | 19/04/2016 | 36 | 306 | MPA-T | -53.9298833 | -61.4956 | 185 |
| N | BBB16 | 29/04/2016 | 38 | 39 | MPA-T | -54.5887333 | -58.5472167 | 135 |
| O | BBB16 | 19/04/2016 | 40 | 320 | BBS | -54.6167667 | -61.4208333 | 415 |
| P | PD BB 17 | 28/04/2017 | 16 | 131 | BBS | -54.600012 | -61.5100102 | 294 |
| Q | PD BB 17 | 1/05/2017 | 23 | 173 | MPA-C | -54.43342 | -59.5033434 | 91 |
| R | PD BB 17 | 1/05/2017 | 24 | 184 | MPA-C | -54.33334 | -59.8950179 | 97 |
| S | PD BB 17 | 9/05/2017 | 25 | 304 | MPA-C | -54.3450069 | -60.3450069 | 104 |
| T | PD BB 17 | 9/05/2017 | 26 | 317 | MPA-B | -54.100002 | -60.7100142 | 120 |
| U | PD BB 17 | 9/05/2017 | 27 | 326 | MPA-B | -54.1066688 | -60.8783509 | 128 |
| V | PD BB 17 | 8/05/2017 | 29 | 283 | MPA-T | -53.8150163 | -61.3200064 | 197 |
| W | PD BB 17 | 8/05/2017 | 30 | 273 | BBS | -53.8216831 | -61.4733428 | 209 |
| X | PD BB 17 | 7/05/2017 | 31 | 269 | BBS | -53.66668 | -61.6366794 | 642 |
| Y | PD BB 17 | 7/05/2017 | 33 | 256 | BBS | -53.466676 | -61.8416835 | 595 |
| Z | PD BB 17 | 30/04/2017 | 21 | 157 | MPA-T | -54.4250085 | -58.5250105 | 138 |
| A´ | GC 15 | 12/12/2015 | 14 | 8b | MPA-C | -54.439467 | -60.652217 | 99 |
| B´ | GC 15 | 13/12/2015 | 17 | 1 | MPA-T | -54.4855 | -59.081217 | 138 |
| C´ | GC 15 | 13/12/2015 | 17b | 2 | MPA-T | -54.45215 | -59.1285 | 138 |
| D´ | PD BB 17 | 8/05/2017 | 28 | 287 | MPA-T | -54.0533344 | -61.0950019 | 140 |
TABLE 2 Geographical position and depth of the stations sampled at Burdwood Bank Neighboring locations (N), Tierra del Fuego (TDF), Patagonian Shelf (PS) and Namuncurá Marine Protected Area (MPA) during “Puerto Deseado 2017” (PD), “GC Pr. García 2015” (GC) and “Shinkai Maru 1978-1979” (SM) cruises
| Cruise | Station | Area | Lat °S | Long. °W | Depth (m) |
| PD | 34 | N | -53.5680114 | -62.9656026 | 516 |
| PD | 35 | N | -53.5747782 | -63.9987033 | 236 |
| PD | 36 | N | -53.7297479 | -64.5076435 | 137 |
| GC | 1 | TDF | -54.894583 | -67.675783 | 75 |
| GC | 2 | TDF | -54.9124 | -67.235733 | 38 |
| GC | 3 | TDF | -54.958333 | -66.919817 | 64 |
| GC | 4 | TDF | -55.065433 | -66.68285 | 38 |
| GC | 4B | TDF | -55.09805 | -66.3373 | 45 |
| GC | 5 | TDF | -55.041333 | -66.074417 | 86 |
| GC | 6 | TDF | -55.006767 | -65.828 | 103 |
| GC | 6B | TDF | -54.956267 | -65.4572 | 56 |
| GC | FH | TDF | -54.8965 | -67.313883 | 25 |
| SM V | 36 | PS | -43.4504234 | -63.4682603 | 72 |
| SM V | 37 | PS | -43.51677 | -61.9843635 | 91 |
| SM X | 40 | PS | -43.45009 | -59.53344 | 145 |
| SM X | 44 | PS | -44.5001 | -62.48343 | 103 |
| SM IV | 55 | PS | -46.55011 | -65.5171034 | 79 |
| SM XI | 55 | PS | -46.51677 | -65.45009 | 72 |
