Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.60 suppl.2 San José Apr. 2012
Description of the Panamá and Iguanita mangrove stands of Bahía Culebra, North Pacific coast of Costa Rica
*Dirección para correspondencia
Abstract
Mangrove forests are abundant and important coastal marine ecosystems that are being impacted by human activity in Costa Rica. There are two mangrove stands (Panama and Iguanita) in Bahia Culebra, Guanacaste, North Pacific coast of Costa Rica. Their forest structure was determined with the Point-Centered Quarter Method (PCQM) during the dry season (December 2007-March 2008). Eleven transects were established at Panama mangrove, with a total of 52 points and 208 quadrats. Two transects were established at Iguanita with a total of 16 points and 62 quadrats given access difficulty. Mapping of both stands was done with two georeferenced MASTER CARTA 2005 images. Images were digitized to 1:5000 scale using the following categories: mangrove forest, low density mangrove, no mangrove, transition to dry forest, sand and water. In the area studied at Panama was 13.7ha, and 40.8ha for Iguanita. Panama is mostly composed of dense mangrove forest (51% of total study area) and dry forest species (35% of total study area). A small area (2%) had dry soil and scarce mangrove trees and the remaining 12% corresponds to water, sand and other areas without vegetation. At Iguanita, 84% was dense mangrove, 5% scarce mangrove trees and the remaining 10% corresponds to water, sand and other areas without vegetation. Five mangrove species were encountered at Panama (Avicennia germinans, Avicennia bicolor, Conocarpus erectus, Laguncularia racemosa, and Rhizophora mangle), and three at Iguanita (A. germinans, L. racemosa, and R. mangle). Species zonation was similar at both stands; with Rhizophora near water channels and inundated areas, Avicennia frequent in drier areas, and Laguncularia (both stands) and Conocarpus (only Panama) more frequent near fresh water input. Densities at both stands (Iguanita= 67.2 and Panama= 8.4 stems/0.1 ha) were lower than reported for the north Pacific of Costa Rica. Complexity index was higher at Iguanita (CI= 86.5) with R. mangle dominance, than Panama (CI= 1.1) with A. germinans dominance. While both stands are in Bahia Culebra, structurally they are very different and seem to be under two different hydrodynamic contexts. Sea level rise related to global climate change might impact both mangrove stands as they would not be able to migrate further inland (given land elevation at the back of Iguanita, and a paved road at Panama). Given the socio-economic and ecological importance of mangrove habitats, further study and continued conservation efforts of Costa Rican mangroves are needed.
Key words: Mangrove, forest structure, mapping, Eastern Tropical Pacific.
Resumen
Los manglares son abundantes e importantes ecosistemas marino-costeros en Costa Rica pero están siendo afectados por la actividad humana. Se analizo la estructura y cobertura de ambos manglares presentes en Bahía Culebra (Panamá e Iguanita), Guanacaste, Pacifico norte de Costa Rica. Se utilizo el PCQM para estructura durante la época seca entre diciembre 2007 y marzo 2008. Se utilizaron dos imágenes MASTER CARTA 2005 georreferenciadas para mapeo. El área aproximada de bosque de manglar en Panamá fue de 13.7ha; y de 40.8ha en Iguanita. Panamá contiene 51% de manglar denso en el área de estudio, 35% bosque seco, 2% sin vegetación y 12% de arena o agua. En Iguanita el 84% del área corresponde a manglar denso, 5% manglar de baja densidad y 10% sin cobertura vegetal o era arena o agua. Se hallaron cinco especies de manglar en Panamá (Avicennia germinans, Avicennia bicolor, Conocarpus erectus, Laguncularia racemosa y Rhizophora mangle); y tres en Iguanita (A. germinans, L. racemosa y R. mangle). En general, la presencia de las especies de manglar siguió un patrón similar en ambos manglares. La densidad total fue menor que en manglares cercanos; y Panamá (8.4tallos/0.1ha) mucho menor que Iguanita (67.2tallos/0.1 ha). El Índice de Complejidad (IC) fue mucho mayor en Iguanita (IC= 86.5), con dominancia de R. mangle, que en Panamá (IC= 1.1), con dominancia marcada de A. germinans. Estructuralmente ambos manglares son muy distintos entre sí y parecen encontrarse en contextos hidrodinámicos diferentes.
Palabras clave: manglares, estructura del bosque, cobertura, Bahía Culebra, Pacifico Tropical del Este.
