Introduction
Globalization through increased trade, transport, travel, and tourism will inevitably increase the intentional or accidental introduction of organisms to new environments (CABI, 2020) and is still steadily growing (Hulme et al., 2008; van Kleunen, Schlaepfer, Glaettli, & Fischer, 2011). These introductions occasionally result in alien species’ invasions (Lockwood, Hoopes, & Marchetti, 2007). Invasive species have established populations outside their native distribution ranges and can spread and affect native ecosystems (Lockwood et al., 2007). These species can trigger ecological imbalances, trophic structure changes, native species displacements, biodiversity loss, genetic diversity reduction of native species, and non-native infectious agents’ transport (Cassemiro, Bailly, da Graça, & Agostinho, 2018; Hobbs, 2000). Thus, the introduction and later invasion of non-native species represent one of the leading causes of biodiversity loss in the world (Cattau, Martin, & Kitchens, 2010). The biggest problem associated with the introduced species is that they have been already established and extended when they are recognized as invasive, making it almost impossible to eliminate or control them (Sato et al., 2010). In some cases, native species declines often co-occur and in the same place as invasion by non-native species, leading many conservationists and researchers to believe that invasions and extinctions are closely linked (Gurevitch & Padilla, 2004). For that reason, a prime objective of invasion biology is predicting which species are likely to become invaders and where they are likely to invade even before introduction outside their native range, which represents a goal of invasion ecologists (Fournier, Penone, Pennino, & Courchamp, 2019).
The ecological niche modeling (ENM) use associations between environmental variables and occurrence locations of the species to predict the potential presence of the species in areas that have those ideal environmental conditions or that are appropriate for the survival of the species (Raxworthy et al., 2003; Raxworthy, Ingram, Rabibisoa, & Pearson, 2007). This modeling has many applications, such as understanding species’ ideal environmental conditions, predicting the existence of unknown species, inferring aspects of the biogeography of species, planning conservation areas, and as in this case, evaluating the potential areas of invasion of species (Peterson, 2006). In this way, ENM tools generates distribution maps of species, which are usefull for designing and leading strategies about control and mitigation of ecological problems generated by invasion processes, such as establishment, spread, and impact (Jiménez-Valverde et al., 2011). The invasion of species can be a highly predictable process if one knows the environmental conditions in the native range (Peterson, 2003; Jiménez-Valverde et al., 2011). The invasive species’ geographic potential elaborated with ENM techniques is usually constructed assuming evolutionary conservatism in ecological niche characteristics. This means that species will follow the same set of ecological rules on invaded distributional areas as they do on their native distributional areas (Peterson, 2006); however, we should take this assumption with caution because both biotic and abiotic factors are different in native and non-native regions (Lockwood et al., 2007). In recent years, evidence that changes between the native and non-native niche exist in fish and other organisms have been found (Lauzeral et al., 2011). However, the ENM allows valid estimates of the geographical distribution of invasive species or their invasiveness (Yiwen, Bi Wei, & Darren, 2016; Srivastava, Lafond, & Griess, 2019).
Pangasianodon hypophthalmus (Sauvage, 1878) family Pangasiidae (Siluriformes) is native to the Mekong and Chao Phraya rivers basin in Southeast Asia. It carries out long-distance movements during hundreds of kilometers between ecosystems in high areas and spawning habitats. This species uses floodplain lands to feed and breed (FAO, 2010; IAvH, s.f.; Tarkan et al., 2020). A female in suitable conditions can produce around 100 000 eggs per kilogram in each oviposition, and its demersal embryos suggest some parental care (e.g., nests on a hard substrate). These hatch in 24 hours, and the larvae drift in favor of the floods, searching for growth zones within flooded areas. Its larvae have an omnivorous diet that includes algae, seeds, crustaceans, zooplankton, and fish (Van Zalinge, Lieng, Ngor, Heng, & Valbo-Jørgensen, 2002). As an adult, it is carnivorous and even cannibalistic. The species has been reported as a predator of native species in some places of Asia (Pallewatta, Reaser, & Gutierrez, 2003).