| SM XI | 57 | PS | -46.5001 | -63.43342 | 115 |
| SM X | 59 | PS | -46.46676 | -61.5001 | 121 |
| SM IV | 60 | PS | -46.48343 | -60.46676 | 155 |
| SM XI | 78 | PS | -49.48343 | -64.48343 | 120 |
| SM V | 79 | PS | -49.4505901 | -62.4675935 | 152 |
| SM V | 81 | PS | -49.5001 | -60.5337734 | 178 |
| SM XI | 82 | PS | -49.46676 | -60.46676 | 188 |
| SM IV | 89 | PS | -50.483343 | -65.50001 | 117 |
| SM XI | 92 | PS | -50.450009 | -63.583345 | 154 |
| SM IV | 93 | PS | -50.516677 | -60.483343 | 154 |
| SM X | 95 | PS | -50.483343 | -59.483343 | 152 |
| SM IV | 96 | PS | -50.533344 | -57.933352 | 143 |
| SM V | 99 | PS | -51.5339401 | -67.5842835 | 100 |
| SM V | 101 | PS | -51.4847636 | -65.5337734 | 134 |
| SM V | 102 | PS | -51.5014336 | -63.3175635 | 180 |
| SM XI | 101 | PS | -51.40008 | -65.48343 | 135 |
| SM XI | 105 | PS | -51.48343 | -61.8335 | 192 |
| SM X | 106 | PS | -51.63346 | -57.30006 | 189 |
| SM IV | 108 | PS | -52.516677 | -67.300006 | 92 |
| SM IV | 111 | PS | -52.483343 | -64.583345 | 183 |
| SM XI | 111 | PS | -52.616679 | -65.516677 | 125 |
| SM IV | 119 | PS | -53.516677 | -66.450009 | 95 |
| SM XI | 120 | PS | -53.416675 | -66.550011 | 92 |
| SM X | 122 | PS | -53.300006 | -64.416675 | 169 |
| SM IV | 128 | TDF | -54.5001 | -64.41675 | 111 |
| SM X | 128 | PS | -54.33334 | -65.466676 | 93 |
| SM X | 133 | MPA | -54.26672 | -60.05001 | 100 |
| SM X | 135 | MPA | -54.5001 | -58.5001 | 133 |
| SM X | 138 | MPA | -54.5001 | -56.58345 | 135 |
| SM V | Ad3 | PS | -49.4177502 | -63.4344202 | 145 |
| SM XI | Ad3 | PS | -44.56678 | -65.01667 | 82 |
| SM XI | Ad5 | PS | -47.06668 | -65.45009 | 70 |
| SM XI | Ad11 | TDF | -54.21671 | -66.55011 | 55 |
| SM XI | Ad14 | PS | -43.55011 | -59.8335 | 116 |
Multidimensional Scaling (MDS) and Cluster Analysis (CA) were applied to a Bray-Curtis similarity index to assess polychaete assemblages in the NMPA - BB slope and Magellan region respectively. Similarity Percentage Analyses (SIMPER) was applied to describe the contribution of species to the dissimilarity between groups of stations. An Analysis of Similarities (ANOSIM) was carried out between samples located in the NMPA and BB slope to assess polychaete assemblages inside and outside the NMPA, and between samples from NMPA, S, N, TDF and PS to analyze polychaete distribution patterns in the Magellan region, considering the null hypothesis of no differences between areas. We used PRIMER version 6.1 (Clarke & Gorley, 2006) with presence-absence data, excluding unique findings.
Voucher specimens of the polychaetes collected during recent cruises were deposited at the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina.