Mangrove forests in the Eastern Pacific extend from the Gulf of California to the northern coast of Peru. While they are abundant on both coasts of the American continent, higher species diversity is found on the Pacific coast (highest in Costa Rica, Panama and Colombia) than the Caribbean (Spalding et al. 2010). Mangroves also show increased abundance along the Pacific of Costa Rica in comparison with its Caribbean coast, which may be related to a wider tidal range and coastal rugosity in the Pacific (Jimenez & Soto 1985, Polania 1993, Cortes & Werhtmann 2009). Mangroves in the Costa Rican Pacific coast cover approximately 41 002ha of coast line (Pizarro & Angulo 1993) in 127 individual stands, representing 99% of total mangrove area for the country (Zamora- Trejos 2006). They are divided into three groups based on predominant environmental conditions: a) North Pacific: least developed mangrove stands due to drier climate, higher salinities than oceanic within the mangrove stand and a marked dry season; b) Central Pacific: transition area, with increased precipitation and mangrove development; and c) South Pacific: greater mangrove extension and larger trees, lower salinities than oceanic values within the stand and increased development due to higher precipitation rates (Jimenez & Soto 1985, Pizarro et al. 2004, Zamora-Trejos & Cortes 2009).
Despite their abundance and having the benefit of full protection status under Costa Rican legislature (Pizarro et al. 2004), Pacific coast mangroves are not exempt from the various pressures that threaten these habitats. Major pressures include habitat loss and degradation from anthropogenic impacts, such as deforestation for aquaculture and coastal development, hydrodynamic flow alterations, and eutrophication (Valiela et al. 2001, Kathiresan & Qasim 2005, Feller et al. 2010). Specific pressures in the Pacific coast of Costa Rica consist mainly of deforestation, salt and shrimp pond construction and functioning, destructive fishing practices, carbon and tannin production, land use change into agriculture and coastal infrastructure development particularly for tourism and marinas, among others (Jimenez 1994, Cordoba-Munoz et al. 1998, Zamora- Trejos & Cortes 2009).
The importance of mangroves as highly productive coastal habitats that serve critical functions is highly acknowledged and praised, such as their role as nursery areas, land consolidation, sediment trapping, coastal and flood protection, among many others (Hogarth 1999, Kathiresan & Qasim 2005). Furthermore, along with seagrasses and salt marshes they serve as critical coastal habitats for carbon sequestration, related to climate change mitigation (Sifleet et al. 2011). However, the study of these habitats in the north Pacific of Costa Rica has been scarce, and further knowledge of the presence and characteristics of these habitats is critical (Zamora-Trejos & Cortes 2009). The present study aims to map and describe the Iguanita and Panama mangrove stands within Bahia Culebra (Panama and Iguanita stands), in order to provide further knowledge and understanding of the mangrove habitats in the north Pacific coast of Costa Rica.
Methodology
Study sites: Panama and Iguanita mangrove stands are located in Bahia Culebra (Culebra Bay), North Pacific coast of Costa Rica, within the Area de Conservacion Tempisque and Area de Conservacion Guanacaste, respectively (SINAC 2010). This region is characterized by a marked dry season in December-April, and wet season May-November. It has mean annual precipitation rates between 1 400-1 500mm/yr, promoting the presence of Tropical Dry Forest (Cordoba-Munoz et al. 1998, IMN 2010). The North Pacific coast of Costa Rica is a seasonal upwelling area (Alfaro et al. 2012). There is high tourism pressure in the area, particularly with a significant amount of visitors to nearby beaches such as Panama, Coco and Hermosa (Cordoba-Munoz et al. 1998; ICT 2010).
Panama mangrove stand (10.35’25”N & 85.39’56”W; Zamora-Tejos & Cortes 2009) is linked to the Panama estuary (IGNCR 1988) behind Panama Beach (Playa Panama), and is located near the entrance of the bay. Bravo and Rivera (1998), reported total mangrove area of 60ha. Iguanita mangrove stand (10°37’05”N & 85°37’07”W; Zamora-Trejos & Cortes 2009) is associated with the Iguanita estuary (Quebrada Grande), and has a reported extension of 100ha (Cordoba-Munoz et al. 1998). It is located in the inner part of the bay, within the Iguanita National Wildlife Refuge (IGNCR 1988, Cordoba-Munoz et al. 1998, SINAC 2010). Both mangroves are directly linked to freshwater river sources as well as the sea (Fig. 1, 2), with an estimated average semidiurnal tidal range of 3m.
Forest structure: To determine mangrove forest structure the Point-Centered Quarter Method (PCQM) was used (Cintron & Novelli 1984). Fieldwork was carried out at low tide intervals on repeated visits to the study sites between December 2007-March 2008 (dry season). At each mangrove, linear transects perpendicular to the coast line were carried out for the width of each stand at intervals of 100m (Panama) and 250m (Iguanita), and multiple points within each transect were analyzed. Distance to initial point was randomly selected, and in order to avoid analyzing the same tree in more than one point subsequent points were distanced at 20m intervals based on observed stand density, therefore avoiding tree overlap. At each point, GPS coordinates were noted when possible and the area surrounding the point was divided into four quadrats. In each quadrat where mangrove trees were present the nearest one with diameter ≥2.5cm was selected. Distance from the centre point to the tree was noted, as well as species, height, and circumference at breast height (avoiding trunk protuberances) (Pool et al. 1977, Cintron & Schaeffer-Novelli 1984).
