Introduction
Brush-footed butterflies (Nymphalidae) represent the second most diverse family of Papilionoidea worldwide, behind the skippers (Hesperiidae). Because of the remarkable diversity and local endemism featured within Nymphalidae, the description and analysis of spatio-temporal patterns of this group are a priority for advancing conservation and systematics. This is particularly true for Mexico, which is a megadiverse country and biodiversity hotspot where Nymphalidae constitute 25 % (413 species) of the country's Papilionoidea fauna sensu lato (Llorente-Bousquets et al., 2014).
Oaxaca is one of the most biodiverse states in Mexico (Flores-Villela & García-Vázquez, 2014; García-Mendoza & Meave del Castillo, 2011; Navarro-Sigüenza et al., 2014), and this pattern is reflected in Nymphalidae. Some 339 species or 82 % of the country's nymphalids are documented from Oaxaca, ranking it second only to the state of Chiapas in nymphalid species richness (Luis-Martínez et al., 2016). This outstanding biodiversity in Oaxaca is attributable to the convergence of five different biogeographic provinces in the state, with the most well-represented of these being the Sierra Madre del Sur, Costa del Pacífico, and Golfo de México (Morrone, 2005; Morrone et al., 2002). The Loxicha Region of Oaxaca lies within the Sierra Madre del Sur, and it is of special faunal interest. Loxicha experienced two main collecting periods in the 20th century, during which numerous species of Nymphalidae were described and named (Luis-Martínez et al., 2020). The MARIPOSA database (Luis-Martínez et al., 2005), contains 1 248 records from the Loxicha Region during that century, with 139 species of Nymphalidae being documented by more than 30 collectors. Particularly notable among those collectors were Eduardo Cecilio Welling with 66 species (265 records), and John Kemner with 78 species (402 records).
Modern description and analysis of the Papilionoidea from the Pacific slope of the Loxicha Region has been the core focus of an ongoing project, begun in 2005, to analyze the butterfly fauna of Oaxaca (Luis-Martínez et al., 2016). To date, the families Papilionidae, Pieridae, and Riodinidae of the Loxicha Region have been analyzed (Arellano-Covarrubias et al., 2018; Luis-Martínez et al., 2020). The present study aims to describe the temporal and spatial patterns of Nymphalidae diversity in the Loxicha Region. Based on a review of the literature and historical records, coupled with sampling from 2005-2014 along an elevational gradient from 80-2 600 m, we document this diversity and make a preliminary comparison of nymphalids in Loxicha to other comparatively well-sampled regions to describe broad-scale patterns.
Historical outline of the collection of Papilionoidea from the Pacific slope and the Loxicha Region: The historical collection and classification of Mexican species of Papilionoidea began at the end of the 19th century and continued through the early 1980's. Collectors generally focused on the Southeastern states, the Atlantic slope, and the states of Guerrero and Morelos due to the high diversity of these areas and to the presence of associated highways (v. gr.Llorente-Bousquets et al., 1986; Llorente-Bousquets & Luis-Martínez, 1993; Llorente-Bousquets et al.,1998; Luis-Martínez et al., 2000; Luis-Martínez et al., 2003; Michán et al., 2004). During this period, sporadic collections were also made in Northern Mexico, mostly carried out by academic institutions of the USA (v. gr.Comstock, 1953; Holland, 1995; Spade et al., 1988; Stanford, 1998). The Pacific slope of Mexico was often ignored by collectors, resulting in relatively few publications except for a butterfly checklist for the Chamela Biological Research Station in the state of Jalisco (Beutelspacher, 1982), notes on the butterflies of some Pacific islands (Vázquez, 1958; Vázquez, 1959; Vázquez, 1960), and a contribution on the fauna of the Pacific slope of Jalisco (v. gr.Comstock & Vázquez, 1961). Additionally, Members of the Mexican Society of Lepidopterology published the results of periodic collections along this slope (v. gr.Maza & Maza, 1981; Maza & Maza, 1983; Maza et al., 1982; Velázquez, 1976; Velázquez & Velázquez, 1975), with the main goal of describing new taxa from unexplored areas.
The first systematic sampling of the Pacific slope extended from the coast (Puerto San Blas) to the mountains (Sierra San Juan) in the state of Nayarit during 1978-1980, spanning an elevational gradient from sea level to 1 300 m. This effort documented 135 species of Nymphalidae, marking the beginning of dedicated research on the distribution of Papilionoidea from the Pacific slope. A decade later, faunistic studies were carried out in the Sierra de Atoyac (Vargas-Fernández et al., 1994) and the Omiltemi region (Luis-Martínez & Llorente-Bousquets, 1993) of the Sierra Madre del Sur (SMS) in the state of Guerrero. The former spanned an elevational range of 300-3 100 m, and the latter a range of 2 300-3 000 m (v. gr.Llorente-Bousquets, 1984; Llorente-Bousquets et al., 2004; Warren & Llorente-Bousquets, 1999).
Further studies on the faunistic composition of Papilionoidea from the Pacific slope were completed during the last three decades in Pedernales, state of Michoacán (Balcázar, 1993); Mismaloya, Jalisco and Bahía de Banderas, Nayarit (Warren & Llorente-Bousquets, 1999); the Sierra de Manantlán, Jalisco-Colima (Vargas-Fernández et al., 1999); and Western Michoacán (Luis-Martínez, 1997; Luis-Martínez, 1999). Additional analyses of the alpha and beta diversity of the Nymphalidae (Tapia-Sedeño, 2013) and the lepidopterofauna of the Loxicha Region, Oaxaca (Arellano-Covarrubias et al., 2018; Luis-Martínez et al., 2020) were also released. Most of these studies focused on the local, regional, or elevational distribution and the phenology of Papilionoidea species, under distinct ecological conditions and across a wide environmental gradient. Some faunal comparisons between different areas have also been made (v. gr.Monteagudo-Sabaté & Luis-Martínez, 2013; Monteagudo-Sabaté et al., 2001; Monteagudo-Sabaté et al., 2014).
When analyzing the fauna of the Pacific region and the Sierra Madre del Sur, the diversity of the state of Morelos must be considered. This state is located mainly in the Eje Neovolcánico and Cuenca del Balsas biogeographic provinces (Morrone et al., 2002). Michoacán and Guerrero also include portions of these two provinces. More than half of Guerrero lies in the Cuenca del Balsas, and the state also encompasses a large portion of the Sierra Madre del Sur, particularly its Pacific slopes (Luna-Reyes et al., 2012). There are 450 species or subspecies of Papilionoidea sensu lato recorded from Morelos, classified in 214 genera. The Nymphalidae is represented in Morelos by 147 species or subspecies, of which 42 are endemic to Mexico (Luna-Reyes, 2020).
Besides these faunistic studies over the past three decades, species lists have been published for 10 of the 11 states in the Pacific slope. Table 1 presents current data for the number of species and endemics in these Mexican states according to Llorente-Bousquets et al. (2014) and Luis-Martínez et al. (2016).
Table 1 Species richness and endemism of Nymphalidae in Mexico and its Pacific-slope states
| State | spp. | E | % | Reference |
| Baja California | 41 | 0 | 0 | Brown et al., 1992 |
| Baja California Sur | 43 | 2 | 5 | Brown et al., 1992 |
| Sonora | 101 | 6 | 6 | Bailowitz et al., 2017 |
| Sinaloa | 106 | 8 | 8 | |
| Nayarit | 144 | 12 | 8 | Llorente-Bousquets et al., 2004 |
| Jalisco | 176 | 18 | 10 | Llorente-Bousquets, Luis-Martínez et al., 1996; Vargas-Fernández et al., 1996; Vargas-Fernández et al., 1999; Warren et al., 1996 |
| Colima | 139 | 15 | 11 | Llorente-Bousquets, Warren et al., 1996 |
| Michoacán | 168 | 15 | 9 | |
| Guerrero | 216 | 22 | 10 | Vargas-Fernández et al., 1994 |
| Oaxaca | 339 | 31 | 9 | Luis-Martínez et al., 2016 |
| Chiapas | 351 | 26 | 7 | Maza & Maza, 1993; Luis-Martínez et al., 2011 |
| Mexico (country) | 413 | 123 | 12 |
spp.: species, E: taxa endemic to the state/country, %: percentage of endemics relative to the total species richness of the state/country. / Note. Chiapas endemism is reduced because species' ranges extend towards Central America as a biogeographical and ecological unit; the same occurs in Baja California.
Materials and methods
Faunistic inventory and sites: To update the Papilionoidea species list for Oaxaca published by Luis-Martínez et al. (2004), systematic sampling was begun in 2005 (Luis-Martínez et al., 2016) which increased scientific knowledge of the geographical and vegetational distribution of the group. This sampling effort focused on areas with the greatest diversity and endemism. The Loxicha Region is one such area, and thus was subject to 267 sampling days over seven years (2005, 2007, 2008, 2011-2014), including 21 sampling sites that span eight municipalities within the Pacific slopes of the Sierra Madre del Sur (Table 2). In 12 of these sites, sampling was systematic. A total of 16 collectors participated in the sampling effort, with 13 to 65 sampling days per site. Sites were grouped by elevational levels and vegetation type for comparison and analysis purposes. Vegetation classification followed Rzedowski (1978) and Llorente-Bousquets (1984), with four types recognized in the Loxicha Region: tropical deciduous forest (TDF), tropical sub-deciduous forest (TSDF), cloud forest (low- and mid-elevation) (CF), and oak-pine forest (OPF). Besides these, a fifth vegetation type was recognized in some sites at 2 000-2 400 m elevation, denominated as oak-pine and high-elevation cloud forest (OPCF). OPCF is similar to OPF but has intermixed elements of CF. Because the OPCF can be easily distinguished from both the OPF and the CF, and is located at different elevations in the region, this vegetation type was considered a distinct category (Arellano-Covarrubias et al., 2018; Luis-Martínez et al., 2020).
Table 2 Sampling sites in the Loxicha Region, Oaxaca, Mexico
| Site | Elevation (m) | Geographic location | Vegetation type | SE/ R /T | |
| Lat North | Long West | ||||
| a. Parque Nacional Huatulco, Río Cacaluta | 80-100 | 15°47'07'' | 96°10'34'' | TDF | 16/ 1 627 /781 |
| b. Parque Nacional Huatulco | 100 | 15°45'20'' | 96°09'19'' | TDF | 24/ 1 869 /977 |
| c. Azulillo | 380-500 | 15°53'25'' | 96°29'27'' | TSDF | 65/ 4 929 /1 755 |
| d. Rancho Hagia Sofía | 410 | 15°52'01'' | 96°21'55'' | TSDF | 49/ 6 672 /3 430 |
| e. Río Molinos* | 530-700 | 15°56'10.8'' | 96°30'41.8'' | CF | 4/ 71 /30 |
| f. Copalita, Río Copalita* | 600-1 200 | 15°56'48'' | 96°20'23.43'' | CF | 21/ 1 200 /450 |
| g. Magdalena, El Lirio | 750-900 | 15°55'11'' | 96°23'34'' | CF | 13/ 1 934 /789 |
| h. Copalita, Los Plátanos* | 900 | CF | 1/ 31 /6 | ||
| i. Copalita, Siete Veneros* | 940 | CF | 24/ 1 066 /457 | ||
| j. San Mateo Piñas* | 1 000 | 15°59'57.50'' | 96°20'4.47'' | CF | 27/ 426 /132 |
| k. Pluma Hidalgo, 4 km NW “La Curva'' | 1 100-1 200 | 15°56'23'' | 96°25'59'' | CF | 41/ 2 243 /528 |
| l. Finca Aurora-Finca San Isidro | 1 100-1 250 | 15°56'30'' | 96°24'13'' | CF | 25/ 1 013 /390 |
| m. Copalita, Llano de Ocote* | 1 200 | CF | 17/ 434 /229 | ||
| n. Portillo del Rayo-Finca El Encanto | 1 200-1 530 | 15°58'38'' | 96°31'11'' | CF | 24/ 1 218 /622 |
| o. La Soledad-Buenavista | 1 470-1 550 | 15°58'18'' | 96°31'54'' | CF | 31/ 1 690 /574 |
| p. La Pasionaria | 1 500-1 650 | 15°66'09'' | 96°25'08'' | CF | 15/ 1 153 /420 |
| q. Puente Arroyo “El Guajolote'' | 2 020-2 150 | 16°03'28'' | 96°30'18'' | OPF-OPCF | 17/ 274 /53 |
| r. San José del Pacífico, 1 km S | 2 280-2 400 | 16°09'28'' | 96°29'21'' | OPF-OPCF | 33/ 771 /126 |
| s. Manzanal-Doncella* | 2 700-2 800 | 16°07'47.9'' | 96°30'12'' | OPF | 1/ 1 /- |
| t. Camino a San Agustín Loxicha* | 2 700-2 800 | 16°07'41.57'' | 96°29'54.2'' | OPF | 2/ 5 /- |
| u. Nevería-La Ciénega* | 2 820-2 850 | 16°12'01'' | 96°20'56'' | OPF | 2/ 19 /- |
* Sites without systematic sampling; SE: sampling effort (days); R: records (specimens); T: specimens collected with Van Someren-Rydon traps; Vegetation types: tropical deciduous forest (TDF), tropical sub-deciduous forest (TSDF), cloud forest (low- and mid-elevation) (CF), oak-pine forest (OPF), and oak-pine and high-elevation cloud forest (OPCF). (Arellano-Covarrubias et al., 2018; Llorente-Bousquets, 1984, Luis-Martínez et al., 2020; Rzedowski, 1978). Note Van Someren-Rydon traps were not used at sites s, t, and u.