Results
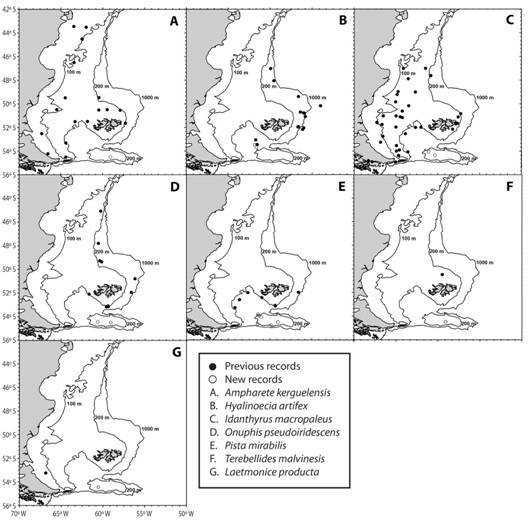
A total of 918 individuals, which correspond to 39 taxa distributed in 22 families, were recorded in samples from NMPA and BB slope (Table 3). Terebellidae and Polynoidae were the most diverse families, represented by five taxa each. Polyeunoa laevis McIntosh, 1885 and Serpula narconensis Baird, 1865 were most frequently collected in the different zones of NMPA and BB slope. Nicolea chilensis (Schmarda, 1861), Trypanosyllis gigantea McIntosh, 1885, Eunereis patagonica (McIntosh, 1885) and Eucranta sp. were commonly registered within the MPA, although present once at the slope of the bank. Chaetopterus antarcticus Kinberg, 1866 (see Moore, Nishi, & Rouse, 2017) was frequent in samples taken at the core zone of NMPA, where the total number of taxa was 22. The number of taxa was 13 and 32 at the buffer and transition zones of NMPA respectively. Samples from the slope included 14 polychaete taxa, with H. artifex and Nicon maculata Kinberg, 1866 as exclusive species. According to the current literature (Bremec & Giberto, 2008; Orensanz et al., 2012; Horton et al., 2018; OBIS, 2018), A. kerguelensis (ampharetid), H. artifex (onuphid), I. macropaleus (sabellariid), L. producta (aphroditid), O. pseudoiridescens (onuphid), P. mirabilis (terebellid) and T. malvinensis (trichobranchid) constitute new records for BB (Fig. 2).
TABLE 3 Polychaete taxa recorded at the sites (see Table 1) sampled at Burdwood Bank Slope (BB-Slope), Transition (MPA-Transition), Buffer (MPA-Buffer) and Core (MPA-Core) areas during the cruises and sites referred in Table 1
| Taxa recorded | MPA-Core | MPA-Buffer | MPA-Transition | BB-Slope | N° Sites |
| Aglaophamus virginis (Kinberg, 1866) | A´ | E,B´ | 3 | ||
| Ampharete kerguelensis Mclntosh, 1885 | B´ | 1 | |||
| Anobothrus sp. | C | 1 | |||
| Boccardia sp. | S | D´,V | 3 | ||
| Chaetopterus antartcticus Kinberg, 1866 | H, I, Q, R | F | 5 | ||
| Eteone aurantiaca Schmarda, 1861 | H, Q, R, S | U | D´, T | 7 | |
| Eucranta sp. | Q, R, S | J, K, T, U | C, D, E, M, D´ | 12 | |
| Eunereis patagonica (Mclntosh, 1885) | Q, R, A´ | J, K, T, U | E, M, N, D´, Z | P | 13 |
| Eunice pennata (Muller, 1776) | C, E | 2 | |||
| Glycera capitata Orsted, 1843 | C, E, B´ | 3 | |||
| Harmothoe sp. | V | X | 2 | ||
| Hermadion magalhaensi Kinberg, 1855 | I | F | L | O | 4 |
| Hyalinoecia artifex Verrill, 1880 | X,Y | 2 | |||
| Idanthyrsus macropaleus (Schmarda, 1861) | S | D´ | 2 | ||
| Laetmonice producta Grube, 1877 | A´ | 1 | |||
| Lanice sp. | E | 1 | |||
| Lumbrineridae unid. | D, N, B´ | 3 | |||
| Maldanidae unid. | B´ | 1 | |||
| Nephtys sp. | F | 1 | |||
| Nicolea chilensis (Schmarda, 1861) | I, R, S | J, K, T, U | E, M, N, D´, Z | W | 13 |
| Nicon maculata Kinberg, 1866 | B, O, A, W | 4 | |||
| Oenonidae unid. | D, N | 2 | |||
| Onuphis pseudoiridescens Averincev, 1972 | A´ | B´ | 2 | ||