Total height was determined using a manual HAGA clinometer, adjusting for eye height of observer. When trees were less than 2m tall, total height was determined directly by using a measuring tape. Rhizophora mangle circumference in the field was measured directly above the highest prop root. For other species, when the trunk was evidently subdivided into multiple trunks below breast height, individual measurements were added for a total estimated tree circumference datum. Diameter at breast height (DBH) was determined from field measurements of tree circumference (diameter= circumference/π). Whenever soil hardness and water availability allowed, a sample of interstitial water was taken at each point (approximately 50ml) digging with a shovel until water presence was evident down to a maximum soil depth of 50cm. Salinity of each water sample was determined in the laboratory using a handheld refractometer.
A total of 11 transects were carried out at Panama mangrove, with a total of 52 points and 208 quadrats analyzed. At Iguanita a total of two transects were carried out (one 150m and the other 250m long), for a total of 16 points and 62 quadrates analyzed. Transects covered the entire Panama stand extension, while at Iguanita, one transect was carried out at the southern section of the mangrove stand (Transect 1) and the other at the middle of the mangrove (Transect 2), all covering the full width of the stand. Both stands were fully explored with the purpose of edge delimitation and field observations, noting GPS coordinates. Subsequent access to Iguanita proved to be extremely difficult, which is evident in the limited number of points analyzed (16 rather than the recommended minimum of 20 points) (Cintron & Schaeffer-Novelli 1984). However, it was considered to be sufficient for a preliminary analysis given overall field and image observations, as well as persistent site access difficulty.
Average height and DBH, as well as number of trees per species were determined for each mangrove stand. Number of points and quadrates with each species, total stand density, absolute density, basal area, as well as relative frequency, dominance, density and importance value for each species were calculated following Cintron & Schaeffer-Novelli (1984). Stand complexity index was determined as: CI= [total stand density (stem/0.1ha) x total basal area (m2/0.1ha) x mean tree height (m) x number of species] x 10-3 (Pool et al. 1977, Jimenez & Soto 1985). Statistical analysis for comparisons between Panama and Iguanita were carried out by applying t-student and chi squared tests (when possible given unavoidable variation encountered between data collected at each mangrove stand per species, and chi squared tests were corrected for variation in sampled area). Salinity comparisons were done with non-parametric Mann-Whitney U test, all using the statistical program PAST (version 2.01) (Hammer et al. 2001).
Mapping: Mapping of Iguanita and Panama mangrove stands was carried out using two MASTER CARTA 2005 images, georeferenced with control points taken in the field in 2007 and 2008, and the 2004 digital atlas road layer (ITCR 2004). Costa Rica Lambert Conformal Conic Proyection and Ocotepeque Fundamental Datum were used. To georreference both images a 1st order polynomial transformation was used, with nearest neighbor as the resample type, cell size of 1m and georeferencing error determined (Root Mean Square, RMS).
Georeferenced images were digitized to 1:5000 scale using the following categories: a) Dense mangrove, which included only mangrove tree species and evident high canopy cover; b) Low density mangrove, mostly areas with dry soil and scarce mangrove trees (particularly dwarf Avicennia trees); c) Transition to dry forest, with both mangrove and dry forest species; d) No mangrove, specific areas within the stands without vegetation; e) Sand; and f) Water. This classification was made based on field and image observations.
Digitizing was carried out for the study area, incorporating field data. Area of each category was calculated using Spatial Statistics Tools extension of the ArcGIS 9.2 software. Geoereferencing and digitizing was done using the same software. Field data and coordinates were used to develop a GIS database to plot species distribution.
Results
Mangrove forest structure: At the Panama mangrove stand total measured distance to center points was 1907m, a total of 170 mangrove trees were analyzed, and five mangrove species were encountered (Avicennia germinans, Avicennia bicolor, Conocarpus erectus, Laguncularia racemosa, and Rhizophora mangle) (Table 1). Total stand density was 8.4stems/0.1ha, and stand complexity index was 1.1. Relative frequency, dominance and density, as well as the importance value, reflect a clear dominance of A. germinans, followed by L. racemosa and C. erectus; and minimal dominance by the remaining two species encountered (Table 2). While most trees were of considerable height (Table 1), abundant dwarf A. germinans trees (<1m height) were encountered in drier areas of consolidated soil, particularly at the back of the mangrove stand.
At Iguanita, total distance using PCQM was 129.4m, 63 trees were analyzed, and a total of three species were found (A. germinans, L. racemosa and R. mangle) (Table 1). An A. bicolor tree was observed in the inner and drier part of Iguanita but none were encountered within transects. Total stand density was 67.2stems/0.1ha, and stand complexity index 86.5. Preliminary analysis indicates dominance by R. mangle (Table 3). Relative frequency, dominance and density from the area studied showed dominance by trees of this species of considerable height (Table 1, 3), reaching up to 40m towards the back of the stand near fresh water river input. At Iguanita, L. racemosa was abundant near the fresh water source for the stand; while A. germinans was only encountered in drier areas of consolidated soil at the back of the mangrove forest, with several dwarf trees present.