Combining the aforementioned biological characteristics and the escape and introduction of exemplary species in places other than their natural distribution justify their classification as an invasive species or a potentially invasive species (Gutiérrez, Lasso, Baptiste, Sánchez-Duarte, & Díaz, 2012; Garcia et al., 2018). Its broad food spectrum, the ease with which it gains weight, and its fast growth have made it possible to introduce this species with productive purposes in different countries such as Cuba, Chile, Colombia, Guatemala, México, the United States, Indonesia, India, Turkey, Puerto Rico, and The Dominican Republican (IAvH, s.f.). However, fish farming of P. hypophthalmus presents problems such as the spreading of pathogens that are difficult to control (Bigarré et al., 2009; Lakra & Singh, 2010; Mitra, Bandyopadhyay, Gong, Goswami, & Bhowmik, 2013), and increasing cost for its production (Lakra & Singh, 2010; Singh & Lakra, 2011).
In Colombia, the species started to be used as an ornamental fish, and in 2008 its meat began to be imported for national consumption in frozen fillets. Currently, it is sold in fresh fish marketplaces (Valderrama, Mojica, Villalba, & Avila, 2016). Pangasianodon hypophthalmus farming has been reported in at least five states of the country: Valle del Cauca, Cauca, Huila, Meta and Santander (Gutiérrez et al., 2012). Since 2015, live specimens have been captured by fishermen in the aquatic ecosystems of Magdalena River, in floodplains of the lower zone, and in main riverbeds and tributary rivers (Valderrama et al., 2016). However, the distribution of this species in Colombia is little-known, and scientists have barely explored the effects on the native fauna. In the Caribbean sub-basins such as the Magdalena, Atrato, Sinú, and Catatumbo rivers, there are other siluriform species that, like P. hypophthalmus, carry out long-distance migrations between small tributaries and floodplains, have carnivorous habits, and use floodplains as breeding and growth areas. Among these species are Pseudoplatystoma magdaleniatum, Sorubim cuspicaudus, and Ageneiosus pardalis, which are of high importance for commercial and artisanal fishing and are under a critical conservation status (P. magdaleniatum) and vulnerable (S. cuspicaudus and A. pardalis) (Mojica, Usma, Álvarez-León, & Lasso, 2012). In these sub-basins, only the Magdalena river basin contributes 70 % of the gross domestic product, and 80 % of the population lives in its territory (The Nature Conservancy, Fundación Alma, Fundación Humedales, & AUNAP, 2016). As a result, this basin presents several environmental conflicts in its aquatic ecosystems (Barletta et al., 2010; Galvis & Mojica, 2007; Jiménez-Segura et al., 2016). Although habitat destruction and human pressures are major causes of this conservation status, potential competitive interactions could occur with introduced species, which might worsen the conservation state of native species. For this reason, our objective was to evaluate the invasive potential of P. hypophthalmus in some of the basins of the Caribbean Sea watershed (1) making a comparison between the native niche and the niche of introduction in the Caribe watershed, (2) transferring the ENM calibrated with the native area of this species in Colombia to calculate the possible area of invasion and (3) comparing the geographical distribution of P. hypophthalmus with the distribution of three native species (P. magdaleniatum, S. cuspicaudus, and A. pardalis) present in rivers of the Caribbean basin.
Materials and methods
Study area: We focused on 5 sub-basins of Caribbean Basin: Magdalena-Cauca, Atrato, Sinú, and Catatumbo. In the Caribbean basin located North of the Andes, 326 fish species were reported, from which 66 % were endemic (DoNascimiento et al., 2017). Of these endemic species, 35 were reported based on specimens captured by artisanal fishermen (Jiménez-Segura, Gutierrez, Ajiaco-Martínez, & Lasso, 2020a); 19 of them have migratory behaviors and move seasonally among low areas and the Magdalena, Atrato, Sinú and Ranchería river tributaries (Jiménez-Segura et al., 2016; López-Casas, Jiménez-Segura, Agostinho, & Pérez, 2016).