| Paraonidae unid. | B´ | 1 | |||
| Phyllodocidae unid. | A´ | B´ | 2 | ||
| Pista mirabilis Mdntosh, 1855 | C, D´, V, B´ | W, P | 6 | ||
| Polyeunoa laevis Mclntosh, 1855 | G, H, I, Q, S, A´ | J, T, U | C, D, M, N, L, D´, V, Z, B´, C´ | B, A, O, W, P | 24 |
| Polynoidae unid. | A´ | B´ | Y | 3 | |
| Sabellidae 1 | I, A´ | B´ | 3 | ||
| Sabellidae 2 | I | 1 | |||
| Sabellidae Fabricinae | Q, R, S | K, T, U | E, D | P | 9 |
| Serpula narconensis Baird, 1865 | H, I, Q, S | J, F, T, U | C, E, M, L, N, Z, B´, C´ | O, W, P | 19 |
| Spionidae unid. | H, R | E | 3 | ||
| Syllidae unid. | I, A´ | J | B´ | O | 5 |
| Terebellidae unid. | Q | 1 | |||
| Terebellides malvinesisBremec & Elias, 1999 | B´ | 1 | |||
| Thelepus sp. | J, F, T, U | N, V | O, W | 8 | |
| Travisia kerguelensis Mclntosh, 1855 | B´ | 1 | |||
| Trypanosyllis gigantea Mdntosh, 1855 | I | J, U | C, E, N, D´, V, Z | P | 10 |
MDS analysis among sites shows one main group of sampling stations from BB slope and the core, buffer and transition areas of NMPA (group 1), from which only two samples from the slope of the bank are excluded (31 and 33, group 2) (Table 1, Fig. 3A). The SIMPER test (presence-absence data) resulted in an average dissimilarity of 99.04 % between both groups. P. laevis, S. narconensis, E. patagonica, N. chilensis, T. gigantea and Eucranta sp. contributed 82 % to the average similarity (0.32) of group 1. H. artifex contributed 100 % to the average similarity (0.50) of group 2. The results of the ANOSIM between stations from NMPA and BB Slope (Global R = 0.398; p = 0.3 %) show no differences in the polychaetes species composition.

Fig. 3 A. Multidimensional Scaling among samples collected in the different zones of Namuncurá Marine Protected Area and Burdwood Bank slope during 2015, 2016 and 2017. B. Cluster analysis among polychaete data (presence-absence) from different Magellan areas: Patagonian Shelf (PS), Tierra del Fuego (TDF), Marine Protected Area (MPA), Burdwood Bank Neighboring Area (N) and Burdwood Bank Slope (S).
The regional comparison was performed with a data matrix of 82 samples from different Magellan areas (Tables 1, 2) and 73 polychaete taxa (Table 4). Cluster analysis (Fig. 3B) grouped NMPA-TDF (similarity > 60 %) and PS in group 1 (~0.50 similarity). Kinbergonuphis dorsalis (Ehlers, 1897), Aglaophamus virginis (Kinberg, 1866), A. kerguelensis, E. patagonica, S. narconensis, Travisia kerguelensis McIntosh, 1885 and P. laevis contributed 65 % to the average similarity (0.15). Deep areas S and N clustered at lower similarity in group 2 (Fig. 3). P. laevis, S. narconensis, E. patagonica, Thelepus sp., H. artifex and N. maculata contributed 87% to the average similarity (0.21). The results of the ANOSIM developed to compare different Magellan areas does not show statistical differences among stations from NMPA, PS, TDF, S and N (Global R = 0.42; p = 0.1 %).