Taxonomic determination: Taxa were identified by comparison with specimens in the Lepidoptera Collection of the Zoology Museum, Facultad de Ciencias, Universidad Nacional Autónoma de México (MZFC), together with the taxonomic expertise of the authors and reference to specialized literature. The list of species follows the taxonomic order proposed by Vargas-Fernández et al. (2016), and Llorente-Bousquets, Luis-Martínez et al. (2006), for the Satyrinae subfamily. All specimens were deposited in the Lepidoptera Collection of the MZFC, which is registered at the Secretaría de Medio Ambiente, Recursos Naturales y Pesca (SEMARNAP) (DFE.IN.071.0798). Specimens were collected under scientific collecting permit FAUT-0148 issued by the Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). Collecting data were entered into the MARIPOSA database (Luis-Martínez et al., 2005).
Nets and Van Someren-Rydon traps: Sampling techniques included 4-6 aerial nets set for eight hours and 10-20 Van Someren-Rydon traps (Rydon, 1964) placed daily 1-2.5 m high along transects, with 50 m between each trap. Traps used a mixture of water, brown sugar, pineapple, and banana as bait. Transects covered distinct microhabitats (open and closed vegetation) to obtain a more representative sample. The number of specimens captured with traps in each locality is presented in Table 2.
Species richness estimation and diversity analysis by vegetation type and elevational level: A species accumulation curve was estimated using data from all specimens collected. Data were randomized (500 runs) with EstimateS 9.1 (Colwell, 2013) and fitted to the Clench model (Soberón & Llorente-Bousquets, 1993) to estimate species richness. Species richness was also estimated for each vegetation type (Table 2) and three elevational levels: 0-750 m, 750-1 800 m, and 1 800-2 850 m (Luis-Martínez et al., 2020). For the elevational analysis, the non-parametric estimation method Chao1 (Colwell & Coddington, 1994) was implemented in SPADE (Chao & Shen, 2010). For each estimation, the 95 % confidence interval (CI) was estimated using the bootstrap technique. Significant differences between the species richness of distinct vegetation types or elevational levels were evaluated by comparison and overlap of the confidence intervals.
Additionally, the real diversity defined as the exponential of the Shannon-Wiener index (H') was estimated for each elevational level along with the 95 % CI, using the method proposed by Chao and Shen (2003) implemented in SPADE (Chao & Shen, 2010). The scale of the real diversity (effective number of species) is linear; therefore, the values of each elevational level can be compared directly using the CI overlap (Jost, 2006).
Species composition similarity analysis: Similarities were estimated in species composition between the Loxicha Region and other regions with roughly equivalent sampling effort on the Pacific and Atlantic slopes of Oaxaca and other states. Besides Loxicha, our analysis included the Sierra de Juárez, Oaxaca (Luis-Martínez et al., 1991), the Sierra Mazateca, Oaxaca (Álvarez-García et al., 2016), the Sierra de Atoyac de Álvarez, Guerrero (Vargas-Fernández et al., 1994), the Sierra de Manantlán, Jalisco-Colima (Vargas-Fernández et al., 1999) and the Sierra de San Juan, Nayarit (MARIPOSA database). A distance matrix was estimated with presence/absence data of the species in each region using the Jaccard index with the “vegan'' package (Oksanen et al., 2019) in R 4.0.0 (R Core Team, 2020). Using the same method, the similarity among 77 sites distributed across Mexico was estimated, including multiple biogeographic provinces and both Pacific and Atlantic slopes.
Natural history: Some biological observations made during fieldwork are presented, which include elevational migrations and species aggregations among other data. These observations are valuable for describing the fine-scale spatial and temporal distribution of some rare or hard-to-find species and could aid future efforts to collect these species.
Results
Species list: Based on a review of published records (1950-2004) and a query of the MARIPOSA database up to the year 2000, 139 taxa of Nymphalidae were historically recorded from the Loxicha Region. Our subsequent field sampling from 2005-2014 across 21 sites in the region (Table 2) increased that list to 189 taxa, including 10 subfamilies, 23 tribes, 15 subtribes, and 85 genera. Our sampling effort resulted in 28 756 records plus 1 248 records from historical collections archived in the MARIPOSA database, for a total of 30 004 records of Nymphalidae for the Loxicha Region. Data from publications and scientific collections are mostly from the 400-1 400 m elevational range, while records from our field work are distributed from 80-2 850 m. All species and subspecies historically recorded for the region were collected during our fieldwork except for Adelpha donysa ssp., Pedaliodes dejecta ssp., and Actinote guatemalena guerrerensis J. Maza, 1982.
The Nymphalidae species documented in the Loxicha Region constitute 46 % of Mexican nymphalid species and 56 % of those recorded from Oaxaca (Luis-Martínez et al., 2016). A total of 10 species and 56 subspecies endemic to Mexico are represented in the Loxicha Region. These endemic taxa were binned into four groups according to their degree of endemism (Fig. 1): group 1, endemic to the Loxicha Region (5 spp.); group 2, endemic to the Sierra Madre del Sur (13 spp.); group 3, endemic to the Pacific slope (23 spp.); and group 4, endemic to Mexico (15 spp.). The taxa exclusive to the Loxicha Region are Memphis wellingi (L. Miller & J. Miller, 1976); Cyllopsis jacquelineae (L. Miller, 1974); Callicore texa loxicha (R. G. Maza & J. Maza, 1983); Chlosyne gaudialis wellingi (L. Miller & Rotger, 1979), and Altinote stratonice oaxaca (J. Miller & L. Miller, 1979).

Fig. 1 Nymphalidae taxa endemic to Mexico that occur in the Loxicha Region, Oaxaca. Numbers correspond to names available in our species list. Dorsal view: left side of the specimen; Ventral view: right side.
Species list of Nymphalidae for the Loxicha Region, Oaxaca. Taxa in bold text are endemics, and superscript numbers correspond to their degree of endemism: 1, endemic to the Loxicha Region; 2, endemic to the Sierra Madre del Sur; 3, endemic to the Pacific slope; 4, endemic to Mexico.
Family NYMPHALIDAE
Rafinesque, 1815
Subfamily Libytheinae Boisduval, 1833
1.Libytheana carinenta mexicana Michener, 1943
Subfamily Danainae Boisduval, 1833
Tribe Euploeini Herrich-Schäffer, 1849
Subtribe Itunina Reuter, 1896
2.Anetia thirza thirza Geyer, (1833)
3.Lycorea halia atergatis Doubleday, (1847)
4.Lycorea ilione albescens (Distant, 1876)
Tribe Danaini Boisduval, 1833
Subtribe Danaina Boisduval, 1833
5.Danaus eresimus montezuma Talbot, 1943
6.Danaus gilippus thersippus (Bates, 1863)
7.Danaus plexippus plexippus (Linnaeus, 1758)
Subfamily Ithomiinae Godman & Salvin, 1879
Tribe Tithoreini Fox, 1940
8.Aeria eurimedia pacifica Godman & Salvin, 1879
9.Tithorea harmonia hippothous Godman & Salvin, 1879
10.Tithorea tarricina duenna Bates, 1864
Tribe Melinaeini Clark, 1947
11. Melinaea lilis flavicans Hoffmann, 1924 3
Tribe Mechanitini Bar, 1878
12.Mechanitis lysimnia utemaia Reakirt, 1866
13.Mechanitis menapis doryssus Bates, 1864
14.Mechanitis polymnia lycidice Bates, 1864
Tribe Oleriini Fox, 1940
15.Oleria paula (Weymer, 1883)
Tribe Dircennini D'Almeida, 1941
16.Dircenna klugii klugii (Geyer, 1837)
17. Episcada salvinia portilla J. Maza & Lamas, 1978 3
18. Pteronymia artena praedicta J. Maza & Lamas, 1982 2
19.Pteronymia cotytto cotytto (Guérin-Méneville, (1844))
2. Pteronymia rufocincta (Salvin, 1869) 3
Tribe Godyridini D'Almeida, 1941
21. Greta annette moschion (Godman, 1901) 3
22. Greta morgane morgane (Geyer, 1837) 3
Subfamily Charaxinae Guenée, 1865
Tribe Anaeini Reuter, 1896
23.Hypna clytemnestra mexicana Hall, 1917
24.Consul electra electra (Westwood, 1850)
25.Consul excellens genini (Le Cerf, 1922)
26.Consul fabius cecrops (Doubleday, (1849))
27.Phantos callidryas (R. Felder, 1869)
28.Siderone galanthis ssp.
29.Zaretis ellops (Ménétriés, 1855)
30.Anaea troglodyta aidea (Guérin-Méneville, (1844))
31. Fountainea eurypyle glanzi (Rotger, Escalante & Coronado, 1965) 3
32.Fountainea glycerium glycerium (Doubleday, (1849))
33. Fountainea nobilis rayoensis (J. Maza & Díaz, 1978) 3
34.Memphis forreri (Godman & Salvin, 1884)
35.Memphis perenna perenna (Godman & Salvin, (1884))
36.Memphis pithyusa pithyusa (R. Felder, 1869)
37. Memphis wellingi L. Miller & J. Miller, 1976 1
Tribe Preponini Rydon, 1971
38. Archaeoprepona amphimachus baroni J. Maza, 1982 2
39. Archaeoprepona demophon occidentalis Stoffel & Descimon, 1974 3
40. Archaeoprepona demophoon mexicana Llorente, Descimon & K. Johnson, 1993 3
41. Archaeoprepona phaedra ssp. 2
42.Prepona laertes octavia Fruhstorfer, 1905
43. Prepona brooksiana ibarra Beutelspacher, 1982 3
Subfamily Morphinae Newman, 1834
Tribe Morphini Newman, 1834
Subtribe Morphina Newman, 1834
44.Morpho polyphemus Westwood, (1850)
45. Morpho helenor guerrerensis Le Moult & Réal, 1962 3
Tribe Brassolini Boisduval, 1836
Subtribe Brassolina Boisduval, 1836
46.Caligo telamonius memnon (C. Felder & R. Felder, 1867)
47.Caligo uranus Herrich-Schäffer, 1850
48.Opsiphanes boisduvallii Doubleday, (1849)
49.Opsiphanes cassina fabricii (Boisduval, 1870)
50.Opsiphanes quiteria quirinus Godman & Salvin, 1881
51.Opsiphanes tamarindi tamarindi C. Felder & R. Felder, 1861
Subfamily Satyrinae Boisduval, 1833
52.Manataria hercyna maculata (Hopffer, 1874)
53. Oxeoschistus hilara ssp. 2
54. Oxeoschistus tauropolis ssp. 3
55. Pedaliodes dejecta ssp. 2
56.Cissia similis (Butler, 1867)
57.Cissia terrestris (Butler, 1867)
58.Cissia sp.