Mangrove species zonation followed a similar pattern at both stands; Rhizophora was encountered near channels and inundated areas, Avicennia was frequent in drier areas, and Laguncularia and Conocarpus (latter only encountered at Panama) were more frequent in areas near fresh water input (Fig. 1, 2). From field observations, it was noted that Panama had very compact, dry and cracked soil with unvegetated areas with visible surface salt accumulation, soil hydration increased near water channels and decreased drastically away from them. Avicennia pneumatophores were more abundant near water channels and more humid areas. Iguanita had less consolidated soils, more consistently hydrated throughout. Rhizophora trees near water channels had very low DBHs and diminished tree heights. The inward extension of Panama was mostly limited by a paved road, while the inward extension of Iguanita was mainly limited by topographic variation of land level (increased elevation).
Comparison of shared species within both mangroves is highly restricted by variation in number of trees encountered at each stand, as was the case for A. germinans (Panama n=83 & Iguanita n=3) (Table 1). Laguncularia trees were significantly taller at Iguanita (Table 1; t=3.1, p<0.05, Iguanita n=22 & Panama n=41) but had similar DBH at both sites (Table 1; t=1.5, p>0.05). While Rhizophora tree height showed no significant variation between stands (Table 1; t=1.5, p>0.05, Iguanita n=37 & Panama n=8), DBH was significantly higher at Iguanita (Table 1; t=3.9, p<0.001).
Collection of interstitial water samples was only possible at Iguanita, as no interstitial water was available in Panama at excavated depths, and soil hardness prevented access deeper than 50 cm during the sampling period. Mean salinity in Transect 1 at Iguanita was 38.2±3.3psu (n=5, mean±SD), with an average water accessibility depth of 19.0±17.1cm. Transect 2 showed a slight decrease in mean salinity 36.3±1.5psu (n=6), and interstitial water was available at a decreased mean depth 9.2±2.0cm. However, no statistical variation in salinity was found between transects (Mann- Whitney U=9.5; p>0.05) or depth (Mann-Whitney U=13.5; p>0.05). Two samples of channel water within each transect revealed salinities of 36psu (Transect 1) and 35psu (Transect 2).
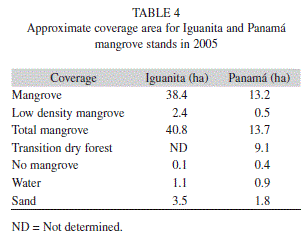
Mapping:Image georeferencing error was RMS= 12.1m for Iguanita and RMS=0.65m for Playa Panama. Variation between locations was due to higher number of field control points for Panama than for Iguanita. Initial estimation of stand length and width yielded approximate distances of 950m x 500 m for Iguanita, and 940m x 330m for Panama. Approximate area of mangrove forest (including both high and low mangrove forest density categories) was 13.7ha at Panama, and 40.8ha at Iguanita (Table 4).
Panama stand is mostly composed of dense mangrove forest (51% of total study area) or dry forest species (35% of total study area). Small areas had dry soil and scarce mangrove trees (2%), as those with no vegetation or covered by water or sand (12%). At Iguanita 84% of the total study area corresponded to dense mangrove forest, 5% had only scarce mangrove trees and 10% of the area had no vegetation or was covered by sand or water (Table 4, Figure 1 & 2). In this case it was not possible to classify the vegetation into mangrove or transition to dry forest, as field data detail did not allow it.
Discussion
Previous mangrove research in the north Pacific of Costa Rica has focused mainly on the mangrove stands of Puerto Soley and Santa Rosa, located further North along the coast, with three studies at each site (Zamora-Trejos & Cortes 2009). The Panama stand had only been considered in a study related to a species of crab (Ucides occidentalis), where forest structure information was limited to mentioning the presence of three mangrove genera (Cabrera- Pena et al. 1994). Although we present a preliminary analysis and field observations of the Iguanita stand - given logistical difficulties in the field - these findings are relevant given that there are no previous publications of this stand other than a general species list (Cordoba-Munoz et al. 1998). Results provided by the present study of Iguanita can be used to carry out further related research in this stand and comparisons with more extensive analyses.
