Introduction
One of the most prevalent birth defects among live births is non-syndromic cleft of the lip with or without palate (NSCL/P), which affects between 1 and 2 births per 1000 in all communities worldwide(1). NSCL/P affects the patient's appearance, mastication, speech, and hearing and can lead psychological issues (2, 3). The anomaly may have a significant negative psychological and financial impact on the patients and their families, which will negatively affect their quality of life (4). Identification of the aetiology of NSCL/P will help in the diagnosis, treatment and prevention of the disease. The aetiology of NSCL/P has been considered to be multifactorial (4, 5). Genetic and environmental factors appear to play a role in the causation of NSCL/P. The aetiology and mechanism of NSCL/P are not entirely known because of the complex pathophysiology of the disease (6). There are several studies that have tried to identify the genes responsible for NSCL/P and the association between gene polymorphisms and the aetiology of NSCL/P (6,7,8).
TPM1 gene belongs to the family of actin- binding proteins known as tropomyosins, which are highly conserved, extensively distributed, and involved in the cytoskeleton of non-muscle cells as well as the contractile system of striated and smooth muscles (9). Two alpha-helical chains organized in a coil make up tropomyosin. It gives the actin filaments stability by being polymerized end to end along their two grooves (10). The encoded protein is a particular kind of alpha-helical chain that makes up the majority of the tropomyosin in striated muscle (11). Together with the troponin complex, it also controls the calcium-dependent interaction between actin and myosin during muscle contraction (12). Alternatively, spliced transcript variants encoding a variety of isoforms have been identified in smooth muscle and non-muscle cells (13). Tropomyosin 1 (TPM1) is a gene that codes for proteins. TPM1 is linked to various diseases including cardiomyopathy, familial hyper- trophy, etc. It is also associated with interleukin- related pathways including the Vitamin D receptor pathway and cytoskeletal signaling (14, 15). Actin binding and cytoskeletal protein binding are GO annotations associated with this gene (16,17,18). TPM3 is a significant paralog of this gene, which evolved due to gene duplication events.
In humans, tropomyosins are encoded by TPM1, TPM2, TPM3 and TPM4 genes. The tropomyosin isoforms may be muscle tropomyosin Isoforms or cytoskeletal tropomyosin isoforms. Different tropomyosin isoforms occupy slightly different positions along actin filaments. Tropomyosin isoforms localize differentially within the cell, regulate actin cytoskeleton functions, vesicle transport, cell migration, cytokinesis and angiogenesis (19). Previous authors have suggested that the lack of cytoskeletal tropomyosin may affect craniofacial development in non-humans (20,21,22) affecting the morphogenesis of the head in Drosophila (20) and causing embryonic death of mice in knockout studies (21, 22). The exact role of TPM1 gene in the development of craniofacial complex is obscure.
Existing studies on the influence of TPM1 gene in the development of non syndromic cleft lip and palate have established some association between a few SNP and NSCL/P in different ethnic population (23,24,25,26). SNPs rs1972041GA, rs197204, rs11071720, rs3803499, rs 1873147 and rs12148828 were studied in the Chinese population for its influence in the development of NSCL/P (24, 25). SNPs rs11071720, rs12148828, rs3803499 and rs1972041 have been evaluated for association NSCL/P in the Iranian polymorphism (23). European-descent population-based studies found significant associations between isolated cleft lip only and fetal SNPs near TPM1 gene (26).
However, the role of TPM1 gene in the development of cleft lip and palate in the South Indian population has not been reported in literature.
Hence this study was proposed to assess if TPM1 gene polymorphism (rs11071720) was responsible for the etiology of non-syndromic cleft lip and palate in the South Indian population. The null hypothesis was that there was no association between TPM1 gene (rs11071720) polymorphism and the development of cleft lip and palate. This aim of the study was to find the association between TPM1 gene polymorphism (rs1107171) and non-syndromic cleft lip and palate in subjects belonging to the South Indian population.
Materials and methods
This retrospective case-control study was conducted in the Department of Orthodontics of our institution. Approval was obtained from the scientific review board of our university with reference number SRB/SDC/FACULTY/22/ ORTHO/052. Ethical clearance was obtained from the ethical board with reference number IHEC/ SDC/FACULTY/22/ORTHO/58. Patients reporting to the cleft and craniofacial unit of our university were screened. Patients with cleft and palate without associated syndromes and a familial history of cleft lip and palate were included in the study. Patients with habits such as pan chewing, tobacco chewing, smoking, diabetes, oral lesions such as submucous fibrosis, leukoplakia, erythroplakia, oral submucous fibrosis, diabetic patients and patients with systemic diseases were excluded from the study. About twenty five patients with NSCL/P who met the inclusion criteria were selected for the study. Twenty five healthy individuals without cleft lip and palate were selected as control. A total of 50 participants, 25 individuals each in group, group 1 (case group) and group 2 (control group) were evaluated.
Sample collection and data extraction
The antecubital fossa was used to collect 5mL of venous blood, which was then disseminated in a sterile tube with a small amount of ethylene- diaminetetraacetic acid. To prevent the formation of a clot, it was carefully blended. In accordance with a modified Miller et al. 1998 procedure, DNA isolation was carried out.
Polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP)
TPM1 gene (rs1107171) polymorphisms were assessed by polymerase chain reaction (PCR) amplification and restriction digestion. Amplification of DNA spanning the rs11071720 polymorphic site of the TPM1 gene was done using PCR. The forward primer was 5’-TACGGGGTTGTTCT- GAGTTGAG- 3’ and reverse primer was 5’-CTAGC- GAGTAAGGGAGTCGGA-3’. The amplification of DNA was performed in 20 µL volumes using 10 ng of genomic DNA, 5 pmol/µL, each of the forward and reverse primers along with PCR Master Mix (Takara, Shiga, Japan). The cycling conditions were as follows: initial denaturation at 94°C for 5 minutes, denaturation at 94°C for 35 seconds, annealing at 60°C for 35 seconds, extension at 72°C for 35 seconds and a final extension at 72°C for 5 minutes. A 5µL volume of PCR product was checked on a 1% agarose gel, and 15µL of PCR product was digested using a restriction enzyme AseI (New England Biolabs, Hitchin, UK). Digestion was carried out at 37°C for 2 hours. The digested product was visualized on 2% agarose gel.
Statistical analysis
Statistical analysis was done with SPSS version 22.0. The Hardy-Weinberg equilibrium (HWE) was tested in each group using Chi-Square test. Allele ratio and genotype distribution of NSCL/P patients and healthy controls were evaluated. The odds ratio (OR) with 95% confidence intervals was used to calculate the risk related to specific alleles or genotypes. A p-value ≤0.05 was considered to be statistically significant.
Results
50 patients were evaluated. Of the 25 patients in group 1, 8 were male and 17 were female. The mean age of the patients included in group 1 was 4.99±5.2 years. In group 2, 25 healthy individuals consisting of 16 males and 9 females were included. The mean age of the patient in the control group was 23.24±6.8 years. The ancestral allele associated with TPM1 gene polymorphism (rs11071720) was the T allele and the variant allele was the C allele with the minor allele frequency 0f 0.48 (https://asia.ensembl.org/Homo_sapiens/ Variation/Explore?db=core;r=15:63049297- 63050297;v=rs11071720;vdb=variation;vf=105952625) Comparison of genotype frequencies of the TPM1 gene polymorphism (rs11071720) with normal healthy subjects did not show a statistically significant difference within group 1 and group 2 between the CC, CT and TT genotype with a p value of 0.8472 (Table 1). The percentage of the TC genotype was 48%, the CC genotype was 32% and TT genotype was 20% in group 1. There was no statistically significant deviation from Hardy Weinberg Equilibrium, the case group χ2ᵈᶠ (p=0.8472) (Table 1). Classification of the genotypes based on genetic models such as dominant, recessive or additive (allelic) did not present any significant association of the polymorphic marker with the disease status (Table 2). Each genomic DNA sample was amplified using allele specific primers (F+R). Agarose gel electrophoretogram showed partial amplification of TPM1 gene with amplicon spanning the polymorphic site (rs11071720) of the TPM1 gene with a o size of 273bp (Figure 1). Agarose gel electrophoretogram showed AseI digested amplicon of TPM1 gene at (rs11071720) suggesting the presence all three alleles, TT< CC and TC (Figure 2).
The comparison of allele frequency among different populations showed that the global allele frequency for the T allele was 51%, which was close to the observation made in our present study among control subjects (44%) (Figure 3).
Table 1 Genotype and allele frequencies of TPM1 gene polymorphism (rs11071720) among the case and control groups recruited in the present study.
| Groups | TT (%) | TC (%) | CC (%) | T | C | HWE (p value)* |
|---|---|---|---|---|---|---|
| Case (N=25) | 5 (20) | 12(48) | 8(32) | 0.44 | 0.56 | 0.8966 |
| Control (N=25) | 6(24) | 10(40) | 9(36) | 0.44 | 0.56 | 0.3464 |
*For departure from Hardy-Weinberg equilibrium (HWE), Chi square with one degree of freedom. The genotype frequency of cases and controls do not differ significantly χ2ᵈᶠ (p=0.8472).
Table 2 The table shows the genotype frequencies classified based on dominant, recessive and allelic models.
| Dominant | - | - | - | - |
|---|---|---|---|---|
| Genotypes | Case | Control | Unadjusted OR (95% CI) | P value |
| TT | 5 | 6 | 0.7917 (0.2067 -3.0316) | 0.7331 |
| TC+CC | 20 | 19 | - | - |
| Recessive | - | - | - | - |
| TT+TC | 17 | 16 | 1.1953 (0.3703 -3.8582) | 0.7654 |
| CC | 8 | 9 | - | - |
| Allele | - | - | - | - |
| T | 22 | 22 | 1.0000 (0.4540 - 2.2027) | 1.0000 |
| C | 28 | 28 | - | - |
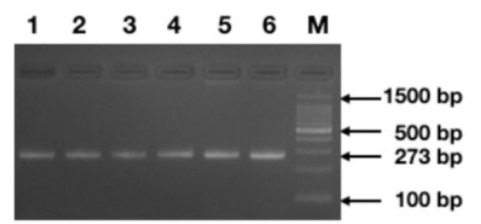
Figure 1 Agarose gel electrophoretogram demonstrating the amplicons of the region spanning the polymorphic site (rs11071720) of TPM1 gene of size 273bp (Lanes 1-6), (M=100 bp. Standard DNA ladder).
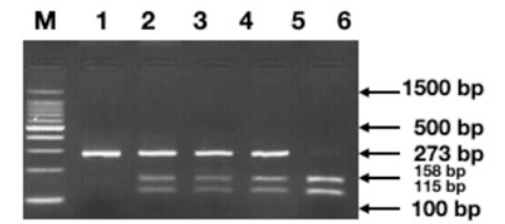
Figure 2 Agarose gel electrophoretogram demonstrating the genotypes classified based on the band patterns obtained after digesting the amplicons with Asel restriction enzyme (Homozygous TT=158+115 bp, Homozygous CC=273bp, Heterozygous TC=115+158+273 bp);. (M=100bp, Standard DNA ladder).
Discussion
Because of the combination of numerous genetic and environmental risk factors, NSCL/P is a multifactorial disease (27). Although the familial inheritance of orofacial clefts has been known, systematic genetic research did not start until Fogh-Anderson who postulated that genetic factors contribute to NSCL/P after finding an increasing incidence of clefting in family members of patients with a cleft and confirmed the genetic component to NSCL/P (28). The risk of developing NSCL/P in first-degree relatives is considered to be 32 times higher than the risk for people without a family history of the disease (29). The concordance percentage of 40-60% in monozygotic twins is higher than the rate of 3-5% in dizygotic twins and also denotes a robust, consistent, and reliable inheritance pattern (30, 31). Whole-genome sequencing in a pair of monozygotic twins with discordant cleft lip and palate subtypes showed that twin 1 had nonsyndromic bilateral cleft lip and palate, while twin 2 had nonsyndromic bilateral cleft lip and unilateral left-sided cleft alveolus (32). There was no oro-facial cleft in either of the parent. Standard cephalometry and whole-genome sequencing were used to examine craniofacial morphologic characteristics and potential genetic variations. The result of this study was that the lack of hereditary differences and the observed disparities in craniofacial morphology between the monozygotic twins may be directly attributed to the effects of the orofacial cleft on growth and/or differences in surgical history (32).
Gene Interactions influencing signaling Pathways Involved in cleft Lip/palate in humans investigated with multidimensional reduction method studied gene-gene and gene-folic acid consumption interactions for the 24 SNPs as well as two additional SNPs previously genotyped in the same study group. Based on these findings, as well as earlier association studies and molecular characterizations of murine models, it was suggested that an interaction network in which interferon regulatory factor 6 plays a key role in the aetiology of NSCL/ was present (33).
The TPM1 gene polymorphism was found to play a role in the development of NSCL/P in the Chinese population and was studied for several tag single nucleotide polymorphisms (tSNPs) of TPM1 (rs11071720, rs3803499, rs12148828 and rs1972041). The SNPs were genotyped by the IPLEX Sequenom MassARRAY platform. SNP rs1972041GA showed a decreased risk of non syndromic oral clefts heterozygotes with an enhanced protective effect of the minor allele G at rs197204 in NSCL/P. No association was observed between rs11071720, rs3803499, and rs12148828 and NSCL/P suggesting that TPM1 gene polymorphism may influence craniofacial development (24).
TPM1 polymorphism was also suggested to contribute to the aetiology of NSCL/P in the Iranian population. TPM1 rs11071720, rs3803499, rs12148828, and rs1972041 polymorphisms were genotyped by the polymerase chain reaction- restriction fragment length polymorphism method. The finding showed that rs11071720 polymorphism significantly increased the risk of NSCL/P in homozygous codominant. The rs12148828 polymorphism was associated with protection against NSCL/P in codominant. The findings showed that rs3803499 polymorphism significantly increased the risk of NSCL/P in codominant. No significant association could be established between rs1972041 polymorphism and NSCL/P (23).
Significant associations were observed in patients with isolated cleft lip only and fetal SNPs near TPM1 and NOG1 (26).In patients with only cleft lip and those with cleft lip and palate, the double doses of TPM1 and other SNPs had larger associations than those for the single-dose alleles. There was a nearly two fold increased risk with the double minor allele doses of TPM1 and other SNPs. However, the associations with the single and double minor allele doses of TPM1 and other SNPs on NSCL/P were significant at nominal levels. TPM1 was suggested to have a stronger association with isolated cleft lip only than cleft lip and palate (26).
TPM1 gene polymorphism (rs1873147) has been suggested to play a role in the development of NSCL/P in a Chinese Han population (25). TPM1 gene polymorphism (rs1873147) showed protective effects against non syndromic oral clefts according to the results of allele and genotype comparison (allelic, heterozygote and dominant comparisons). The protective effect of rs1873147 was on cleft palate only (25).TPM1 gene polymorphism was found to be associated with other abnormalities in humans. Association between TPM1 gene polymorphisms and idiopathic congenital talipes equinovarus risk in a Chinese population suggested that the TPM1 (rs4075583 G>A polymorphism) is connected with ICTEV risk in a southern Chinese population, although further research and mechanistic studies are required to validate this association (34).
Single nucleotide polymorphisms in the 3’ UTR region of the TPM1 gene was found to be associated with dilated cardiomyopathy (35). The risk of development of dilated cardiomyopathy in the rs6738 locus G allele carriers were greater than A allele carriers and age and gender had no effect on the association of TPM1 gene SNPs with dilated cardiomyopathy. The spectrum of mutation in hypertrophic cardiomyopathy genes among Tunisian patients, showed that the mutation were not extremely frequent to account for the majority of cases (36). Significance of sarcomere gene mutation in patients with dilated cardiomyopathy of Kazakh heritage may have the TPM1 and TNNT2 genes at risk for the condition (37). Patients with idiopathic dilated cardiomyopathy had a considerably greater prevalence of sarcoma gene mutations than that had previously been reported (37). The beginning of dilated cardiomyopathy may be influenced by at least one type of genetic mutation, according to familial transmission. Therefore, extensive gene sequencing of the patient is required in order to facilitate early disease detection and enable prompt therapy and prevention.
Conclusion
The present study found no significant association between rs11071720 polymorphism of the TPM1 gene with the phenotype NSCL/P in the South Indian population. However, taking into consideration the functions of the TPM1 gene, numerous other polymorphisms and pathogenic mutations can be screened among the patient group to identify the genetic marker associated with NSCL/P in the South Indian population. The sample size could also be increased to provide concrete evidence about the associations between the marker selected and the disease phenotype.
