Data of presence: The georeferenced records of the wildlife of P. hypophthalmus both in the native and introduced ranges and of P. magdaleniatum, S. cuspicaudus, and A. pardalis were compiled from the review of scientific articles, reports of field observations, and databases available online as Freshwater Biodiversity Data Portal (Biofresh, 2012), the Global Biodiversity Information Facility (GBIF, 2019; GBIF, 2020a; GBIF, 2020b; GBIF, 2020c) and the platform InvBasa through SIB Colombia (InvBasa UN, 2020; SiB Colombia, 2020). Databases were reviewed and curated, applying verification procedures of duplicate records or taxonomic uncertainty (Chapman, 2005). Records that did not coincide with water sources were relocated to the nearest pixel (Domisch, Wilson, & Jetz, 2016).
Environmental data: We selected 70 environmental variables from the freshwater systems that have a relationship with the analyzed species’ biology (for example, flow, substrate type, and surrounding vegetation (Nori & Rojas-Soto, 2019). The variables are available on the EarthEnv website (Domisch, Amatulli, & Jetz, 2015) and have a 1 km resolution global coverage. This dataset includes earth covers, climatic and topographic variables, and soil characteristics. Besides, we included a flow layer average for the periods 1960-2015 (Barbarossa et al., 2018), which is available globally at a resolution of 1 km (Digital Appendix 1).
The accessible area or mobility (M) can condition the models’ evaluation methods and their accuracy (Barve et al., 2011). Therefore, we defined each species-area by making an intersection between each species’ presence records with a map of watersheds HydroBASINS 1.0 Level 6 (Lehner & Grill, 2013). To evaluate the range of possible invasion of P. hypophthalmus in Colombia, we selected the largest M within the native species (A. pardalis) (Digital Appendix 2). All variables were trimmed with the M defined using the raster package (Hijmans et al., 2015) in the program R (R Core Team, 2014).
Comparison between the native and the introduction niche of P. hypophthalmus: The niche comparisons were made by following Broennimann et al. (2012) proposal,which consisted of getting values of niche similarity in the environmental space using Schoener’s D similarity metric (Warren, Glor, & Turelli, 2008). This technique uses the first two axes of principal component analysis (PCA) that reduce the 71 environmental variables to know the environmental conditions occupied by P. hypophthalmus. We calculated the native niche stability proportion observed in the exotic niche and the expansion of the species’ new environments in the non-native niche. Subsequently, we tested for niche similarity by assessing random changes of the niches within available conditions in the study area, i.e., assessing if the niche of the species is more similar than expected by chance under a specific null model (Warren et al., 2008; Warren, Glor, & Turelli, 2010). We made all analyses using available tools in the Ecospat package (Di Cola et al., 2017) in the software R (R Core Team, 2014).
Modeling and projection of the ecological niche: We built the ENM for each species using 71 environmental variables and the Maximum Entropy algorithm (Maxent) (Phillips, Dudík, & Schapire, 2004; Phillips, Anderson, & Schapire, 2006) through the ENMeval package (Muscarella et al., 2014) in the R program (R Core Team, 2014). We selected Maxent because of its capability to mitigate redundant variables’ contributions (Elith et al., 2011; Feng, Park, Liang, Pandey, & Papes, 2019). Also, the algorithm has shown to remain stable both in the precision of the prediction and the total predicted area present using different categories of sample sizes (Phillips & Dudík, 2008; Wisz et al., 2008; West, Kumar, Brown, Stohlgren, & Bromberg, 2016).
In the construction of the P. hypophthalmus model, we used the Jackknife method (Pearson, Raxworthy, Nakamura, & Peterson, 2007) with 31 folds given the low number of obtained records, while for native species, we used the random kfold method, 10 000 background points by default, and the combination of feature classes (Linear, Quadratic, Linear-Quadratic, Hinge, Linear-Quadratic-Hinge) with ten regularization values (0.5 to 5, each 0.5). The best model for each species was selected, bearing in mind that the lowest ΔAIC (the best models taking into account the smallest number of parameters, Muscarella et al., 2014) and the logistic output were used for all models. Finally, we converted the suitability map to binary map using the 10P cut-off threshold value (tenth percentile) for the P. hypophthalmusand MTP (minimal training presence) threshold value for the native species.