TABLE 4 Polychaete taxa recorded at Magellan areas: Patagonian Shelf (PS), Tierra del Fuego (TDF), NMPA, BB Slope (BBS) and neighboring locations (N) during the cruises referred in Tables 1 and 2
| PS | TDF | AMP | BBS | N | |
| Aglaophamus virginis (Kinberg, 1866) | X | X | X | ||
| Ampharete kerguelensis McIntosh, 1885 | X | X | X | ||
| Amphicteis gunneri antarctica Hessle, 1917 | X | ||||
| Anobothrus sp. | X | ||||
| Armandia sp. | X | ||||
| Boccardia sp. | X | ||||
| Chaetopterus antarcticus Kinberg, 1866 | X | X | X | ||
| Cistenides ehlersi (Hessle, 1917) | X | X | |||
| Drilonereis tenuis (Ehlers, 1900) | X | ||||
| Eteone aurantiaca Schmarda, 1861 | X | X | |||
| Eucranta sp. | X | ||||
| Eunereis patagonica (McIntosh, 1885) | X | X | X | X | |
| Eunice frauenfeldi Grube, 1866 | X | ||||
| Eunice pennata (Muller, 1776) | X | ||||
| Euphrosine armadilloides Ehlers, 1900 | X | ||||
| Euzonus sp. | X | ||||
| Glycera americana Leidy, 1855 | X | ||||
| Glycera capitata Orsted, 1843 | X | X | |||
| Glycera papillosa Grube, 1857 | X | X | X | ||
| Glycinde sp. | X | ||||
| Harmothoe sp. | X | X | X | ||
| Hemipodus sp. | X | ||||
| Hermadion magalhaensi Kinberg, 1855 | X | X | |||
| Hyalinoecia artifex Verril, 1880 | X | ||||
| Idanthyrsus macropaleus (Schmarda, 1861) | X | X | X | X | |
| Kinbergonuphis dorsalis (Ehlers, 1897) | X | X | X | ||
| Laetmonice producta Grube, 1877 | X | ||||
| Lanice sp. | X | ||||
| Lumbriclymenella robusta Arwidsson, 1911 | X | ||||
| Lumbriclymeninae | X | ||||
| Lumbrineriidae sp. 1 | X | X | |||
| Lumbrineriidae sp. 2 | X | ||||
| Lumbrineris cingulata Ehlers, 1897 | X | ||||
| Magelonidae unid. | X | ||||
| Maldaniidae unid. | X | X | X | ||
| Melinna cristata (M. Sars, 1851) | X | ||||
| Neanthes kerguelensis (McIntosh, 1885) | X | ||||
| Nephtys magellanica Augener, 1912 | X | ||||
| Nephtys sp. | X | X | |||
| Nereididae unid. | X | X | X | ||
| Nicolea chilensis (Schmarda, 1861) | X | ||||
| Nicomache sp. | X | ||||
| Nicon maculata Kinberg, 1866 | X | ||||
| Nothria anoculataOrensanz, 1974 | X | X | |||
| Notocirrus lorum Ehlers, 1897 | X | X | |||
| Notocirrus virginis (Kinberg, 1865) | X | ||||
| Oenonidae unid. | X | ||||
| Onuphis pseudoiridescens Averincev, 1972 | X | X | |||
| Ophelina syringopyge (Ehlers, 1901) | X | ||||
| Orbiniidae unid. | X | X | |||
| Paraonidae unid. | X | X | |||
| Perkinsiana antarctica (Kinberg, 1867) | X | X | |||
| Phyllocomus crocea Grube, 1877 | X | ||||
| Phyllodoce patagonica (Kinberg, 1866) | X | ||||
| Phyllodocidae unid. | X | X | |||
| Phylo felix Kinberg, 1866 | X | X | |||
| Pista corrientis McIntosh, 1885 | X | X | |||
| Pista mirabilis McIntosh, 1885 | X | X | X | ||
| Polyeunoa laevis McIntosh, 1885 | X | X | X | X | |
| Polynoidae unid. | X | X | X | X | |
| Potamilla antarctica (Kinberg, 1866) | X | ||||
| Sabellidae sp. 1 | X | X | X | ||
| Sabellidae sp. 2 | X | ||||
| Sabellidae Fabricinae | X | X | |||
| Serpula narconensis Baird, 1865 | X | X | X | X | X |
| Sphaerodoridae | X | ||||
| Spionidae unid. | X | ||||
| Syllidae unid. | X | X | X | X | |
| Terebellidae unid. | X | X | |||
| Terebellides malvinensisBremec & Elias, 1999 | X | X | |||
| Thelepus sp. | X | X | X | X | X |
| Travisia kerguelensis McIntosh, 1885 | X | X | X | ||
| Trypanosyllis gigantea McIntosh, 1885 | X | X | X |
Discussion
This research gives faunistic information on polychaetes from BB, including NMPA -a wide area delineated by the 200m isobath- and the slope of the bank. Data from recent monitoring cruises, particularly developed to conduct benthic sampling in the study area, have led to baseline knowledge about the invertebrate assemblages inhabiting a variety of substrates. A preliminary inventory of the most conspicuous benthic species collected during 2013 in NMPA and BB slope included nearly 250 taxa (Schejter et al., 2016), of which 19 were polychaetes mostly identified to family level. In the present study, a total of 39 polychaete taxa were identified, 20 of them to species level and previously recorded from other Subantarctic or Antarctic locations (Clarke & Johnston, 2003; Orensanz et al., 2012; Horton et al., 2018; OBIS, 2018). However, some of them, collected in different zones of NMPA and/or BB slope (A. kerguelensis, H. artifex, I. macropaleus, L. producta, O. pseudoiridescens, P. mirabilis and T. malvinensis) constitute new records for BB. Recent investigations showed that BB is home to more than 90 species of small organisms belonging to Peracarida (Doti et al., 2014; Chiesa et al., 2015), more than 280 species of small mollusks (Zelaya & Guller, 2018) -most of them new records for the area or new species to science- and two new coral (Cairns, 2012) and sponge (Schejter, Bertolino et al., 2017) species. The bathymetric ranges of Asteroidea were extended for two species while the geographic distribution was updated for seven of them, being their first record in the NMPA (Fraysse et al., 2018). A new genus and species of cheilostome bryozoan showing an obligate association with a hermit crab was also described for the area (Lopez‑Gappa, Liuzzi, & Zelaya, 2017). It becomes clear that studies on polychaetes, and invertebrates in general, should continue in order to properly assess species richness in the MPA.