59.Cissia themis (Butler, 1867)
60. Cyllopsis clinas (Godman & Salvin, 1889) 2
61. Cyllopsis diazi L. Miller, 1974 4
62.Cyllopsis hedemanni hedemanni R. Felder, 1869
63. Cyllopsis jacquelineae L. Miller, 1974 1
64. Cyllopsis nayarit (R. L. Chermock, 1947) 4
65.Cyllopsis pyracmon pyracmon (Butler, 1867)
66.Cyllopsis suivalenoides L. Miller, 1974
67.Euptychia fetna Butler, 1870
68.Hermeuptychia hermes (Fabricius, 1775)
69. Megisto rubricata pseudocleophes L. Miller, 1976 4
70. Paramacera xicaque rubrosuffusa L. Miller, 1972 2
71.Pindis squamistriga R. Felder, 1869
72.Taygetis kerea Butler, 1869
73. Taygetis mermeria griseomarginata L. Miller, 1978 3
74. Taygetis uncinata Weymer, 1907 4
75.Taygetis virgilia (Cramer, 1776)
76.Taygetis weymeri Draudt, 1912
77. Gyrocheilus patrobas patrobas (Hewitson, 1862) 4
Subfamily Apaturinae Boisduval, 1840
78.Asterocampa idyja argus (Bates, 1864)
79.Doxocopa laure laure (Drury, 1773)
80.Doxocopa pavon theodora (Lucas, 1857)
Subfamily Biblidinae Boisduval, 1833
Tribe Cyrestini Guenée, 1865
81.Marpesia chiron marius (Cramer, 1779)
82.Marpesia petreus ssp. nov.
83.Marpesia zerynthia dentigera (Fruhstorfer, 1907)
Tribe Biblidini Boisduval, 1833
Subtribe Biblidina Boisduval, 1833
84.Biblis hyperia aganisa Boisduval, 1836
85.Mestra dorcas amymone (Ménétriés, 1857)
Subtribe Ageroniina Doubleday, (1847)
86. Hamadryas amphinome mazai Jenkins, 1983 3
87. Hamadryas atlantis lelaps (Godman & Salvin, 1883) 3
88.Hamadryas februa ferentina (Godart, (1824))
89.Hamadryas glauconome glauconome (Bates, 1864)
90. Hamadryas guatemalena marmarice (Fruhstorfer, 1916) 4
Subtribe Epicaliina Guenée, 1865
91.Eunica alcmena alcmena (Doubleday, (1847))
92.Eunica monima (Stoll, 1782)
93.Eunica tatila tatila (Herrich-Schäffer, (1855))
94. Catonephele cortesi R. G. Maza, 1982 3
95. Catonephele numilia immaculata Jenkins, 1985 2
96. Myscelia cyananthe cyananthe C. Felder & R. Felder, 1867 4
97. Myscelia cyaniris alvaradia R. G. Maza & Díaz, 1982 3
98.Myscelia ethusa ethusa (Doyère, (1840))
Subtribe Epiphilina Jenkins, 1987
99. Nica flavilla bachiana (R. G. Maza & J. Maza, 1985) 2
100. Temenis laothoe quilapayunia R. G. Maza & Turrent, 1985 3
101. Bolboneura sylphis beatrix R. G. Maza, 1985 3
102. Epiphile adrasta escalantei Descimon & Mast, 1979 4
103. Pyrrhogyra edocla paradisea R. G. Maza & J. Maza, 1985 3
104.Pyrrhogyra neaerea hypsenor Godman & Salvin, 1884
Subtribe Callicorina Orfila, 1952
105. Diaethria anna mixteca J. Maza, 1977 2
106. Diaethria astala asteroide R. G. Maza & R. F. Maza, 1985 2
107. Callicore texa loxicha R. G. Maza & J. Maza, 1983 1
108.Cyclogramma pandama (Doubleday, (1848))
Subtribe Eubagina Burmeister, 1878
109.Dynamine dyonis Geyer, 1837
110.Dynamine postverta mexicana D'Almeida, 1952
111.Dynamine theseus (C. Felder & R. Felder, 1861)
Subfamily Limenitidinae Behr, 1864
Tribe Limenitidini Behr, 1864
Subtribe Limenitidina Behr, 1864
112.Adelpha barnesia leucas Fruhstorfer, 1915
113.Adelpha basiloides (Bates, 1865)
114.Adelpha bredowii Geyer, 1837
115. Adelpha diocles ssp. 3
116. Adelpha donysa ssp. 2
117.Adelpha fessonia fessonia (Hewitson, 1847)
118.Adelpha iphicleola iphicleola (Bates, 1864)
119.Adelpha iphiclus iphiclus (Linnaeus, 1758)
120.Adelpha leuceria leuceria (Druce, 1874)
121. Adelpha leucerioides ssp. 3
122.Adelpha lycorias melanthe (Bates, 1864)
123.Adelpha naxia naxia (C. Felder & R. Felder, 1867)
124.Adelpha paraena massilia (C. Felder & R. Felder, 1867)
125.Adelpha phylaca phylaca (Bates, 1866)
126.Adelpha pithys (Bates, 1864)
127.Adelpha serpa celerio (Bates, 1864)
Subfamily Nymphalinae Rafinesque, 1815
Tribe Coeini Scudder, 1893
128.Historis acheronta acheronta (Fabricius, 1775)
129.Historis odius dious Lamas, 1995
130.Pycina zamba zelys Godman & Salvin, 1884
Tribe Nymphalini Rafinesque, 1815
131.Colobura dirce dirce (Linnaeus, 1758)
132.Smyrna blomfildia datis Fruhstorfer, 1908
133.Smyrna karwinskii Geyer, (1833)
134.Hypanartia dione disjuncta Willmott, J. Hall & Lamas, 2001
135.Hypanartia godmanii (Bates, 1864)
136.Hypanartia lethe (Fabricius, 1793)
137.Hypanartia trimaculata autumna Willmott, J. Hall & Lamas, 2001
138.Nymphalis antiopa antiopa (Linnaeus, 1758)
139.Vanessa atalanta rubria (Fruhstorfer, 1909)
140.Vanessa cardui (Linnaeus, 1758)
141.Vanessa virginiensis (Drury, 1773)
Tribe Victorinini Scudder, 1893
142.Siproeta epaphus epaphus (Latreille, (1813))
143.Siproeta stelenes biplagiata (Fruhstorfer, 1907)
144.Anartia fatima fatima (Fabricius, 1793)
145.Anartia jatrophae luteipicta Fruhstorfer, 1907
Tribe Junoniini Reuter, 1896
146.Junonia coenia Hübner, (1822)
147.Junonia evarete nigrosuffusa Barnes & McDunnough, 1916
148.Junonia genoveva ssp. nov.
Tribe Melitaeini Herrich-Schäffer, 1843
Subtribe Nova
149. Chlosyne cynisca (Godman & Salvin, 1882) 4
150.Chlosyne erodyle ssp.
151. Chlosyne gaudialis wellingi L. Miller & Rotger, 1979 1
152.Chlosyne hippodrome hippodrome (Geyer, 1837)
153.Chlosyne janais janais (Drury, 1782)
154.Chlosyne lacinia lacinia (Geyer, 1837)
155.Chlosyne marina marina (Geyer, 1837)
156.Chlosyne melanarge (Bates, 1864)
157.Chlosyne theona theona (Ménétriés, 1855)
158.Microtia elva elva Bates, 1864
Subtribe Phyciodina Higgins, 1981
159.Phyciodes graphica graphica (R. Felder, 1869)
160.Phyciodes mylitta thebais Godman & Salvin, 1878
161. Phyciodes pallescens (R. Felder, 1869) 4
162.Phyciodes phaon phaon (Edwards, 1864)
163.Phyciodes tharos tharos (Drury, 1773)
164.Tegosa guatemalena (Bates, 1864)
165. Anthanassa ardys ardys (Hewitson, 1864) 4
166.Anthanassa argentea (Godman & Salvin, 1882)
167.Anthanassa atronia (Bates, 1866)
168.Anthanassa frisia tulcis (Bates, 1864)
169. Anthanassa nebulosa alexon (Godman & Salvin, 1889) 4
170. Anthanassa otanes oaxaca Beutelspacher, 1990 4
171. Anthanassa ptolyca amator (Hall, 1929) 4
172. Anthanassa sitalces cortes (Hall, 1917) 4
173.Anthanassa texana texana (Edwards, 1863)
174.Eresia phillyra phillyra Hewitson, 1852
Subfamilia Heliconiinae Swainson, 1822
Tribe Acraeini Boisduval, 1833
175. Altinote stratonice oaxaca (J. Miller & L. Miller, 1979) 1
176. Actinote guatemalena guerrerensis J. Maza, 1982 2
Tribe Heliconiini Swainson, 1822
Subtribe Heliconiina Swainson, 1822
177.Agraulis vanillae incarnata (Riley, 1926)
178.Dione juno huascuma (Reakirt, 1866)
179.Dione moneta poeyii Butler, 1873
180.Dryas iulia moderata (Riley, 1926)
181.Dryadula phaetusa (Linnaeus, 1758)
182.Eueides aliphera gracilis Stichel, 1903
183.Eueides isabella eva (Fabricius, 1793)
184.Heliconius charithonia vazquezae W. P. Comstock & F. M. Brown, 1950
185. Heliconius erato cruentus Lamas, 1998 3
186.Heliconius hortense Guérin-Méneville, (1844)
187.Heliconius ismenius telchinia Doubleday, 1847
Tribe Argynnini Swainson, 1833
Subtribe Euptoietina Simonsen, 2006
188.Euptoieta claudia daunius (Herbst, 1798)
189.Euptoieta hegesia meridiania Stichel, 1938
Alpha diversity: Considering only the data obtained during our fieldwork, 186 Nymphalidae species were collected. When species from the literature review and historically collected specimens were added, the list reached 189 species. The Clench model estimated 188 species (Fig. 2) while Chao1 estimated 193 species. Therefore, the Clench model underestimated the region's species richness, while the Chao1 estimator was potentially more reliable. According to Chao1, our list is 98 % complete.
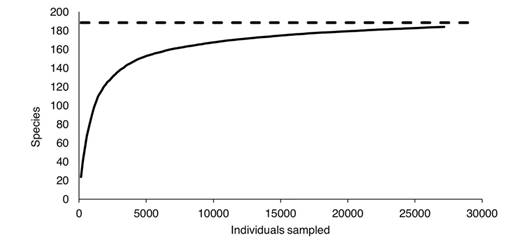
Fig. 2 Species accumulation curve of Nymphalidae in the Loxicha Region, Oaxaca, Mexico fitted to the Clench model. The asymptote (dashed line) corresponds to 188 estimated species.
Across the 11 states of Mexico's Pacific slope from Baja California to Chiapas (Table 1), the nymphalid species richness of the Loxicha Region alone (189 spp.) surpasses all states except for Guerrero (216 spp.) and Chiapas (351 spp.). The high species richness in Guerrero is attributable to the biogeographic provinces Planicie Costera del Pacífico and Sierra Madre del Sur occupying most of the state, with the SMS having substantial faunal endemism. In Chiapas, the high species richness can be explained by the convergence of Atlantic and Pacific slopes in the state, as well as the presence of tropical evergreen forest, a vegetation type that supports more than 50 % of Mexican species of Papilionoidea (Salinas-Gutiérrez et al., 2004).
Species composition similarity across regions: We compared nymphalid faunal richness across eight regions with differing elevational gradients, comprising six from the Pacific slope and two from the Atlantic slope (Table 3). All regions have roughly similar sampling effort, and species estimation methods indicate that more than 90 % of the species have been recorded from each, thus validating the comparison. The Loxicha Region is the most diverse for Nymphalidae on the Pacific slope, and the second most diverse in Oaxaca, behind only the Sierra de Juárez (259 spp.) of the Atlantic slope. Identical patterns exist for Papilionidae and Pieridae (Luis-Martínez et al., 2020).
Table 3 Species richness of Nymphalidae across eight regions in Mexico
| Region | spp. | Elevation (m) | Slope |
| 1. Sierra de Juárez, Oaxaca | 259 | 100-3 100 | Atlantic |
| 2. Sierra Mazateca, Oaxaca | 185 | 100-2 200 | Atlantic |
| 3. Loxicha Region, Oaxaca | 189 | 80-2 600 | Pacific |
| 4. Sierra de Atoyac, Guerrero | 170 | 300-3 100 | Pacific |
| 5. Morelos (state) | 147 | 1 000-3 000 | Pacific -CB |
| 6. Michoacán, SMS portion | 134 | 800-1 800 | Pacific |
| 7. Sierra de Manantlán, Jalisco-Colima | 144 | 250-1 750 | Pacific |
| 8. Sierra de San Juan, Nayarit | 135 | 0-1 350 | Pacific |
1: Luis-Martínez et al., 1991; 2: Álvarez-García et al., 2016; 3: this article; 4: Vargas-Fernández et al., 1994; 5: Luna-Reyes et al., 2012 and Luna-Reyes, 2020; 6 and 8: MARIPOSA database; 7: Vargas-Fernández et al., 1999. CB: Cuenca del Balsas.