Ecological niche overlap of native species with non-native species: We estimated geographical overlap between the P. hypophthalmus and each native species summing their binary maps of distribution in QGIS software (QGIS, 2020). Then, we calculated the number of pixels corresponding to the spatial overlap among areas of predicted distribution of each species.
Results
Data of presence: We analyzed a total of 31 records for the native area of P. hypophthalmus and 25 in the non-native area, 57 records for P. magdaleniatum, 93 for S. cuspicaudus, and 159 for A. pardalis (Digital Appendix 3, Digital Appendix 4, Digital Appendix 5, Digital Appendix 6).
Comparison between the native niche and introduction niche of P. hypophthalmus: For the comparisons between the introduction and the native niche, we obtained PCA that explains 53.36 % of the variance, with a Schoener’s D value of 0.125, an expansion of 24 %, and stability of 76 % (Fig. 1A). On the other hand, similarity tests show that native and introduced range niches are more similar to what is expected by chance (P = 0.047; Fig. 1B). Therefore, the niche of P. hypophthalmus has significantly conserved non-native area.
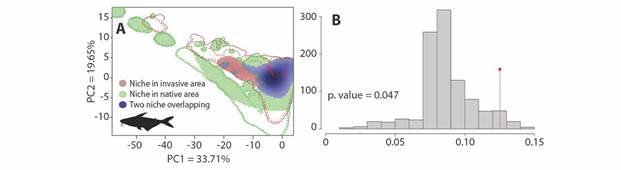
Fig. 1 Comparisons of the native and introduced niche of P. hypophthalmus. A. Test’s PCA results proposed by Broennimann et al. (2012). The shadow in the blue zone represents the species’ density, while the continuous and dotted red and green contours represent 100 % and 25 % respectively of the available environment in both zones. B. Niche similarity index histograms, the red bar the value of Schoener’s D. The histogram corresponds to the niche similarity of the non-native niche in the Colombian region.
Ecological niche modeling: P. hypophthalmus is widely distributed in the Asian tropical region in Laos, Thailand, Vietnam and Cambodia (Fig. 2A). The ecological niche model made for its natural distribution zone gives a probability value of the area under the curve (AUC) of 0.94, indicating that the model had a good performance. The selected variables provided a satisfactory prediction of the species distribution (Table 1).
Table 1 Selected model for each of the species
| Species | Feature classes | Regularization multiplier | AICc | AUCtrain | AUCtest | Number of parameters | Cut-off threshold |
| P. hypophthalmus | L | 4.5 | 619.92 | 0.9541 | 0.9426 | 9 | 10P |
| P. magdaleniatum | L | 2.5 | 1048.7 | 0.9445 | 0.9001 | 18 | MTP |
| S. cuspicaudus | H | 3.5 | 1765.6 | 0.9298 | 0.8989 | 24 | MTP |
| A. pardalis | LQH | 5 | 2842.3 | 0.0495 | 0.9389 | 24 | MTP |
L = Linear, H = Hinge, LQH = Linear-Quadratic-Hinge.
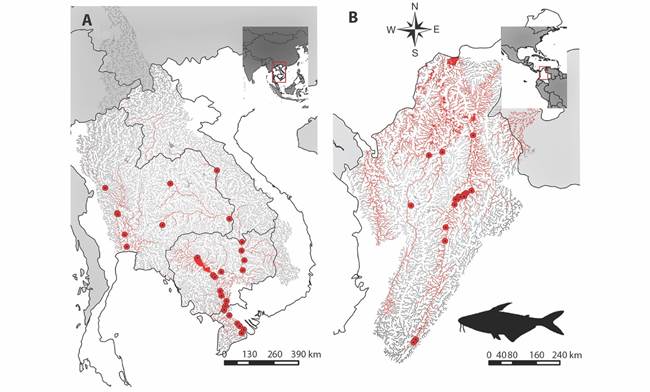
Fig. 2 Potential distribution of P. hypophthalmus represented in red lines. A. Its native area in Laos, Thailand, Vietnam and Cambodia. B. Its non-native area in Colombia. The red dots represent occurrences.