Some of the polychaete species were frequently collected and were distributed throughout the depth range (91-785 m) considered in this sampling (P. laevis, S. narconensis, E. patagonica, Thelepus sp.), while others were only collected within NMPA: C. antarcticus, N. chilensis, T. gigantea, Eucranta sp., Boccardia sp., Hermadion sp., Nephtys sp. and Sabellidae (two species). In fact, MDS shows a similar polychaete assemblage characterizing the study area (three zones of NMPA and slope locations), and H. artifex characterizing two locations at the NW deepest slope of the bank. Due to the sampling procedure, the taxa registered are mainly epibenthic or associated with other large colonial organisms collected in the area. In general, sponges were conspicuous components (40 % to 88 % of the total catch) in locations of the MPA, and cnidarians (mainly corals) were a dominant group in the catches at stations located at > 300 m depth (Schejter, Genzano, Gaitán, Perez, & Bremec, 2018a). The high diversity both of sponges (López Gappa & Landoni, 2005; Schejter, Bertolino, Calcinai, Cerrano, & Pansini, 2012) and cnidarians (López-González, Rodriguez, & Vert, 2003; Margolin et al., 2014) in BB was previously reported. These sessile and three dimensional branched organisms are habitat-forming, as they provide substrate and refuge to a variety of associated species (see Buhl-Mortensen et al., 2010). In this sampling, the most frequent free living polynoid P. laevis, nereidid E.patagonica and syllid T. gigantea, as well as the epibiotic serpulid S. narconensis, were mostly associated with octocorals or sponges. Ongoing studies registered 109 taxa, of which 19 were polychaete species, associated with octocorals (Thouarella sp., Dasystenella sp., Bayergorgia sp.) and hydroids (Amphisbetia sp., Sertularella sp., Symplectoscyphus sp., Grammaria sp.,Abietinella sp., Halecium sp., Acryptolaria sp., Plumularia sp.) between 90-650 m depth in BB (Martin Sirito, 2019). P. laevis, widely distributed in the SW Atlantic (northwards up to Buenos Aires) and the Magellan, Sub-Antarctic and Antarctic regions, is often associated with corals (Barnich, Gambi, & Fiege, 2012), which are also present and frequent beyond the shelf break of Argentina (Portela et al., 2012; Portela et al., 2015). Interspecific relationships between polychaetes and colonial hosts, like Cnidaria and Porifera, are numerous and include commensalism, endobiosis or epibiosis that facilitate, for instance, feeding strategies (Martin & Britayev, 1998). Polychaetes can have a large variety of feeding modes (Fauchald & Jumars, 1979); they were assumed to consume all suspended sources and the biofilm in a quantitative food-web analysis of a cold-water coral community in NE Atlantic, where biodiversity appears to be higher than in surrounding soft sediments (Oevelen et al., 2009). The nature and functionality of the associations between polychaetes and hosts are fields open to further research in Argentinean waters.