Similarity analysis shows two main groups (Fig. 3), corresponding to the arrangement of mountain ranges in Mexico and the consequent isolation of the lepidopterofauna. The first group comprises the Sierra Mazateca and the Sierra de Juárez, on the Atlantic slope of Oaxaca. The second group is composed of six regions on the Pacific slope or in the Amacuzac-Cuenca del Balsas, which are divided into two subgroups with a similarity value of more than 50 %. The first subgroup contains the regions of the Southern Sierra Madre del Sur: Atoyac and Loxicha, which share 66 % of their species (142 spp.). The second subgroup corresponds to the regions located North of the Costa del Pacífico, in the Western Eje Neovolcánico (San Juan-Manantlán), the Northern Sierra Madre del Sur (SMS-Michoacán), and Morelos which includes parts of two biogeographic provinces: Cuenca del Balsas and Eje Neovolcánico.
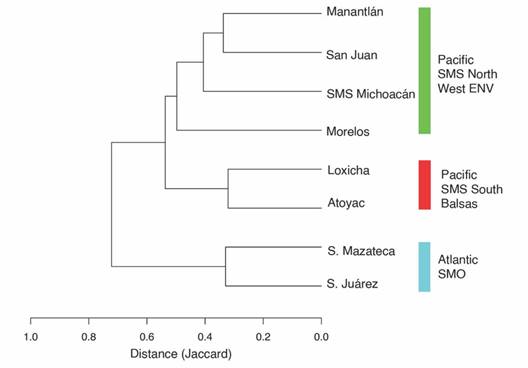
Fig. 3 Similarity of Nymphalidae species composition across eight regions in Mexico. The distance matrix was estimated with the Jaccard index. For full names of the regions see Table 3.
Species composition distances between Loxicha and other regions are correlated with geographic distances, but also with biogeographic regionalization (Fig. 3). The two regions with the greatest faunistic distance are those from the Atlantic slope (the Sierra de Juárez and Sierra Mazateca), even though they are the closest geographically. The same pattern was observed with Morelos, which belongs to the Cuenca del Balsas: although Morelos is closer to Loxicha than regions like San Juan and Manantlán, these two have lower faunistic distances. Considering only the four regions in the SMS, faunistic distances are correlated with geographic distances, with Atoyac being the closest and most similar to Loxicha while also being the farthest and most distinct relative to San Juan.
Species richness by site: The MARIPOSA database contains records of Nymphalidae species from more than 5 000 sites across Mexico. However, only 40 of those sites have over 100 species recorded, eight of which are on the Pacific slope while the other 32 are distributed elsewhere in Mexico (Table 4). From the Loxicha Region, only Azulillo and Rancho Hagia Sofía exceed 100 species, placing them in sixth and fourth place at a national scale, respectively. Areas like Acahuizotla, Guerrero (158 spp.) and Candelaria Loxicha, Oaxaca (138 spp.) also have high species richness but are not specific sites, instead being extended areas that include several sites with wide variation in elevation and vegetation type. These extended or historical “sites'' served as geographic references for decades prior to this study, where collected specimens from other sites were gathered and labeled. Additional such historical “sites'' across Mexico include Xalapa, Catemaco, and Presidio in the state of Veracruz (Luis-Martínez et al., 1996), Chiltepec, Oaxaca (Luis-Martínez et al., 1991), and some others on the Atlantic slope.
Table 4 Sites with more than 100 species of Nymphalidae along the Atlantic and Pacific slopes of Mexico
| State | R | Site | spp. | State | R | Site | spp. |
| Atlantic slope | |||||||
| Veracruz | 3 | Xalapa* | 193 | San Luis Potosí | Tamazunchale | 109 | |
| Veracruz | 1 | Córdoba | 180 | Chiapas | Santa Rosa | 108 | |
| Veracruz | 1 | Presidio | 170 | Veracruz | 3 | Parque Francisco Javier Clavijero1 | 107 |
| Chiapas | San Antonio Buena Vista | 162 | Oaxaca | 4 | Cerro Armadillo1 | 106 | |
| Oaxaca | 4 | San José Chiltepec | 156 | Veracruz | Cuetzalapan | 104 | |
| Oaxaca | 4 | Metates1 | 152 | Veracruz | 2 | Popoctépetl | 104 |
| Veracruz | 3 | Barranca de Cayoapa*1 | 149 | Oaxaca | 4 | Soyolapan El Bajo1 | 103 |
| Puebla | 10 | Tequezquitla | 145 | Oaxaca | Matías Romero | 102 | |
| Veracruz | 1 | Fortín de las Flores*1 | 146 | Oaxaca | La Gringa, Sta. María Chimalapa1 | 102 | |
| Veracruz | 2 | Catemaco | 142 | Veracruz | 1 | Orizaba | 102 |
| Veracruz | 2 | Dos Amates | 138 | Veracruz | 2 | Tapalapan | 102 |
| Veracruz | 1 | Presidio, Ixhuatlán del Café* | 134 | ||||
| Veracruz | 3 | Teocelo1 | 132 | Pacific slope | |||
| Puebla | 10 | Barranca de Patla* | 132 | Guerrero | 8 | Acahuizotla* | 158 |
| Oaxaca | 4 | La Esperanza1 | 131 | Oaxaca | 11 | Candelaria Loxicha* | 138 |
| Chiapas | Santa Rosa, Comitán | 130 | Oaxaca | 11 | Rancho Hagia Sofía1 | 121 | |
| Chiapas | Zona Arqueológica Yaxchilán1 | 129 | Chiapas | San Jerónimo, Tacaná | 117 | ||
| Oaxaca | 4 | Puerto Eligio1 | 110 | Jalisco | 6 | La Calera1 | 115 |
| Veracruz | 2 | Laguna de Catemaco | 117 | Oaxaca | 11 | Azulillo1 | 109 |
| Tabasco | Cerro del Coconá1 | 115 | Guerrero | 5 | Río Santiago1 | 106 | |
| Oaxaca | 4 | Naranjal Chiltepec | 115 | Guerrero | 5 | El Faisanal1 | 105 |
*: extended or historical “sites'' that combine records from multiple distinct sites under a single name (see text); 1: sites collected by MZFC members; R: sites that lie in the most diverse regions of Mexico (see text).
Of the 40 sites with more than 100 species of Nymphalidae (Table 4), 16 have been sampled by members of the MZFC, and 30 are located in 11 of the regions with highest diversity of Papilionoidea in Mexico. Of these high-diversity regions, nine were recognized by Luis-Martínez et al. (2003): 1, Orizaba-Córdoba-Fortín de las Flores corridor; 2, Tuxtlas; 3, Xalapa-Coatepec-Teocelo corridor; 4, Sierra de Juárez; 5, Sierra de Atoyac; 6, Sierra de Manantlán; 7, Mismaloya-Bahía de Banderas corridor; 8, Acahuizotla; and 9, Sierra de San Juan. Recently, two more high-diversity regions were recognized: 10, La Sierra Norte de Puebla (MARIPOSA database), and 11, Loxicha (Arellano-Covarrubias et al., 2018; Luis-Martínez et al., 2016; Luis-Martínez et al., 2021; data presented here).
Species composition similarity across sites: Of the 77 sites included in the species composition similarity analysis (Table 5), 62 are on the Pacific slope and 15 on the Atlantic slope, 44 have been sampled by members of the MZFC, 32 are located between 0-750 m, 27 between 800-1 750 m, and six above 1 800 m elevation. Five vegetation types are included: TDF, TSDF, OPF, OF, and CF. The latter includes three elevation-delimited subtypes: low-CF (750-1 199 m), medium-CF (1 200-1 800 m), and high-CF (> 2 000 m). Additionally, we recognize six ecotones: OF-CF, OPF-CF, TDF-TSDF, TDF-TSDF-CF, TSDF-CF and TSDF-OPF. Twenty-four sites are in the CF, and seven more exist in an ecotone that includes CF. Therefore, CF is the most well-represented vegetation type across the sites (48 % of the sites).
Table 5 Sites included in the species composition similarity analysis for Mexican Nymphalidae
| State | Site | Spp. | Abbreviated name | Elevation | Vegetation type |
| Atlantic slope | |||||
| San Luis Potosí | Tamazunchale | 109 | SLP_Tam_350 | 350 | TEGF |
| Puebla | Tequezquitla | 145 | PUE_Teq_650 | 650 | TSDF-CF |
| Puebla | Barranca de Patla | 132 | PUE_Bpa_600 | 600 | TSDF-CF |
| Veracruz | Barranca de Cayoapa1 | 149 | VER_Bca_600 | 600 | CF |
| Veracruz | Catemaco | 142 | VER_Cat_250 | 250 | TEGF |
| Veracruz | Córdoba | 180 | VER_Cor_900 | 900 | CF |
| Veracruz | Fortín de las Flores1 | 146 | VER_FFl_900 | 900 | CF |
| Veracruz | Xalapa | 193 | VER_Jal_1350 | 1 350 | CF |
| Veracruz | Teocelo1 | 132 | VER_Teo_1200 | 1 200 | CF |
| Oaxaca | San José Chiltepec | 156 | OAX_Chi_100 | 100 | TEGF |
| Oaxaca | La Esperanza1 | 131 | OAX_Esp_1750 | 1 750 | CF |
| Oaxaca | Metates1 | 152 | OAX_Met_900 | 900 | CF |
| Oaxaca | Puerto Eligio1 | 110 | OAX_Pel_650 | 650 | TEGF |
| Chiapas | San Antonio Buena Vista | 162 | CHIS_SAB_1350 | 1 350 | CF |
| Chiapas | Santa Rosa, Comitán | 130 | CHI_SRC_1800 | 1 800 | CF |
| Pacific slope | |||||
| Nayarit | Compostela1 | 41 | NAY_Com_800 | 800 | TDF |
| Nayarit | Jumatán1 | 78 | NAY_Jum_300 | 300 | TDF |
| Nayarit | La Bajada1 | 61 | N_Baj_250 | 250 | TSDF |
| Nayarit | La Yerba, Tepeltite1 | 72 | NAY_Yer_900 | 900 | CF |
| Nayarit | Mecatán1 | 47 | NAY_Mec_300 | 300 | TSDF |
| Nayarit | Palapita1 | 66 | NAY_Pal_650 | 650 | TSDF |
| Nayarit | Pintadeño1 | 23 | NAY_Pin_750 | 750 | OF |
| Nayarit | San Blas1 | 45 | NAY_SBl_50 | 50 | MAN |
| Nayarit | Singayta1 | 59 | NAY_Sin_50 | 50 | OP |
| Nayarit | Venustiano Carranza1 | 69 | NAY_Vca_1250 | 1 250 | CF |
| Jalisco | Ahuacapán1 | 85 | JAL_Ahu_900 | 900 | TDF |
| Jalisco | Estación de Biología, UNAM, Chamela | 50 | JAL_Cha_100 | 100 | TDF |
| Jalisco | La Calera1 | 115 | JAL_Cal_650 | 650 | TDF |
| Jalisco | Los Mazos1 | 76 | JAL_Maz_1600 | 1 600 | CF |
| Jalisco | Puerto Vallarta | 49 | JAL_Pva_50 | 50 | TDF |
| Jalisco | Zenzontla1 | 77 | JAL_Zen_800 | 800 | TDF |
| Colima | Agua Dulce1 | 86 | COL_Agu_250 | 250 | TSDF |
| Colima | Platanarillo1 | 88 | COL_Pla_350 | 350 | TSDF |
| Michoacán | Arteaga | 70 | MICH_Art_900 | 900 | TDF |
| Michoacán | Chiquihuitillo | 75 | MICH_Chi_260 | 260 | TDF |
| Michoacán | Los Chorros del Varal1 | 72 | MICH_Cho_900 | 900 | TSDF |
| Michoacán | P.H. Cupatitzio | 83 | MICH_CUP_1000 | 1 000 | TSDF-PF |
| Michoacán | Rancho “El Zorrillo'' | 92 | MICH_Rzo_750 | 750 | TDF |
| Michoacán | Rancho “El Zorrillo'', Cañada Húmeda | 70 | MICH_RzC_750 | 750 | TDF-TSDF |
| Morelos | 3 km al NE de Chiautla | 41 | MOR_Shia_1000 | 1 000 | TDF |
| Morelos | Km 1.5 Carretera Tetecala-Coatlalco | 56 | MOR_TC_1100 | 1 100 | TDF |
| Morelos | Cañón de Lobos | 51 | MOR_CL_1200 | 1 200 | TDF |
| Morelos | 1.5 Km al E de Palo Grande | 57 | MOR_PG_1200 | 1 200 | TDF |
| Morelos | Sierra de Huautla | 58 | MOR_SH_1000 | 1 000 | TDF-TSDF |
| Guerrero | Agua Salada | MOR_AS_760 | 760 | TDF | |
| Guerrero | Coapango | 74 | GRO_Coa_1330 | 1 330 | TDF |
| Guerrero | Las Vías | 72 | GRO_LV_1200 | 1 200 | TDF |
| Guerrero | Cascada de las Granadas | 81 | GRO_CdeG_1370 | 1 370 | TDF |
| Guerrero | Los Amates | 65 | GRO_Ama_980 | 980 | TDF |
| Guerrero | Quetzalapa | 66 | GRO_Que_850 | 850 | TDF |
| Guerrero | Piedras Negras | 54 | GRO_PN_1300 | 1 300 | TDF |
| Guerrero | Palmillas | 57 | MOR_Pal_1050 | 1 050 | TDF |
| Guerrero | Acahuizotla | 158 | GRO_Aca_900 | 900 | TDF-TSDF-CF |
| Guerrero | El Faisanal1 | 94 | GRO_Fai_1250 | 1 250 | TSDF-CF |
| Guerrero | El Iris1 | 24 | GRO_Iris_2000 | 2 000 | OF-CF |
| Guerrero | La Golondrina1 | 45 | GRO_LGo_1800 | 1 800 | CF |
| Guerrero | Las Parotas1 | 78 | GRO_Par_350 | 350 | TSDF |
| Guerrero | Los Retrocesos | 71 | GRO_Ret_1600 | 1 600 | CF |
| Guerrero | Nueva Delhi1 | 82 | GRO_Nde_1350 | 1 350 | CF |
| Guerrero | Puente de Los Lugardo1 | 94 | GRO_PLL_800 | 800 | TSDF |
| Guerrero | Puerto del Gallo1 | 38 | GRO_Pga_2350 | 2 350 | BPE-CF |
| Guerrero | Río Santiago, 4 km W1 | 106 | GRO_Rsa_680 | 680 | TSDF |
| Oaxaca | Azulillo1 | 109 | OAX_Azu_380 | 380 | TSDF |
| Oaxaca | Copalita, Río Copalita | 58 | OAX_Cop_600 | 600 | CF |
| Oaxaca | Copalita, Siete Veneros | 54 | OAX_Ven_940 | 940 | CF |
| Oaxaca | Finca Aurora-Finca San Isidro1 | 63 | OAX_FAS_1100 | 1 100 | CF |
| Oaxaca | La Pasionaria1 | 72 | OAX_Pas_1500 | 1 500 | CF |
| Oaxaca | La Soledad-Buenavista1 | 70 | OAX_Sbu_1470 | 1 470 | CF |
| Oaxaca | Magdalena, El Lirio1 | 95 | OAX_Mag_750 | 750 | CF |
| Oaxaca | Parque Nacional Huatulco1 | 68 | OAX_PNH_100 | 100 | TDF |
| Oaxaca | Parque Nacional Huatulco, Río Cacaluta1 | 74 | OAX_PNHC_80 | 80 | TDF |
| Oaxaca | Pluma Hidalgo, 4 km NW “La Curva'' 1 | 95 | OAX_Phi_1100 | 1 100 | CF |
| Oaxaca | Portillo del Rayo-Finca El Encanto1 | 88 | OAX_PRa_1200 | 1 200 | CF |
| Oaxaca | Puente Arroyo “El Guajolote''1 | 74 | OAX_Gua_2020 | 2 020 | OPF |
| Oaxaca | Rancho Hagia Sofía1 | 121 | OAX_Rha_410 | 410 | TSDF |
| Oaxaca | San José del Pacífico, 1 km S1 | 61 | OAX_Pac_2280 | 2 280 | OPF-CF |
| Chiapas | San Jerónimo, Tacaná | 117 | CHIS_Sje_750 | 750 | CF |
1: sites collected by MZFC members; CF, cloud forest; MAN, mangrove; OF, oak forest; OP, Orbygnia palmar; OPF, oak-pine forest; TDF, tropical deciduous forest; TEGF, tropical evergreen forest; TSDF, tropical sub-deciduous forest.
The similarity analysis showed two groups corresponding to the sites of the Atlantic and Pacific slopes, respectively (Fig. 4). Sites of the Atlantic slope, in the low part of the phenogram (1), are associated with the vegetation and environmental conditions of the Gulf of Mexico (East of Chiapas). This group has 163 exclusive taxa, six of which are distributed in 85 % of the sites of the group: Archaeoprepona demophon centralis (Fruhstorfer, 1905), Catonephele mexicana Jenkins & R. G. Maza, 1985, Fountainea eurypyle confusa (A. Hall, 1929), Hamadryas amphinome mexicana (Lucas, 1853), Morpho helenor montezuma Guenée, 1859, and Taygetis thamyra (Cramer, 1779). The second group comprises the sites in the Pacific slope (2), divided into four subgroups. In the first subgroup are the sites of the highlands of the Sierra de Atoyac de Álvarez (La Golondrina, El Iris, and Puerto del Gallo) (3). No exclusive species were recorded for these three sites. Interestingly, although these sites are geographically close to the sites of Balsas and SMS, they segregate from other sites of the Pacific because of their high-elevation species assemblage (1 800-2 350 m). In the second subgroup are the sites of the biogeographic provinces Costa del Pacífico, Northern Sierra Madre del Sur, and Western Eje Neovolcánico (4). Six species are exclusive to this subgroup: Bolboneura sylphis sylphis (Guérin-Méneville, 1844), Chlosyne rosita montana A. Hall, 1924, Cyllopsis pallens L. Miller, 1974, Fountainea halice tehuana (A. Hall, 1917), Polygonia interrogationis (Fabricius, 1798), and Texola elada hepburni (Godman, 1901), but all are restricted to just 4-12 % of the sites in the subgroup. A third subgroup is formed by the sites in the Balsas province of Guerrero and Morelos (6). These sites support mostly TDF and lie at elevations from 760-2 280 m. Of the three exclusive species to Balsas, Zischkaia lupita (Reakirt, (1867)) is in 79 % of the sites of this subgroup, while Chlosyne cyneas cynisca (Godman & Salvin, 1882) and Cyllopsis pertepida pertepida (Dyar, 1912) are documented only in 7 % of the sites. The fourth subgroup includes the Loxicha Region and is composed of the sites of Oaxaca and Guerrero in the SMS (5). Exclusive species recorded in at least 80 % of the sites of this subgroup are Archaeoprepona amphimachus baroni, Catonephele numilia immaculata, Diaethria astala asteroide, and D. anna mixteca. This subgroup is divided into two sets of sites: those with low-elevation CF, and those with TSDF between 350-900 m. However, the high-elevation site El Guajolote (2 202 m) is similar to these sites, possibly because of its geographical proximity. The other set of sites are those within the SMS with mid-elevation CF, between 600-1 600 m. A clear distinction exists between the sites of Oaxaca and those of Guerrero. These results show that the position of the sites in the phenogram corresponds to their geographic location but also to their respective elevations (Fig. 4).
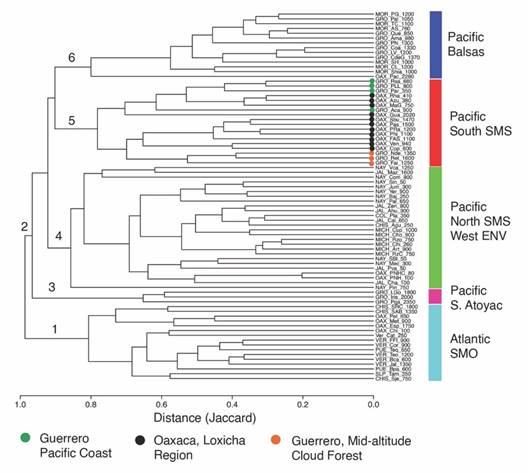
Fig. 4 Nymphalidae species composition similarity across 77 sites in Mexico (15 in the Atlantic and 62 in the Pacific slopes). The distance matrix was estimated with the Jaccard index. Groups: 1. Atlantic slope; 2. Pacific slope; 3. Sierra de Atoyac high-lands; 4. Costa del Pacífico, Northern Sierra Madre del Sur, and Western Eje Neovolcánico; 5. Sierra Madre del Sur (Guerrero-Oaxaca); 6. Cuenca del Balsas (Guerrero-Morelos).
Diversity of the Loxicha Region relative to other regions of the Sierra Madre del Sur and Costa del Pacífico: The Mexican Pacific slope, particularly in Guerrero and Oaxaca, stands out for its remarkable diversity and endemism at both the species and genus level. In this area, only two faunistic studies exist that cover a complete elevational gradient, and that have an inventory completeness level higher than 95 % according to the species richness estimators. The first study was carried out in the Sierra de Atoyac, Guerrero, from 300-3 100 m (Vargas-Fernández et al., 1994) and the second one is the present study from 80-2 850 m (Table 6). Including both inventories, the diversity of Nymphalidae in the area is 214 species including 11 subfamilies and 84 genera. Of these 214 species, 88 % occur in the Loxicha Region and 78 % in the Sierra de Atoyac. Species richness is higher in Loxicha for nine of the 11 subfamilies, with the greatest difference being in Nymphalinae (11 sspp.); only Satyrinae has a higher species richness in the Sierra de Atoyac, with nine subspecies.
Table 6 Species richness and endemism of Nymphalidae subfamilies for two regions of the Pacific slope of the Sierra Madre del Sur, Mexico (modified from Luis-Martínez et al., 2021)
| Subfamily | Loxicha Region, Oaxaca | Sierra de Atoyac, Guerrero | ||||
| Genera | Species | Subspecies | Genera | Species | Subspecies | |
| Libytheinae | 1 | 1/0 | 1/0 | 1 | 1/0 | 1/0 |
| Danainae | 3 | 6/0 | 6/0 | 3 | 5/0 | 5/0 |
| Ithomiinae | 9 | 2/1 | 15/6 | 7 | 2/1 | 12/8 |
| Charaxinae | 10 | 4/1 | 21/8 | 9 | 3/0 | 16/6 |
| Morphinae | 3 | 3/0 | 8/1 | 3 | 2/0 | 7/1 |
| Satyrinae | 12 | 16/5 | 26/12 | 12 | 20/8 | 35/16 |
| Apaturinae | 2 | 3/0 | 3/0 | 1 | 1/0 | 1/0 |
| Biblidinae | 16 | 5/1 | 31/15 | 14 | 6/1 | 29/14 |
| Limenitidinae | 1 | 3/0 | 16/3 | 1 | 2/0 | 13/2 |
| Nymphalinae | 16 | 12/2 | 47/8 | 15 | 12/1 | 36/5 |
| Heliconiinae | 9 | 2/0 | 15/3 | 7 | 19/0 | 12/3 |
| TOTAL Richness/Endemism | 82 | 57/10 | 189/56 | 73 | 73/11 | 167/55 |
Considering both transects, 13 species endemic to Mexico were recorded: Pteronymia rufocincta, Cyllopsis caballeroiBeutelspacher, 1982, C. clinas, C. diazi, C. jacquelineae, C. nayarit, C. perplexa L. Miller, 1974, Paramacera copiosa L. Miller, 1972, Taygetis uncinata, Catonephele cortesi, Chlosyne cynisca, Chlosyne eumeda (Godman & Salvin, 1894), and Phyciodes pallescens. At the subspecies level there are 66 endemics, constituting 31 % of the Nymphalidae recorded in these areas. At a national scale, Loxicha and the Sierra de Atoyac contain 43 % of the species or subspecies of Nymphalidae endemic to Mexico. Of these endemics, 56 are present in the Loxicha Region and 55 in the Sierra de Atoyac, with 64 % of the endemics shared between the two (Llorente-Bousquets, Trujano-Ortega et al., 2006; Luis-Martínez et al., 2003; Luis-Martínez et al., 2016; Luis-Martínez et al., 2021). As with the endemics of the Loxicha Region, endemics of the Sierra de Atoyac were grouped into four categories: 1, endemic to the Sierra de Atoyac (3 sspp: Drucina championi ssp., Eunica malvina almaeVargas, Llorente & Luis, 1996, and Eueides isabella nigricornis R. G. Maza, 1982); 2, endemic to the Sierra Madre del Sur (17 sspp.); 3, endemic to the Pacific slope (23 sspp.); and 4, endemic to Mexico (12 sspp.). These numbers are very similar to those of the Loxicha Region, with differences of at most three species. In both regions, Nymphalinae has the highest species richness and Biblidinae has most of the endemic taxa. Libytheinae, Danainae, and Apaturinae have the lowest richness, a common pattern in America (Lamas, 2004; Pelham, 2008). Additionally, in Loxicha these three subfamilies have no endemic taxa.
Phenology, elevational patterns, and exclusivity of species in the transect
Phenology: Temporal distribution of the species richness and abundance of Nymphalidae is influenced by season (Fig. 5). Richness and abundance both reach their maximum values in October (the end of the rainy season), after which both parameters suddenly drop to their lowpoints in December. The increase in species richness is more or less steady, with just a few more species in the rainy season compared with the dry season; in contrast, abundance has a steep increase from the dry to the rainy season. April shows a slight increase in species richness but a decrease in abundance, suggesting an increase of rare species during this month, compared with other months.

Fig. 5 Phenology of Nymphalidae abundance and species richness in the Loxicha Region, Oaxaca, Mexico based on seven years of sampling data. February was not sampled during this study.
Elevational diversity patterns: Species richness and diversity (e H ) of Nymphalidae decrease abruptly at elevations above 1 800 m, with just 33 % of the total species assemblage compared with 79 % at low elevations and 82 % at mid elevations (Fig. 6). Although observed species richness is higher at the 750-1 800 m level (151 spp.) than at the 0-750 m level (146 spp.), diversity estimates show no significant difference between these two levels. However, diversity is higher at low elevations, which may be because of a higher dominance of Hermeuptychia hermes and Cissia similis at mid elevations which reduces overall diversity.
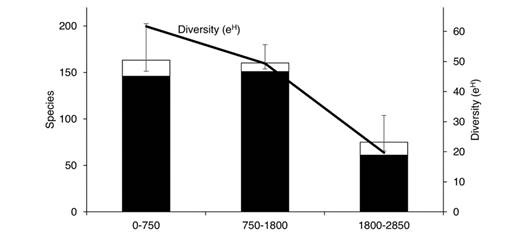
Fig. 6 Observed (black) and estimated (white) Nymphalidae species richness and diversity (exponential of Shannon-Wiener index) across three elevational levels in the Loxicha Region, Oaxaca, Mexico.
Elevational abundance patterns: Abundance is highest at low elevations with 58 % of the total (15 861 records), decreases at mid elevations with 39 % (10 520 records), and is just 3 % (796 records) at high elevations. Each level has dominant or characteristic species, but the overall proportions of those dominant species are highest in the mid and high elevations. The most abundant species in each level belong to the Satyrinae subfamily. At low elevations, Cissia similis (607 records) and Hermeuptychia hermes (869 records) account for 5.1 and 5.4 % of the specimens, respectively; at mid elevations, H. hermes is also the most abundant species with 1 273 records (12 %); at high elevations, 22 % of the specimens (177 records) are Paramacera xicaque rubrosuffusa (Satyrinae) and 15 % (122 records) are Anthanassa a. ardys (Nymphalinae).
A total of 35 species were collected from all three elevational levels (Appendix 1), but these species showed the following three distinct distribution patterns. Pattern A: Dione moneta poeyii, Paramacera xicaque rubrosuffusa, and Vanessa virginiensis mainly occur at high elevations with just one to three records from low elevations. Pattern B: 19 spp. (v. gr. Anartia f. fatima, Cissia similis, Smyrna blomfildia datis) with the opposite pattern, being abundant at low elevations and decreasing at mid elevations, with just a few specimens at high elevations. Pattern C: 13 species (v. gr. Anthanassa a. ardys, Chlosyne h. hippodrome, Hermeuptychia hermes) that are abundant at mid elevations with fewer records at both low and high elevations. Most of the taxa (104, 56 %) are present in two elevational levels, mainly in the low and mid elevations with just 10 species in both the mid and high elevations (v. gr. Anthanassa atronia, A. otanes oaxaca) (see Appendix 1). Remarkably, eight species Adelpha f. fessonia, Anartia jatrophae luteipicta, Chlosyne m. marina, Danaus eresimus montezuma, Doxocopa l. laure, Phyciodes g. graphica, P. mylitta thebais, and Pteronymia artena praedicta were collected only in the low and high elevations, implying that they are also present in the mid elevations. Finally, 45 species are restricted to a single elevational level (Table 7). Of these, 17 species are exclusive to 0-750 m elevation, with the most abundant being Bolboneura sylphis beatrix, Hamadryas g. glauconome, and Hypna clytemnestra mexicana; 20 species are exclusive to mid elevations where Anthanassa argentea and Cyllopsis diazi comprised most of the records; and eight species are distributed only in the highest elevations where Adelpha bredowii, Nymphalis a. antiopa, and Phyciodes t. tharos were the most abundant.
Table 7 Exclusive species of each elevational level (m)
| Taxa | 0-750 | 750-1 800 | 1 800-2 850 |
| Taxa | 0-750 | 750-1 800 | 1 800-2 850 |
| Danainae | |||
| Lycorea ilione albescens | 9 | ||
| Danaus p. plexippus | 1 | ||
| Ithomiinae | |||
| Episcada salvinia portilla | 22 | ||
| Pteronymia c. cotytto | 9 | ||
| Charaxinae | |||
| Hypna clytemnestra mexicana | 159 | ||
| Memphis p. perenna | 33 | ||
| Memphis wellingi | 1 | ||
| Archaeoprepona phaedra ssp. | 2 | ||
| Prepona brooksiana ibarra | 2 | ||
| Morphinae | |||
| Caligo uranus | 1 | ||
| Opsiphanes quiteria quirinus | 1 | ||
| Opsiphanes t. tamarindi | 6 | ||
| Satyrinae | |||
| Oxeoschistus hilara ssp. | 5 | ||
| Oxeoschistus tauropolis ssp. | 2 | ||
| Cyllopsis clinas | 1 | ||
| Cyllopsis diazi | 124 | ||
| Cyllopsis h. hedemanni | 87 | ||
| Cyllopsis nayarit | 5 | ||
| Megisto rubricata pseudocleophes | 2 |
Diversity patterns by vegetation type: The vegetation type with the most observed Nymphalidae species is the CF (147 species); however, according to estimations the species richness in the TSDF, the CF, and the OPCF are not significantly different (Fig. 7). Interestingly, the OPCF has just 9 % of the abundance of both the TSDF and the CF. Below these three types, the TDF supports 47 % of the species in the region with 90 estimated taxa. The species richness in the OPF is much lower than the other vegetation types, with no rare species, so the total richness estimate is also low (six spp.). Estimations indicate that all vegetation types were adequately sampled. The difference between estimated and observed species is higher in the OPCF, suggesting either lower sampling effort or greater numbers of rare species in this vegetation type.
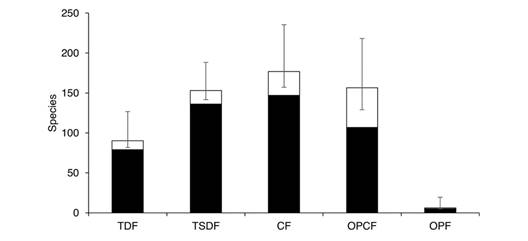
Fig. 7 Observed (black) and estimated (white) species richness by vegetation types of Nymphalidae in the Loxicha Region, Oaxaca, Mexico. TDF = tropical deciduous forest, TSDF = tropical sub-deciduous forest, CF = cloud forest (low- and mid-elevation), OPCF = oak-pine and high-elevation cloud forest, OPF = oak-pine forest.
Abundance patterns by vegetation type: The TSDF and the CF present the highest and most similar abundance of Nymphalidae, with 11 703 (43 %) and 10 903 (40 %) records, respectively. Abundance in other vegetation types is distinctly different, with just 3 496 records (13 %) in the TDF and 1 028 (4 %) in the OPCF. This family is even scarcer in the OPF, with less than 1 % of the overall abundance (47 records) in the Loxicha Region.
Dominant species in most vegetation types mainly belong to the Satyrinae. Dominance is low in most vegetation types, except in the OPF where Nymphalis a. antiopa (24 specimens) and Paramacera xicaque rubrosuffusa (17 specimens) comprise 87 % of the records. Paramacera xicaque rubrosuffusa is also the most abundant species in the OPCF with 15 % of the recorded specimens, followed closely by Anthanassa a. ardys with 14 % of the abundance in this vegetation. The most abundant species in the CF and the TSDF is also a member of the Satyrinae: Hermeuptychia hermes, with 12 and 7 % of the records, respectively. The TDF is the only vegetation type where Satyrinae are not dominant, but is also the vegetation where dominance is lowest, with Microtia e. elva and Hamadryas g. glauconome comprising only 9 % and 8 % of the records, respectively.
Most species (80 %) were collected in multiple vegetation types, although some have just one or two records in the vegetation at the highest or lowest elevations. The only species found in all five vegetation types was Smyrna blomfildia datis, with one record in the OPF. Thirty-seven species are exclusive to a vegetation type (Table 8), half of which are rare species with one or two records. Fig. 8, Fig. 9, Fig. 10, Fig. 11, and Fig. 12 show the elevational distribution by vegetation type of these 37 species (Table 8); all species with 95 % or more of their records in a single vegetation type were also included.
Table 8 Nymphalidae species exclusive to each vegetation type in the Loxicha Region, Oaxaca, Mexico
| Taxa | TDF | TSDF | CF | OPCF | OPF |
| Taxa | TDF | TSDF | CF | OPCF | OPF |
| Danainae | |||||
| Lycorea ilione albescens | 9 | ||||
| Danaus p. plexippus | 1 | ||||
| Ithomiinae | |||||
| Dircenna k. klugii | 134 | ||||
| Episcada salvinia portilla | 22 | ||||
| Pteronymia c. cotytto | 9 | ||||
| Charaxinae | |||||
| Memphis perenna perenna | 33 | ||||
| Memphis wellingi | 1 | ||||
| Archaeoprepona phaedra ssp. | 2 | ||||
| Prepona brooksiana ibarra | 2 | ||||
| Morphinae | |||||
| Caligo uranus | 1 | ||||
| Opsiphanes quiteria quirinus | 1 | ||||
| Opsiphanes t. tamarindi | 6 | ||||
| Satyrinae | |||||
| Oxeoschistus tauropolis ssp. | 2 | ||||
| Cyllopsis clinas | 1 | ||||
| Cyllopsis h. hedemanni | 87 | ||||
| Megisto rubricata pseudocleophes | 2 | ||||
| Taygetis virgilia | 1 | ||||
| Gyrocheilus p. patrobas | 4 | ||||
| Apaturinae | |||||
| Asterocampa idyja argus | 18 | ||||
| Biblidinae | |||||
| Marpesia zerynthia dentigera | 18 | ||||
| Myscelia c. cyananthe | 11 | ||||
| Bolboneura sylphis beatrix | 211 | ||||
| Pyrrhogyra edocla paradisea | 1 | ||||
| Limenitidinae | |||||
| Adelpha barnesia leucas | 3 | ||||
| Adelpha bredowii | 15 | ||||
| Adelpha diocles ssp. | 1 | ||||
| Nymphalinae | |||||
| Pycina zamba zelys | 4 | ||||
| Smyrna karwinskii | 2 | ||||
| Hypanartia trimaculata autumna | 42 | ||||
| Nymphalis a. antiopa | 24 | ||||
| Vanessa cardui | 1 | ||||
| Junonia genoveva ssp. nov. | 5 | ||||
| Chlosyne cyneas cynisca | 1 | ||||
| Phyciodes pallescens | 1 | ||||
| Phyciodes p. phaon | 24 | ||||
| Phyciodes t. tharos | 19 | ||||
| Anthanassa texana texana | 8 | ||||
| Heliconiinae | |||||
| Dryadula phaetusa | 4 | ||||
| Heliconius ismenius telchinia | 1 |
Vegetation types: TDF = tropical deciduous forest, TSDF = tropical sub-deciduous forest, CF = cloud forest (low- and mid-elevation), OPCF = oak-pine and high-elevation cloud forest, OPF = oak-pine forest. For each species listed, the habitat preference value is 90-100 %.
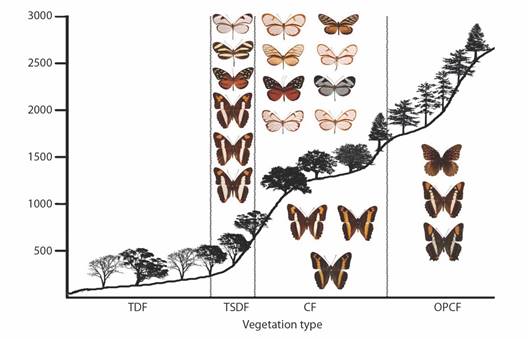
Fig. 8 Elevational profiles and characteristic species of Limenitidinae, Danainae, and Ithomiinae for each vegetation type in the Loxicha Region, Oaxaca, Mexico. TDF = tropical deciduous forest, TSDF = tropical sub-deciduous forest, CF = cloud forest (low- and mid-elevation), OPCF = oak-pine and high-elevation cloud forest.
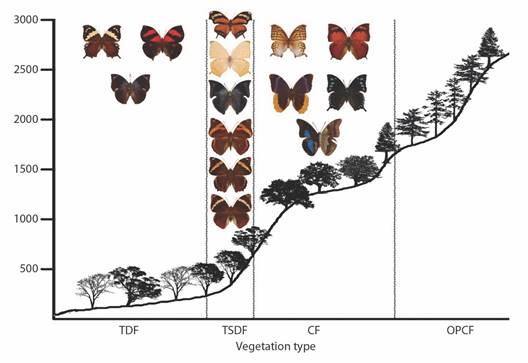
Fig. 9 Elevational profiles and characteristic species of Charaxinae and Morphinae for each vegetation type in the Loxicha Region, Oaxaca, Mexico. TDF = tropical deciduous forest, TSDF = tropical sub-deciduous forest, CF = cloud forest (low- and mid-elevation), OPCF = oak-pine and high-elevation cloud forest.
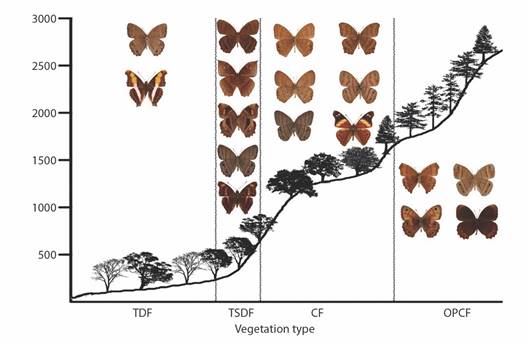
Fig. 10 Elevational profiles and characteristic species of Satyrinae and Apaturinae for each vegetation type in the Loxicha Region, Oaxaca, Mexico. TDF = tropical deciduous forest, TSDF = tropical sub-deciduous forest, CF = cloud forest (low- and mid-elevation), OPCF = oak-pine and high-elevation cloud forest.
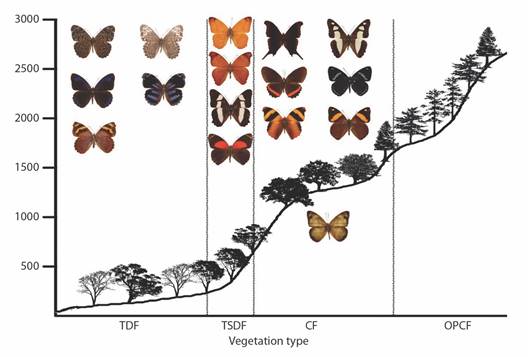
Fig. 11 Elevational profiles and characteristic species of Biblidinae for each vegetation type in the Loxicha Region, Oaxaca, Mexico. TDF = tropical deciduous forest, TSDF = tropical sub-deciduous forest, CF = cloud forest (low- and mid-elevation), OPCF = oak-pine and high-elevation cloud forest.
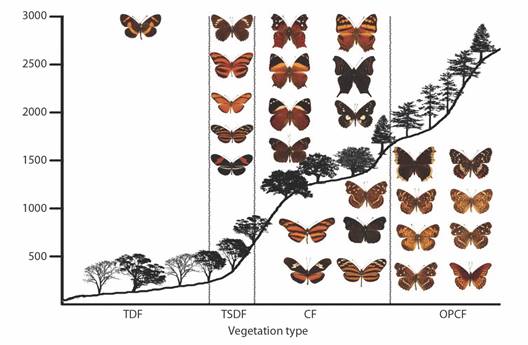
Fig. 12 Elevational profiles and characteristic species of Nymphalinae and Heliconiinae for each vegetation type in the Loxicha Region, Oaxaca, Mexico. TDF = tropical deciduous forest, TSDF = tropical sub-deciduous forest, CF = cloud forest (low- and mid-elevation), OPCF = oak-pine and high-elevation cloud forest.
Efficiency and efficacy of Van Someren-Rydon traps: During our fieldwork, 94 species of seven subfamilies of Nymphalidae were collected with Van Someren-Rydon traps (Appendix 2). These taxa represent 50 % of the species recorded and 41 % of the specimens. Some species were captured with both traps and aerial nets, but abundance was higher in traps (11 543; 73 %). Only 18 species (173 specimens) were collected exclusively in traps. Biblidinae was the subfamily with most species recorded in traps with 24 spp., followed by Satyrinae and Charaxinae with 23 and 21 species, respectively; these three subfamilies account for 74 % of the specimens collected in traps (Fig. 13). In terms of abundance, Satyrinae was the subfamily with the most records (40 % of the specimens), followed by Nymphalinae (21 %). Comparing the relative abundance and species richness solely of trapped specimens for each subfamily, two patterns can be recognized: A. Satyrinae and Nymphalinae had greater abundance than richness; B. Charaxinae, Biblidinae, and Morphinae presented a relatively higher richness than specimens, but still lower abundance than subfamilies in pattern A (Fig. 13).

Fig. 13 Relative abundance (black) and species richness (grey) of seven subfamilies of Nymphalidae collected in Van Someren-Rydon traps in the Loxicha Region, Oaxaca, Mexico.
The efficiency and efficacy of the traps at each elevational level (0-750 m, 750-1 800 m, and 1 800-2 850 m) were evaluated based on the species and specimens captured relative to the totals. The percentage of specimens collected with traps decreased at higher elevations (Appendix 2; Fig. 14), and this was more evident for Apaturinae and Nymphalinae. However, traps reduce to almost 40 % of their efficiency at the higher elevations. On the other hand, efficacy measured as the % of recorded species of each subfamily, remained constant along the elevational gradient. Traps captured 100 % of the species of Charaxinae and Morphinae at all levels (Fig. 15). Efficacy was lower at higher elevations for Apaturinae and Biblidinae, and slightly lower for Satyrinae. Finally, Van Someren-Rydon traps are less effective in capturing species of Nymphalinae, since percentages are lower than 40 % in all elevational levels, although this efficacy stays constant along the gradient. Of note, 16 species of Limenitidinae were recorded in the Loxicha Region but only two were collected in traps, with an efficiency of 19 specimens out of the 298 collected of those species: Adelpha basiloides (5/227) and Adelpha i. iphiclus (14/71).
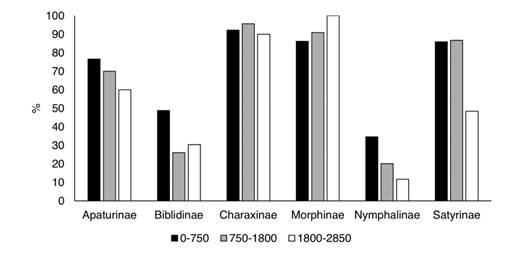
Fig. 14 Percentage of specimens of each Nymphalidae subfamily captured in Van Someren-Rydon traps by elevational level, relative to all specimens captured in that level, in the Loxicha Region, Oaxaca, Mexico.
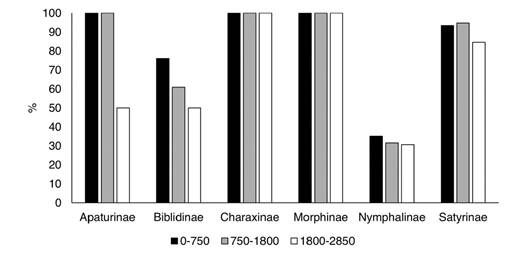
Fig. 15 Percentage of species of each Nymphalidae subfamily recorded in Van Someren-Rydon traps by elevational level, relative to all species recorded in that level, in the Loxicha Region, Oaxaca, Mexico.
Natural history remarks on selected taxa of the Loxicha Region: During our fieldwork, some nymphalid species were particularly abundant or showed special behaviors. We summarize that natural history data for each relevant species below.
Hypna clytemnestra mexicana. Found in the understory of the TDF, mainly in humid and dark microhabitats. Easily collected with traps in two sites: Parque Nacional Huatulco, and Río Cacaluta within Parque Nacional Huatulco. More abundant in the rainy season (July-November).
Consul excellens genini. Recorded and collected in OPF, mostly on humid cliffs where elements of CF are present. Attracted to fermented fruit. Recorded mainly in San José del Pacífico.
Chlosyne gaudialis wellingi. Flight faster than other species of this genus, and flies unusually high in search of flowers three to four meters above ground level, in sunny open places. Unusually low abundance relative to other species of Chlosyne, which tend to be very abundant.
Pycina zamba zelys. In Mexico, this taxon has been recorded at just a few sites and with low abundance. Only four specimens were collected, the first records for the Pacific slope. All specimens were collected on a wet wall along a road where a stream formed puddles. Many other species were also collected in this microhabitat: Pycina zamba zelys specimens were licking the wet wall five or six meters above ground level, as were specimens of Hypanartia dione disjuncta, H. godmanii, H. lethe, H. trimaculata autumna, Historis a. acheronta, Epiphile adrasta escalantei, Diaethria anna mixteca, D. astala asteroide, Marpesia chiron marius, Siproeta e. epaphus, and S. stelenes biplagiata, among other common taxa that exploit this special microhabitat.
Altinote stratonice oaxaca. This species is associated with low elevation CF from 1 000-2 500 m, making large elevational migrations at the end of the summer. Present in open spaces, with a slow flight, visiting bushes with white composite flowers.
Archaeoprepona demophoon mexicana. An elusive species, mainly occurring in the forest canopy but sometimes in the understory associated with open spaces like roads or riverbanks. Along rivers it can be found feeding on minerals dissolved in wet sand and shows similar behavior along roads with some humidity on the ground. Feeds mostly on decomposing fruit and exudates of certain trees. Rapid fliers that quickly flee when disturbed, often into the forest canopy. Males are territorial and chase away other males.
Bolboneura sylphis beatrix. Mostly alongside roads or streams. If near rivers, it is likely to be found on moist sand feeding on dissolved minerals; if along roads, likely feeding on small flowers. Flight is slow and halting, generally about 50 cm above ground level. Groups of more than 10 specimens can be found alongside streams. When resting usually perches on leaves. Recorded only in TDF.
Heliconius erato cruentus. Found mainly in open spaces and along roads and small paths. Flight slow and halting, always less than one meter above ground level. Often feeds at roadside flowers, but sometimes also attracted to decomposing fruit. Gregarious, forming groups to overnight in protected places.
Microtia elva elva. Frequently found along open roads with some shade. Flight low and halting, at most 50 cm above ground level. Feeds mostly on small composite flowers along roadsides. When disturbed, usually flies just a few meters away.
Discussion
More than 60 % of Mexico's surface is covered by mountains, but faunal surveys of Lepidoptera across elevational gradients are scarce, except for those done by members of the MZFC (Arellano-Covarrubias et al., 2018; Luis-Martínez & Llorente-Bousquets, 1990; Luis-Martínez et al., 1991; Luis-Martínez et al., 2020; Vargas-Fernández et al., 1994; Vargas-Fernández et al., 1999). This type of analysis provides important insight into various biological processes. Such insights include the recognition of disjunct distributions across mountain archipelagos due to speciation and endemism processes, and the ability to detect changes of species distribution due to climate change, especially in stenotopic species vulnerable to extinction.
Of all elevational gradients in Mexico, the Loxicha Region is the most systematically sampled area for Papilionoidea sensu lato, particularly for the Nymphalidae (Table 2). Many experienced collectors have sampled this region for decades (Álvarez-García et al., 2016; Arellano-Covarrubias et al., 2018; Luis-Martínez et al., 2000; Luis-Martínez et al., 2003; Luis-Martínez et al., 2020; Michán et al., 2004; Pozo et al., 2008). Estimates of Nymphalidae species richness in the Loxicha Region show that it has the highest percentage of inventory completeness for any region in Mexico (Vargas-Fernández et al., 1994; Vargas-Fernández et al., 1999). Furthermore, it has the highest species richness of any region on the Pacific slope (Table 1 and Table 3) and the second richest in the country, behind only the Sierra de Juárez (Table 3).
Sampling in the Loxicha Region began in 1960, based on historical specimens that we checked and on publications that described a dozen taxa from those specimens. However, even with Loxicha's great species richness and endemism, the present work is the first systematic study that provides a detailed regional description of the diversity and distributional patterns of Nymphalidae, mirroring similar works published previously for Riodinidae (Arellano-Covarrubias et al., 2018), Papilionidae, and Pieridae (Luis-Martínez et al., 2020).
Species richness of Nymphalidae by site: Comparison of the diversity on both the Pacific and Atlantic slopes confirms that the latter has higher species richness (v. gr.Andresen, 2008; Ceballos, 1995; Flores-Contreras & Luna-Reyes, 2017; González-Ramírez et al., 2017; Luis-Martínez et al. 2003; Salinas-Gutiérrez et al. 2004). Of sites with 100 or more species of Nymphalidae, only 20 % are on the Pacific slope. Moreover, among these Pacific slope “sites'' are Acahuizotla, Guerrero and Candelaria Loxicha, Oaxaca, which are larger areas that historically encompassed a group of sites with distinct elevations and vegetation types but labeled under a single site name. These areas were used as reference points for collectors for three decades (1950-1980) of the 20th century. Therefore, actual species richness of these reference sites is lower than cited, further reducing to 15 % the Pacific slope sites with over 100 species. In the last 40 years, more than 10 faunistic studies on Papilionoidea were done on the Pacific slope by members of the MZFC (Llorente-Bousquets et al., 1996; Llorente-Bousquets et al., 2004; Luis-Martínez & Llorente-Bousquets, 1993; Luis-Martínez, 1997; Luis-Martínez, 1999; Luis-Martínez, 2001; Vargas-Fernández et al., 1994; Vargas-Fernández et al., 1996; Vargas-Fernández et al., 1999; Warren & Llorente-Bousquets, 1999; Warren et al., 1996; Warren et al.,1998), but only six sites exceed 100 species. Of these, only four are in Sierra Madre del Sur (two in the Sierra de Atoyac and two in the Loxicha Region), which is the mountain range with the greatest diversity on the Pacific slope despite lacking tropical evergreen forest vegetation. San Jerónimo, Tacaná, Chiapas is the only Mexican site with more than 100 species that lacks a systematic faunistic study; data from this site were obtained from national and international specimen collections (MARIPOSA database).
Species composition similarity across regions: The similarity analysis reflects how Lepidoptera are associated with mountain archipelagos, as described by Llorente-Bousquets (1984) and Halffter (1987). In Mexico, the states of Guerrero and Oaxaca support the most faunistically similar mountain habitat “islands'' (Llorente-Bousquets, 1984). These two isolated regions each have exclusive or endemic species, yet they share at least 50 % of their species, forming geographic and genealogic relations between them. Other areas are disjunct, each with its own biogeographic history based on patterns of biotic provinces defined by Morrone et al. (2002) and Morrone (2005). The distinction between the diurnal Lepidoptera on the Atlantic and Pacific slopes had been further revealed using panbiogeographic methods (Llorente-Bousquets, Trujano-Ortega et al., 2006; Luis-Martínez et al., 2006; Oñate-Ocaña et al., 2006; Vargas-Fernández et al., 2006).
The faunistic similarity of Nymphalidae shows a division in the Pacific slope and SMS in two parts, a Northwest section (Nayarit, Jalisco, Michoacán) and a Southeast section (Guerrero, Oaxaca), with the latter including the Loxicha Region. This division of the SMS in two subprovinces was proposed by Santiago-Alvarado et al. (2016) based on data from 32 species endemic to the SMS. No distribution was consistent with the SMS as a whole. Later, Morrone (2017) named and described three subprovinces in the SMS: the West subprovince with the Jalisciense and Jalisciense-Manantlán districts, the Central subprovince with the Michoacán district, and the Oriental subprovince comprising the Guerrerense and Tierras Altas de Oaxaca districts. Although Nymphalidae data do not reflect the separation of these three subprovinces, when analyzing the data at a site-level scale, Nymphalidae of the Southwest SMS are shown to be more similar to the Balsas province fauna than to the fauna of the Northeast SMS.
Species composition similarity of sites: Diurnal Lepidoptera associated with the CF at all elevational levels are an excellent model for the study of complex dispersion, vicariance, and speciation patterns, due to the disjunct distribution and isolation of this vegetation community (Rzedowski, 1978). Generally, CF fauna distribution patterns are consistent with the geographic barriers that delimit each of the mountain ranges of Mexico. An integrated phylogeographic analysis that included birds, mammals, and plants concluded that the evolution of the biota of the CF in Mesoamerica resulted from a complex combination of distinct histories of range expansion, isolation, and biotic differentiation (Ornelas et al., 2013). A similar process seems to have occurred with the Papilionoidea sensu lato and particularly with Nymphalidae, based on distribution patterns resolved by multiple studies (Llorente-Bousquets, 1984; Llorente-Bousquets & Escalante, 1994; Llorente-Bousquets & Luis-Martínez, 1998).
Nymphalidae assemblages are closely related to vegetation type and elevation but are also influenced by geographic history and distance. Sites are grouped in six sets according to their similarity (Fig. 4). The first one corresponds to the Atlantic slope, while the other subgroups represent the diverse faunas of the Pacific slope distributed in distinct physiographic units and biotic provinces. These results concur with the data published in faunistic studies and generic reviews, describing the distribution of fauna in three elevational levels of the CF based on the proposal of Llorente-Bousquets (1984). Sites on the Pacific slopes (Fig. 4, subgroups 3 to 6) are grouped similarly to the elevational gradients (Fig. 3). The Sierra Madre del Sur (Guerrero and Oaxaca portion) (Fig. 4, subgroup 5) is more similar to the Cuenca del Balsas (Fig. 4, subgroup 6), than to the sites in the Costa del Pacífico, Northern Sierra Madre del Sur, and Western Eje Neovolcánico provinces (Fig. 4, subgroup 4). Notably, the sites above 1 800 m in the Sierra de Atoyac de Álvarez (Fig. 4, subgroup 3, La Golondrina, El Iris, and Puerto del Gallo) are separated from subgroups 4 and 6, and from the sites above 1 800 m in the Loxicha Region (Puente Arroyo “El Guajolote'' and San José del Pacífico, 1 km S), even though these two regions are geographically close. Puente Arroyo “El Guajolote'' is part of the subgroup of low elevation Guerrero-Oaxaca sites below 900 m, while San José del Pacífico is separated from other sites of the Sierra Madre del Sur, placed near the sites of the Cuenca del Balsas, as the most distinct site of this group. These similarity patterns of the high-elevation sites of Atoyac and Loxicha suggest that these are isolated communities, with a distinctive fauna, different from nearby low-elevation sites as well as from other high-elevation sites.
In general, the faunistic composition of Nymphalidae agrees with the biogeographic history of Mexico and its proposed provinces (Morrone, 2005; Morrone et al., 2002). First, there is a clear distinction between the faunas of the Atlantic and the Pacific. At a finer scale, a division of the Sierra Madre del Sur into a Northern and Southern portion was recognized. Within the Southern portion of SMS, sites are grouped by elevation and vegetation type, with high elevation sites separated from the rest. Therefore, distribution patterns of Nymphalidae in Mexico are produced at large scales by historical elements and at smaller scales by ecological elements.
Phenology: In the Loxicha Region, the temporal distribution of Nymphalidae species richness and abundance is correlated directly with the summer-associated rainy season (Fig. 5), which is common in most of the regions in Mexico (Álvarez-García et al., 2016; Luis-Martínez & Llorente-Bousquets, 1990; Luis-Martínez & Llorente-Bousquets, 1993; Luis-Martínez et al., 1991; Vargas-Fernández et al., 1994; Vargas-Fernández et al., 1999). In the Loxicha Region, nymphalid species richness and abundance are higher from July to October. This pattern is echoed in the Sierra de Juárez (Oaxaca), the Sierra de Atoyac (Guerrero), the Sierra de Manantlán (Jalisco-Colima), and the Sierra de San Juan (Nayarit), although the month with the highest species richness differs among the regions. In Loxicha, October had the highest richness. In comparison, September is the month with most species recorded in the Sierras de Juárez, Manantlán, and San Juan, while in the Sierra de Atoyac most species were recorded in July. As rainfall decreases so too does species richness, with April and May being both the driest months and having among the lowest species richness in all regions.
Diversity and abundance by vegetation type: Stenoic species of Nymphalidae have a wider distribution across different vegetation types (Table 8) than the stenoic species of Papilionidae, Pieridae, and Riodinidae, which mostly occur in the TSDF and CF (Arellano-Covarrubias et al., 2018; Luis-Martínez et al., 2020). The elevational profiles (Fig. 8, Fig. 9, Fig. 10, Fig. 11, and Fig. 12) show that the stenoic species of both Satyrinae and Nymphalinae are characteristic of each vegetation type. In contrast, Charaxinae and Biblidinae lack stenoic species in the OPCF but are represented in the lower-elevation vegetation types. The TDF is the vegetation type with the fewest stenoic species, with only 11 characteristic species of four subfamilies: Charaxinae, 3 spp.; Satyrinae, 1 sp.; Apaturinae, 1 sp.; Biblidinae, 5 spp.; and Nymphalinae, 1 sp. Comparing with other families, Riodinidae has a single characteristic species of the TDF (Arellano-Covarrubias et al., 2018). In Papilionidae only the TSDF has a significant number of stenoic species with five of the 10 species recorded, while the number of stenoic species of Pieridae does not differ among vegetation types (Luis-Martínez et al., 2020).
The observed species richness and abundance of Nymphalidae show that the OPCF is the most diverse vegetation type. Although the estimated species richness for the OPCF is not significantly different from that of the CF and the TSDF, the OPCF abundance is lower, with nine exclusive species. Despite being originally considered a combination of two vegetation types (Luis-Martínez et al., 2020), these nymphalid results support the recognition of the OPCF as a distinct ecological unit. We suggest the analysis of other taxonomic groups to further characterize this vegetation type. In comparison, the CF presented the highest number of exclusive species with 16 taxa, although the strong dominance of some species decreases its diversity to a value lower than that of the OPCF. Considering the diversity and exclusive species of Nymphalidae that they support, we recommend that the OPCF, CF, and the TSDF be considered conservation priorities.
Van Someren-Rydon traps: Fruit-feeding butterflies constitute approximately 50 % of all Nymphalidae species (Santos et al., 2011). Similarly, other authors have reported 40-55 % of fruit-feeding Nymphalidae species from tropical forests, with some variation in the composition among the subfamilies (Daily & Ehrlich, 1995; DeVries & Walla, 2001; DeVries et al., 1999; Freire-Jr. et al., 2021a; Pinheiro & Ortiz, 1992; Uehara-Prado et al., 2004; Vargas et al., 1994; Vargas et al., 1999). In the last two decades, this guild has been used in analyses of forest fragmentation, conservation, monitoring, community structure, and ecology, because these species are more easily sampled (Freire Jr., Oliveira, et al., 2021a; Freitas et al., 2003; González-Valdivia et al., 2016; Pozo, 2006; Pozo et al., 2005; Pozo et al., 2009). The growing importance of this guild underscores the relevance of the Van Someren-Rydon trap efficiency and efficacy data presented here.
During our fieldwork, all species of Charaxinae, Morphinae, and Apaturinae were collected in traps, along with most species of Satyrinae (92 %) and Biblidinae (77 %). In contrast, only two species of Limenitidinae were collected in traps (13 %). Apparently, Limenitidinae are only occasional visitors to traps, probably looking for water as some Ithomiinae also do, since neither of these two subfamilies belong to the fruit-feeding guild (Meave del Castillo & Luis-Martínez, 2000). Traps are especially useful for collecting Charaxinae because these species frequent open spaces above the forest canopy, while Satyrinae frequent the understory less than one meter above ground (Freire Jr., Ribeiro, et al., 2021). Across all Nymphalidae, 19 % of the taxa were collected exclusively with this technique, and for 69 % of the species documented, 75 % of the specimens were trapped. We thus emphasize that Van Someren-Rydon traps are critical for accurately documenting the species richness, abundance, and elevational and vegetational distributions of Nymphalidae.
Ethical statement: The authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