Seven variables contributed to obtaining the final model and predicting the geographic areas with environmental suitability characteristics for P. hypophthalmus in the basin of Mekong and Chao Phraya rivers (Digital Appendix 7). Among these variables, the first one is the elevation (elevation_01) and was the most influential factor in the model, followed by the percentage of thick fragments in the soil (fraction > 0.2 mm; soil_minimum_06) and the minimum percentage of the substrate type (R horizon or bedrock; soil_minimum_10). Therefore, based on the response curves and geographic representation (Digital Appendix 8), we identified that the species is located in lower areas of the basin where the composition of the riverbed is mainly small fragments (sands, clays, silts). Those conditions represent a total of 19 938 km2 of the basins of its native distribution. When we projected the model to Colombia (Fig. 2B), we found that the species can cover a potential area of 27 801 km2 of the Magdalena, Cauca, Atrato, and Catatumbo river basins, representing 48.26 % of the total extension of water bodies in the area (57 597 km2).
In native tree ecological niche models for native species, all AUC test values are greater than 0.898, indicating good performance (Table 1). The variable elevation (elevation_01) made the most significant contribution to the three models, placing the three species in lowland areas. The R horizon’s minimum percentage was the second most important variable for the S. cuspicaudus and P. magdaleniatum models, indicating that these species prefer areas with low percentages of rocky material or bedrock. Additionaly, the second most important variable was the coldest month in the case of the species A. pardalis. This indicates that higher values fo temperature (~ 20 °C) favored the presence of the species; however, a low percentage of R horizon values also influenced the final model (Digital Appendix 9, Digital Appendix 10, Digital Appendix 11, Digital Appendix 12, Digital Appendix 13, Digital Appendix 14).
Ecological niche overlap of native species with non-native species: The geographical overlap analysis shows that the P. hypophthalmus distribution area in Colombia covers 91.79 % of the suitable environmental zones of the A. pardalis; this means that P. hypophthalmus overlaps 17 400 km2 of the native species’ distribution (Fig. 3A, Fig. 3D). In the case of S. cuspicaudus species, we found an 82.17 % overlap where both species overlap in 20 045 km2 (Fig. 3B, Fig. 3E), while for P. magdaleniatum there is an overlap of 91.72 %, which is equivalent to 10 342 km2 (Fig. 3C, Fig. 3F).
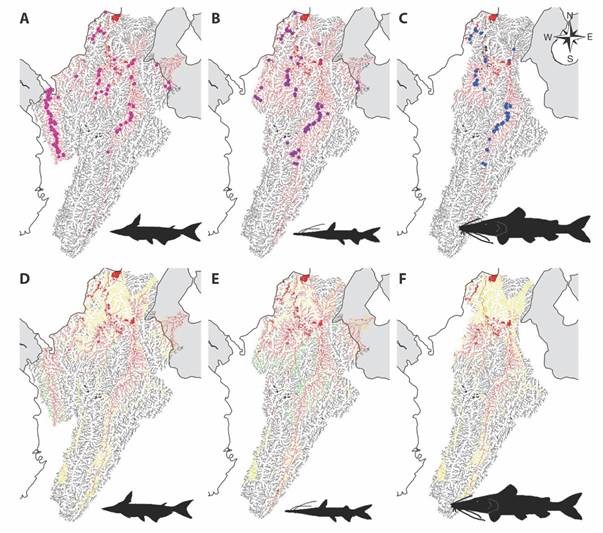
Fig. 3 Potential distribution of three native species represented in red lines: A. A. pardalis. B. S. cuspicaudus. C. P. magdaleniatum. And their areas of distribution overlapping the distribution of P. hypophthalmus: D. A. pardalis. E. S. cuspicaudus. F. P. magdaleniatum. The red color symbolizes the overlap between the native and non-native species; the yellow color is the area for the P. hypophthalmus species, and the green color the range of the native species.
Discussion
Our results suggest a climatic niche conservatism in P. hypophthalmus. It shows that this species is in similar climatic characteristics in both their native and invasive ranges. We also found that around 45 % of our study area is susceptible to invasion. These results have important implications because they are the first demonstration of a possible invasion range of P. hypophthalmus, and the risk that they represent for other species with their same behavior.
Environmental Niche Models can provide a realistic proxy about the geographic range of a fish species, and they can be used for several purposes, including conservation action (Valencia-Rodríguez et al., 2021). In biological invasions, niche models and their transfers to non-native geographical zones require caution in their interpretation (Owens et al., 2013). Thus, a fundamental assumption for allowing geographic predictability between native distribution areas and invaded areas is the niche conservatism analysis of the introduced species. According to this assumption, the species usually conserves its ancestral niche in the invaded regions (Wiens & Graham, 2005; Broennimann et al., 2007; Liu, Wolter, Xian, & Jeschke, 2020). On the one hand, we found that P. hypophthalmus shows conservatism of ecological niche; for that reason, we could transfer the calibrated ENM from the native to the non-native area with a confidence approximation of the possible invasion in our study area. On the other hand, we obtained an expansion estimation of the niche that could indicate that P. hypophthalmus occupies new habitats in Colombia that are not represented in its native range. These results would imply that the P. hypophthalmus fundamental niche is broader than the niche represented in their native area. By contrast the expansion detected may also be explained by the low numbers of records in its native zone, which would lead to incomplete representation of the niche; however, the records used to train the P. hypophthalmus model in its native area correspond with the known distribution area of the species (FAO, 2010).
The selection of environmental predictors is a fundamental step to produce ENMs; if predictors are biologically informative, they represent more realistic species-environment relationships (Parra, Graham, & Freile, 2004), and thus models are projected more accurately into novel environments. It has even been suggested that elevation, slope, precipitation, soil features, temperature, and vegetation, define the type of aquatic ecosystems (Baron et al., 2003). Therefore the variables selected to build our models estimate important environmental preferences of the four species analyzed. Altitude takes on particular importance because freshwater species are confined to water bodies with specific characteristics. The elevation is a direct conditioner of characteristics, such as flow, substrate, or vegetation (Nori & Rojas-Soto, 2019). Notably, basins with lower altitude values correspond with high values of suitability predictions, which represents topographic variability that facilitate migration and / or prevent dispersal process within the hydrographic network (Castillo-Torres, Martínez-Meyer, Córdova-Tapia, & Zambrano, 2017). Another important environmental variable that explained the potential distribution of the four species was the percentage of coarse fragments in the river bed. This variable is a factor that determines the nutritional and chemical conditions of the rivers, and it characterizes the structure and dynamics of the aquatic habitat (Baron et al., 2003). Followed by this, variables related to temperature, such as the minimum temperature of the coldest month, influenced the models, being associated directly with the organisms’ fitness and, by extension, determines the place where the species are distributed and how their community varies. Also, another variable selected was the precipitation seasonality which determines the flow pattern of rivers, lakes, and wetlands, which influence a large part of the habitat of freshwater species (Baron et al., 2003).
The presence of P. hypophthalmus in Colombia has been previously recorded from field observations (Valderrama et al., 2016). The individuals of P. hypophthalmus free in natural environments may be either by accidental escape from aquaculture or direct introductions from sport fishers or aquarists (Gutiérrez et al., 2012; Lasso et al., 2020). Consequently, in this study, the ENM allowed the identification of new areas suitable for the colonization of this species. The models indicated that the species’ niche conditions are widely represented in the Colombian inter Andean valleys. It corresponds with low areas with sediments and sedimentary rocks (fragments such as sand, clay, silt, and consolidations of said fragments, respectively; Jaramillo, 2002). The proposed models indicate that the colonization of P. hypophthalmus in Colombia is possible. Its survival is confirmed by present records of P. hypophthalmus in Magdalena-Cauca, Sinú, Atrato, San Jorge, Cesar, and Catatumbo rivers basins. The spread and colonization of the aquatic ecosystems in Colombian basins by the P. hypophthalmus could be possible because the species has a high tolerance to changes in environmental conditions, mainly the resistance to low oxygen levels, salinity, pH changes, temperature, and turbidity fluctuations (Singh & Lakra, 2011; Faruk, Patwary, & Hasan 2012; Garcia et al., 2018; Islam, Uddin, Uddin, & Shahjahan et al., 2019).
Pangasianoson hypophthalmus is a species that is easy to farm and popular among aquaculturists due to its resistance to water quality variations, fast growth, good survival rates, and economically attractive market size (Ali, Haque, & Belton, 2013; Islam et al., 2019). Given this scenario and its invasive potential, authorities in countries such as Mexico do not allow or encourage the introduction and cultivation in their territories (Mendoza-Alfaro, Luna-Peña, & Arias-Gámez, 2013; Delegación SADER Tamaulipas, 2017). Pangasianodon hypophthalmus has a great capacity for displacement due to its migratory nature and high fecundity. How we illustrate P. hypophthalmus presents life history characteristics similar to the native species analyzed here (Maldonado-Ocampo et al., 2005; Jiménez-Segura, Palacio, & López, 2009; Jiménez-Segura, Palacio, & Leite, 2010; Jiménez-Segura et al., 2020b). These assessments of species’ life features can offer rigorous scientific support for rapid classification of extinction or invasion risks and efforts to prevent invasion because of determining which species and sources of emerging invaders are worthy of scrutiny, more attention to management or policies must be applied (Liu, Comte, & Olden, 2017). Comparing the P. hypophthalmus niche with the native species indicates that the four species share environmental conditions that spatially converge in the evaluated basins’ lower and middle zones as high-water temperature and substrates with a high percentage of small fragments. (e.g. silts, clays). P. hypophthalmus is distributed almost entirely in the available space presented by the three native species, even though the modeled distribution range of P. hypophthalmus in Colombia is underestimated. Its convergence could trigger competition for space and food, and this species can even become a predator of juvenile and adult organisms of other species of fish consumed by native species (Gutiérrez et al., 2012; Raman et al., 2013). Competition and predation would cause mortality in native species and could even affect recruitment, and the result of this situation would be reductions in the genetic diversity of the native species (Raman et al., 2013).
The finding of conserved climatic niche for invasive species has major implications for predicting future invasion risks. Because it permits a model transferability using niche models. This kind of study represents a cost-effective strategy focused on identifyinging climatic conditions occupied in their native range but (not yet) colonized in their introduced range (Liu et al., 2020). We identify that P hypophthalmus present a niche conservatism, and for that reason we identify areas with a high risk of being invaded. That is why we call on environmental authorities because it is important to develop short-term actions to avoid this species’ dispersal. In this case, it is necessary to control its cultivation and use in aquariums. Although they may seem drastic measures, they should be considered by the Colombia’s environmental and fisheries authorities. Posing that the legalization of the use of the species is the only way to control its current illegal exploitation is not an appropriate justification since the control measures for fish farmers and aquarists have historically been non very effective. Eradication through hunting and the control prohibition of its eventual cultivation directly in rivers must be accompanied by educational programs to help consumers understand the impact non-native species have on aquatic biodiversity, and to encourage fish farmers to use good practices to reduce the impact of their activities on natural aquatic environments; also, invest more resources in research for developing fish culture with native species. This is an environmental decision; the precautionary principle should always prevail, in the absence of more robust information on the invasive capacity of P. hypophthalmus in Colombia’s river networks and on its real effect on a highly endemic aquatic biodiversity.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