All mangrove species encountered in this study have been previously reported for the mangrove vegetation of the North Pacific region of Costa Rica (Soto & Jimenez 1982, Jimenez & Soto 1985, Jimenez 1994). The higher number of species encountered at Panama (five) in relation to Iguanita (three), might be due to a more heterogeneous environment within the Panama stand from hydrodynamic variations resulting in very arid areas, and more homogenous inundation at Iguanita. However, further study of Iguanita is needed before the presence of other species is discarded. At Panama, Rhizophora, Avicennia, and Laguncularia had been previously reported by Cabrera-Pena et al. (1994), while C. eructus is a new report for the stand. At Iguanita, previous reported species also included C. erectus, Rhizophora racemosa (Cordoba-Munoz et al. 1998) (neither encountered in this study), and A. bicolor (Cordoba-Munoz et al. 1998) (of which only one tree was encountered, located outside of the randomly selected transects). Variation in species previously reported and those found in this study indicates that further analysis of the Iguanita stand is needed before a definite species listing can be established.
Rhizophora harrisonii and Pelliciera rhizophorae were not encountered at either Panama or Iguanita, contrary to their reported presence in nearby mangrove stands - R. harrisonii at Puerto Soley (Soto & Jimenez 1982) and Tamarindo (Pizarro & Angulo 1993); and P. rhizophorae in Potrero Grande (Cordoba- Munoz et al. 1998), Tamarindo (Pizarro & Angulo 1993), and Tempisque (Jimenez & Soto 1985). However, P. rhizophorae is rarely encountered in high salinity mangrove stands such as those present in the north Pacific coast (Jimenez & Soto 1985). On the other hand, R. harrisonii might be a hybrid of R. mangle and R. racemosa, and accurate species identification in the field may be challenging (Duke et al. 2002). Further detailed study of Rhizophora at both stands, including reproductive morphology and phenology, should be carried out to elucidate species variation.
The complexity index (CI) is a tool for quantitatively comparing forest structural complexity (Pool et al. 1977) which varied between both stands, Iguanita had much higher complexity (CI=86.5) than Panama (CI=1.1). Furthermore, Panama CI is also lower than those reported for Santa Rosa (CI=4.9) (Pool et al. 1977), and Puerto Soley (CI=17.3) (Jimenez & Soto 1985), which are the closest studied mangrove areas (further North). Even with data limited to the preliminary analysis, Iguanita is more complex than most mangroves in the area and has values closer to mangrove stands further south (with higher precipitation rates) such as: Tamarindo (CI=30.7), Pochote (CI=30.7), Quepos (CI=65.3), and Sierpe (CI=54.3, the most developed stand in the Pacific of Costa Rica given significantly increased precipitation rates) (Jimenez & Soto 1985). Furthermore, including the three additional species reported for Iguanita, but not encountered within transects during this study, would further increase CI to 173.0. Therefore, complete species listings of the entire mangrove stands, not just randomly selected sections, need to be fully developed and a broader study of Iguanita carried out before final CI comparisons can be made. Moreover, the different methodologies used for studying the stands (PCQM used in this study vs. parcel and transect methods used in previous studies) may have an impact on results and could affect comparisons among stands (Cintron & Schaeffer-Novelli 1984).
Overall, mangrove species zonation followed a similar pattern at both stands, and coincides with previous reports in Costa Rica, with Avicennia species being more tolerant to dry hypersaline conditions, and Rhizophora abundant near hydrated unconsolidated soil and water channels (Soto & Jimenez 1982, Jimenez & Soto 1985, Jimenez 1994). Species variation seemed related to freshwater influence (salinity variation) and inundation patterns. Dwarf Avicennia trees, as observed in both stands, have been previously encountered in very dry areas further north (Puerto Soley), where marked seasonal precipitation and lack of tidal flooding for weeks to months leads to increased interstitial salinity, corresponding with decreased tree height, basal area and leaf size (Soto & Jimenez 1982, Soto & Corrales 1987). Salinities encountered during this study at Iguanita were not as high as those at Puerto Soley (163psu in dryer areas) (Soto & Jimenez 1982). However, only humid areas at Iguanita were sampled given limited interstitial water availability. Water shortage frequently coincided with areas dominated by Avicennia and unvegetated salt crusts, which might indicate much higher interstitial water salinities (at greater soil depths than were sampled in this study) as it has been reported that above 60psu Avicennia is the dominant mangrove (Jimenez 1994). Further study of the interstitial water salinity gradient, fresh water input, tidal range and seasonal variations need to be carried out at both stands.
Densities at both stands (Iguanita= 67.2 and Panama= 8.4stems/0.1ha) were lower than those reported for Puerto Soley (170.8stems/0.1ha) (Soto & Jimenez 1982), and Santa Rosa (105stems/0.1ha) (Pool et al. 1977). This might be related to lower tree height in stands further North due to diminished precipitation, allowing higher densities in shorter forests. Although, higher densities were also found in Barranca (central Pacific) (110stems/0.1ha) a stand dominated by P. rhizophorae. Comparing overall stand density among differing species dominance might not be adequate, as well as comparison based on different sampling techniques. A mangrove stand in Osa (south Pacific coast) dominated by R. mangle with mean height of 34m presented a density of 36stems/0.1ha (Pool et al. 1977), which is intermediate between Iguanita and Panama, highlighting the great difference encountered between both mangroves within the same bay (Bahia Culebra) and climate.
Lower total densities in Panama in relation to Iguanita could be indicative of a more mature mangrove stand, as it is considered that as a mangrove forest matures density decreases while tree diameter increases (Cintron & Schaeffer-Novelli 1984). However, tree density and diameter comparisons between both stands may not be appropriate given that preliminary analysis of Iguanita showed dominance by Rhizophora within the sampled area, and Panama was dominated by Avicennia (of which tree height and diameter data was not comparable given critical variation in total trees encountered). Furthermore, Rhizophora tree heights did not vary between stands but diameters were higher at Iguanita, while Laguncularia trees were taller at Iguanita but had similar diameters at both stands. Nonetheless, basal area at both sites (Iguanita=25.1 & Panama= 2.7m2/0.1ha) was higher than at Santa Rosa (2.32m2/0.1ha) (Pool et al. 1977), and Puerto Soley (1.97m2/0.1ha), which may be due to decreased tree height at this last location (Soto & Jimenez 1982). Mean and maximum Laguncularia heights at Panama coincide with those for the North Pacific (12m) (Jimenez & Soto 1985); yet they are much higher at Iguanita (mean=21.2m; maximum=40.7m). Density variation between studied stands may therefore be due to species density variation (related to hydrodynamic differences) and not stand maturity. Importance value (IV) for Avicennia at Panama (51%) coincides with IV for this species at Puerto Soley. However, other important species at Puerto Soley were R. mangle and R. harrisonii, while at Panama they were L. racemosa and C. erectus. This seems to indicate a higher freshwater input at Panama, as Laguncularia and Conocarpus are more abundant at lower salinities (Soto & Jimenez 1982). Furthermore, the tallest A. germinans at Puerto Soley were 5m (Soto & Jimenez 1982); while at Panama they were up to 25m (maximum height of A. germinans was 25.5m, and 27.8m for A. bicolor). Dominance by R. mangle in the area studied at Iguanita, does not coincide with previously reported forest structure for the northern Pacific of dry climate other than at Santa Rosa with Rhizophora being 68% dominant, yet with a lower mean height of 10m (Pool et. al. 1977). Meanwhile, maximum Rhizophora height at Puerto Soley was 19m (Soto & Jimenez 1982), 18m at Panama and much higher with 41m at Iguanita.
The question remains if comparisons based on preliminary analysis of Iguanita indicating marked forest structure variations between both stands could be related to overall hydrodynamic characteristics of each location, as higher and more frequent inundation at Iguanita, located in the inner part of the Bay, may be leading to Rhizophora dominance. North Pacific mangrove stands are considered to be fringe type mangroves (Pool et al. 1977, Jimenez & Soto 1985) of high salinity and Avicennia dominance (Jimenez & Soto 1985), which matched well with the characteristics found at Panama. However, Iguanita could be more of a riverine type stand, under intense water flow variations and nutrient input, presenting high vegetative development (Cintron & Schaeffer- Novelli 1983) and canopy height (Pool et al. 1977). Given the difference between both stands, with dominance of tall Rhizophora trees at Iguanita, and tall Avicennia trees at Panama, Bahia Culebra might be a particular transition area between the Central Pacific mangroves (of higher precipitation) and those of more arid climates in the north Pacific region as defined by Jimenez & Soto (1985).
Mangrove stand area estimates from this study (40.8ha Iguanita & 13.7ha Panama) are lower than previous reports of 100ha for Iguanita (Cordoba-Munoz et al. 1998) and 60ha for Panama (Bravo & Rivera 1998). However, the noted higher area at Iguanita in relation to Panama is maintained. Estimated stand area in the present study was carried out using field coordinates and observations, and defines mangrove area as only containing dense or low density mangrove species. This methodological approach may explain part of the difference in estimated areas from previous reports. However, while it is still considered that both stands have high mangrove vegetation, no doubt related to the overall protection of mangrove forests in Costa Rica, they are still under significant pressure due to tourist facility development and associated negative impacts. Mangroves previously present at the nearby Playas del Coco have now completely disappeared, most likely related to large infrastructure development at this site (per. obs.). Historical image analysis is needed to elucidate if there has been a reduction in mangrove cover at Iguanita and Panama, and continued conservation of these habitats is critical.
Even though both Iguanita and Panama mangrove stands are within Culebra Bay, structurally they are very different form one another and seem to be under two different hydrodynamic contexts. Considering that global mean sea level has risen approximately 1-2mm/ year over the last 100 years (Gornitz 1995, Domingues et al. 2008) and climate change is thought to generate continuous sea level rise in coming years (Titus & Narayanan 1995), increased inundation might result in mangrove retreat near the shoreline and landward migration (Cohen & Lara 2003). Unfortunately, both stands studied are limited inland by geographical (increased land elevation), and anthropogenic (roads) barriers that would prevent inward mangrove extension. For this reason, the protection of buffer zones at the back of Costa Rican mangroves is considered of great importance, to increase their chances of survival and conservation. Furthermore, given the social, economic and ecological importance of mangrove habitats, further studies at these and other mangroves in the country are urgently needed.
Acknowledgments
This project was funded by Ecodesarrollo Papagayo and by the Vicerrectoria de Investigacion and CIMAR at the Universidad de Costa Rica. We thank the Escuela de Biologia, Universidad de Costa Rica, for equipment loaned. We would like to thank J.A. Sibaja-Cordero for his help with statistical analysis, all those who helped in the field, and three anonymous reviewers whose comments greatly improved this manuscript.
References
Alfaro, E.J., J. Cortes, J.J. Alvarado, C. Jimenez, A. Leon, C. Sanchez-Noguera, J. Nivia-Ruiz & E. Ruiz- Campos. 2012. Clima y variabilidad climatica de la temperatura subsuperfical del mar en Bahia Culebra, Guanacaste, Costa Rica. Rev. Biol. Trop. 60 (Suppl. 2): 159-171. [ Links ]
Bravo, J. & L. Rivera. 1998. Mapas de Humedales de Costa Rica e Informacion Complementaria. SINAC, MINAE–UICN, San Jose, Costa Rica. Escala 1:200 000. [ Links ]
Cabrera-Pena, J., F. Vives-Jimenez & Y. Solano-Lopez. 1994. Tamanos y proporcion sexual de Ucides occidentalis (Crustacea: Gecarcinidae) en un manglar de Costa Rica. Uniciencia 11: 97-99. [ Links ]
Cintron, G. & Y. Schaeffer-Novelli. 1983. Introduccion a la ecologia del manglar. UNESCO, Montevideo, Uruguay. [ Links ]
Cintron, G. & Y. Schaeffer-Novelli. 1984. Methods for studying mangrove structure, p. 91-113. In: S.C. Snedaker & J.G. Snedaker (eds.). The Mangrove Ecosystem: Research Methods. UNESCO, Paris. [ Links ]
Cohen C.M.L. & R.J. Lara. 2003. Temporal changes of mangrove vegetation boundaries in Amazonia: Application of GIS and remote sensing. Wetlands Ecol. Manag. 11: 223-231. [ Links ]
Cordoba-Munoz, R., J.C. Romero-Araya & N.J. Windevoxhel- Lora. 1998. Inventario de los humedales de Costa Rica. UICN, MINAE, SINAC, Embajada Real de los Paises Bajos, San Jose, Costa Rica. [ Links ]
Cortes, J. & I.S. Wehrtmann. 2009. Diversity of marine habitats of the Caribbean and Pacific of Costa Rica, p. 1-45. In I.S. Wehrtmann & J. Cortes (Eds.). Marine Biodiversity of Costa Rica, Central America. Springer + Business Media, Berlin, Germany. [ Links ]
Domingues, C.M., J.A. Church, N.J. White, P.J. Gleckler, S.E. Wijffels, P.M. Barrer & J.R. Dunn. 2008. Improved estimates of upper-ocean warming and multidecadal sea-level rise. Nature 453: 1090-1093. [ Links ]
Duke, N.C., E. Yuk Ying Lo & M. Sun. 2002. Global distribution and genetic discontinuities of mangroves - emerging patterns in the evolution of Rhizophora. Trees 16: 65-79. [ Links ]
Feller, I.C., C.E. Lovelock, U. Berger, K.L. McKee, S.B. Joye & M.C. Ball. 2010. Biocomplexity in mangrove ecosystems. Annu. Rev. Mar. Sci. 2: 395-417. [ Links ]
Gornitz, V. 1995. Sea-level RSC: a review of recent past and near future trends. Earth Surf. Proc. Land. 20: 7-20. [ Links ]
Hammer, O., D.A.T. Harper & P. Ryan. 2001. PAST: Paleontological Statistics software package for education and data analysis (Version 2.01. May 2010). Palaentol. Electr. 4: 1-9. [ Links ]
Hogarth, P.J. 1999. The Biology of Mangroves. Oxford University Press, Oxford and New York. [ Links ]
IGNCR. 1988. Hoja cartografica Carrillo Norte 3047 I. Instituto Geografico de Costa Rica, Ministerio de Transportes, San Jose, Costa Rica. Edicion 3-IGNCR. Escala 1: 50 000. [ Links ]
ITCR. 2004. Road Layer, Digital Atlas of Costa Rica. Lambert Conformal Conic Proyection, Ocotepeque Fundamental Datum. 1:200 000. Instituto Tecnologico de Costa Rica. Cartago, Costa Rica. [ Links ]
Jimenez, J.A. 1994. Los manglares del Pacifico Centroamericano. EFUNA, Heredia, Costa Rica. [ Links ]
Jiménez, J.A. & R. Soto. 1985. Patrones regionales en la estructura y composición florística de los manglares de la Costa Pacífica de Costa Rica. Rev. Biol. Trop. 33: 25-37. [ Links ]
Kathiresan, K. & S.Z. Qasim. 2005. Biodiversity of Mangrove Ecosystems. Hindustan Publ., New Delhi, India. [ Links ]
Pizarro, F. & H. Angulo. 1993. Diagnostico de los manglares de la costa Pacífica de Costa Rica: Informe para la Comisión Nacional de Manglares. UICN. San José, Costa Rica. [ Links ]
Pizarro, F., L. Piedra, J. Bravo, J. Asch & C. Asch. 2004. Manual de procedimientos para el manejo de los manglares en Costa Rica. Grupo de Trabajo en Humedales- Costa Rica. Programa Nacional de Humedales. Editorial Fundación UNA, Heredia, Costa Rica. [ Links ]
Polania, J. 1993. Mangroves of Costa Rica. Part I, p. 129-137. In L.D. Lacerda (Ed.), Conservation and Sustainable Utilization of Mangrove Forests in Latin America and Africa Regions. ITTO/ISME Project PD 114/90 (F), Okinawa, Japan. [ Links ]
Pool, D.J., S.C. Snedaker & A.E. Lugo. 1977. Structure of mangrove forests in Florida, Puerto Rico, México and Costa Rica. Biotropica 9: 195-212. [ Links ]
Sifleet, S., L. Pendleton & B.C. Murray. 2011. State of the Science on Coastal Blue Carbon. A Summary for Policy Makers. Nicholas Institute Report, Duke University. Durham, U.S.A. [ Links ]
Soto, R. & L.F. Corrales. 1987. Variaciones de algunas caracteristicas foliares de Avicennia germinans (L.) L. en un gradiente climatico y de salinidad. Rev. Biol. Trop. 35: 245-256. [ Links ]
Soto, R. & J.A. Jimenez. 1982. Analisis fisionomico y estructural del manglar de Puerto Soley, La Cruz, Guanacaste, Costa Rica. Rev. Biol. Trop. 30: 161-168. [ Links ]
Spalding, M., M. Kainuma & L. Collins. 2010. World Atlas of Mangroves. Earthscan. London, United Kingdom. [ Links ]
Titus, J.G. & V.K. Narayanan. 1995. The Probability of Sea Level Rise. United States Environmental Protection Agency, Washington, DC, USA. [ Links ]
Valiela, I., J.L. Bowen & J.K. York. 2001. Mangrove forests: one of the world/s threatened major tropical environments. BioScience 51: 807-815. [ Links ]
Zamora-Trejos, P. 2006. Manglares, p. 23-39. In: V. Nielsen- Muñoz & M.A. Quesada-Alpizar (eds). Informe Técnico Ambientes Marino Costeros de Costa Rica. Comisión Interdisciplinaria Marino Costera de la Zona Económica Exclusiva de Costa Rica. CIMAR, CI, TNC, San José, Costa Rica. [ Links ]
Zamora-Trejos, P. & J. Cortes. 2009. Los manglares de Costa Rica: el Pacifico Norte. Rev. Biol. Trop. 57: 473-488. [ Links ]
Internet references
ICT. 2010. Instituto Costarricense de Turismo. San José, Costa Rica (accessed: 29 April 2010, http://www.visitcostarica.com/ict). [ Links ]
IMN. 2010. Instituto Meteorológico Nacional de Costa Rica. International Airport Daniel Oduber Station in Liberia, Costa Rica (accessed: 29 April 2010, http://www.imn.ac.cr). [ Links ]
SINAC 2010. Sistema Nacional de Áreas de Conservación, Ministerio de Ambiente Energía y Telecomunicaciones. San José, Costa Rica (accessed: 29 April 2010 http://www.sinac.go.cr). [ Links ]
*Correspondencia a:
Jimena Samper-Villarreal. Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Ciudad de la Investigación, Universidad de Costa Rica, San Pedro, 11501-2060 San José, Costa Rica; jimena_samper@yahoo.com.
Jorge Cortes. Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Ciudad de la Investigación, Universidad de Costa Rica, San Pedro, 11501-2060 San José, Costa Rica; jorge.cortes@ucr.ac.cr. Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, Costa Rica.
Catalina Benavides-Varela. Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Ciudad de la Investigación, Universidad de Costa Rica, San Pedro, 11501-2060 San José, Costa Rica; tabebuiaguayacan@gmail.com. Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, Costa Rica.
1. Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Ciudad de la Investigación, Universidad de Costa Rica, San Pedro, 11501-2060 San José, Costa Rica; jimena_samper@yahoo.com; jorge.cortes@ucr.ac.cr; tabebuiaguayacan@gmail.com
2. Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, Costa Rica.
Received 28-VII-2011. Corrected 24-I-2012. Accepted 29-II-2012.