Other polychaete species were exclusively collected in particular locations of the study area. The parchment worm Chaetopterus antarcticus inhabited bottoms at nearly 100 m depth of NMPA, where Porifera and Bryozoa were conspicuous, together with other invertebrates (ophiuroids, serpulids, brachiopods, hydroids, peracarids, etc.) (Schejter, Genzano et al., 2018). These worms are active, mucus-net suspension feeders (Jumars, Dorgan, & Lindsay, 2014), usually collected within their U-shaped tubes, typical of an infaunal habit (Fauchald & Jumars, 1979; Rouse, 2001). This species is distributed throughout the shelf break frontal system along the 100m isobath and up to 37 °S (Bremec & Lasta, 2002) in habitats characterized by sandy soft-bottoms, like more than 90 % of the Argentinean continental shelf (Parker, Paterlini, & Violante, 1997), as well as in a submarine canyon located at 43º35’ S - 59º33’ W, 325 m depth (Bremec & Schejter, 2010). On the other hand, deep locations at NW BB slope, characterized by muddy sediments and the presence of pennatulaceans and scleractinid corals (Schejter, Acuña, Garese, Cordeiro, & Pérez, 2018), were inhabited by the onuphid species H. artifex. These tubiculous worms are carnivorous or carrion feeders and carry the tubes with them in a clearly discretely motile fashion, often in soft sediments (Jumars et al., 2014). Although information on the types of bottom at BB is still lacking (Falabella, 2018), the heterogeneity of the substrate and the patchiness of the benthic life in the study area appeared evident by means of underwater photographs acquired during a cruise in 2015 (Schejter, Martín, & Lovrich, 2017). Ongoing research into the unknown bottom habitats will permit the appreciation of their true need for conservation.
The polychaete assemblages of BB (NMPA and slope) were compared with those of other Magellanic areas: Beagle Channel, Patagonian Shelf and three neighboring locations. The present analysis does not indicate statistical differences in the polychaete assemblage among locations, and species already known from the Magellanic Biogeographical Province (Orensanz et al., 2012; Bremec et al., 2000; Bremec et al., 2010), were registered at BB for the first time. In an extensive area of the Argentinean shelf between 39º S and 55º S, in bottoms deeper than 100 m, the most frequent species were K. dorsalis, I. macropaleus, S. narconensis, N. maculata, T. gigantean, E. patagonica and T. kerguelensis (Bremec et al., 2010). Sediment composition on the continental shelf is dominated by sands and silts of < 2 mm grain size, at depths between 50 and 200 m (Bastida, Urien, Lichtschein, Roux, & Arias, 1981; Bastida, Roux, & Martinez, 1992). Other Magellanic habitats, like channels and fjords in the Pacific (42° S-55° S) and the Straits of Magellan (52° S -70° W), are heterogeneous and patchy with different types of sediments and consequently dominance and diversity of austral species is higher when compared with the homogeneous shelf habitats (Mariani et al., 1996; Gambi & Mariani, 1999; Bremec et al., 2000; Montiel, Gerdes, Hilbig, et al., 2005). The polychaete assemblage registered at BB shows affinities with that distributed on the Argentinean continental shelf, on soft homogeneous bottoms. Although it is interesting to point out that most species are distributed in Antarctic waters (Clarke & Johnston, 2003), much taxonomic work is needed to properly establish biogeographical connections. Souto (2014), after an exhaustive historical compilation and analysis of spatial distribution of polychaetes in Argentinean waters, found that Acrocirridae, Alciopidae, Ampharetidae, Arenicolidae, Capitellidae, Chrysopetalidae, Cossuridae, Euphrosinidae, Flabelligeridae, Polynoidae, Maldanidae and Phyllodocidae, among others, have been scarcely registered due to poor sampling coverage. It must be pointed out that high endemism in the distribution of species was showed in a recent revision on austral orbiniids (Blake, 2017).
The present results indicate strong connections between the fauna collected at NMPA and the polychaete assemblages of other Magellan areas dominated by soft bottoms. However, deeper slope areas of BB are inhabited by large species (i.e. H. artifex) not registered in the NMPA. Moreover, the slope area of BB was recently highlighted because cold-water coral ecosystems were recorded in several sites, meeting the characteristics of Vulnerable Marine Ecosystems (VME) (Schejter, Genzano, et al., 2018), which must be protected from fishing gears that destroy seafloor habitat (see Hall, 1999). The biogeographic importance of the BB as connection for benthic polychaete species between South America and the Antarctic Peninsula merits future investigation. Trophic relationships and the role of epibiotic relationships in enhancing biodiversity in BB seabeds should also be explored.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio