Introduction
The key role of endodontic therapy relies on its ability to decrease the presence of microorganisms. The complete disinfection of root canals guarantees the success of this therapy. Essential factors for achieving this aim are the use of ideal chemical irrigants and an adequate instrumentation system; however, there are some factors that make the total disinfection of root canal systems impossible (1). The root canal is a unique environment where biological selection drives the type and course of infection. In an anaerobic environment, microbial interactions and nutrient availability are factors that define the composition of the root canals' microbial flora (2). Some microorganisms present in the root canals can survive after the endodontic therapy, for instance, Candida albicans and Enterococcus faecalis. In fact, these microorganisms have been strongly correlated with endodontic failure or secondary infections (3,4,5).
C. albicans is a yeast that has been reported to colonize the dentine, causing infection in root canals system; it can compete for space and nutrients with other microorganisms of the endodontic flora (6) and form hyphae, allowing invasion through pores and incomplete walls (7). E. faecalis is a Gram-positive coccus and facultative anaerobe able to survive in extreme environments, such as alkaline pH and temperatures ranging from 10-60°C, allowing it to cause periradicular infection including persistent endodontic infections. It can also interact with other strict anaerobes such as Porphyromonas, Prevotella, and Fusobacterium (8,9,10).
Indeed, there is a wide range of instrumentation systems whose objective is to achieve the best conditions for root canals, decrease the bacterial concentration in a minimal amount of time, and provide adequate characteristics of endodontic files, such as greater resistance and flexibility as well as decrease the incidence of fractures to the instrumentation systems. The WaveOne Gold system has a variable taper in the active portion that aids to improve flexibility and allows a more conservative preparation of the root canal system at the coronal zone. In addition, this system has a reciprocal movement of 150 degrees of rotation in an anti-clockwise direction, serving to cut dentine and then rotate the file 30 degrees in a clockwise direction to release the instrument (11). Reciproc is a single instrumentation system for the preparation of root canals that employs reciprocal rotational movements. This instrument cuts in an anti-clockwise direction with approximately 120 degrees of movement and then relieves the tension of the instrument with clockwise movement (12). Twisted File Adaptive (TFA) is an instrumenta- tion system that employs unique motion technology that automatically adapts to the pressure of the instruments. This system uses interrupted movements both clockwise and anti-clockwise in a continuous rotation, thus allowing better removal of debris in oval root canals. Additionally, when the canal is modeled due to greater pressure in the metal, the movement of the instrument changes to an oscillating mode (13).
The aim of this study was to evaluate whether the WaveOne Gold and Reciproc single-file instru- mentation systems produce a reduction in the microbial load of a mixed biofilm and the cleaning of the apical third compared to the Twisted File Adaptive system (multiple-file system). The propo- sed null hypothesis was that there would be no diffe- rence between the single and multiple-file systems in reducing the microbial load of mixed biofilm.
Materials and methods
Samples
First and second lower molars were extrac- ted from patients with dental caries, periodontal disease, and/or for orthodontic reasons at the Oral Surgery Clinic of the Faculty of Dentistry. All patients signed an informed consent form, and this study was approved by the Ethics Commit- tee in accordance with Helsinki Declaration. Teeth without previous endodontic treatment, without internal, external, or apical resorption, and without calcified canals were included in this study. The crown of each tooth was cut using a low-speed saw (Isomet, Buehler, Lake Bluff, IL), and an X-ray of the dental roots was taken to measure the curvature using Autocad software (Autodesk, San Rafael, CA). Dental roots measuring less than 25 degrees were accepted for the study. Overall, seventy mesial dental roots were included in this study (n=20 per group and 10 controls). The standardization of the roots to 15mm was done with a K #10 endodontic file (Dentsply Sirona, York, PA), and afterwards, the roots were instrumented serially with a K #20 file (Dentsply Sirona). The roots were cleaned as described in the protocol by Haapasalo & Orstavik (13) which includes 5.25% NaOCl, 17% ethylene diamine tetra-acetic acid (EDTA) and distilled water. Then, the roots were sterilized with moist heat in an autoclave (KitLab USA, Chicago, IL) for 15 minutes at 121°C.
In vitro formation of mixed biofilm: C. albicans and E. faecalis were isolated from the root canals of patients with endodontic secondary infections at the endodontic clinic, and the biochemical identification of the microorganisms was made using the API test (bioMérieux sa, Marcy l'Etoile, France) according to the manufacturer's instructions. In vitro formation of the mixed biofilm on the dental roots was made in static form over 31 days with Brain Heart Infusion (BHI) culture medium (BD Bioxon, Estado de México, México) and Dextrosa-Sabouraud (DS) (BD Bioxon) (75% and 25%, respectively); additionally, 50µl of human serum was added at the start of biofilm formation for C. albicans growth (14). The culture medium was changed every two days, and Gram stain was used for monitoring the microbial growth.
In vitroinstrumentation of root canals
After 31 days of biofilm formation, the dental roots were placed in a device filled with silicone-based impression material (Speedex putty, Coltene, Alstätten, Switzerland) and the operative field disinfection protocol was performed using these solutions: 5.25% NaOCl, 10% Na2SO3 and 30% H2O2. The pre-instrumentation samples inside root canals were taken with sterile paper tips #20 and were placed in BHI/DS culture medium for 24h at 37°C. Then, the instrumentations were made by one operator according to the manufacturer's instructions. The Twisted File Adaptive (TFA) system (SybronEndo, Coppell, TX) was performed with adaptive movement by an electric motor (Elements Motors, Axis/SybronEndo, Coppell, Texas), at 500rpm with #15 file up to work length, diameter D1 of 0,25mm and 6% conicity, while the Reciproc (VDW, Munich, Germany) and WaveOne Gold (Dentsply Sirona Endodontics, Ballaigues, Switzerland) systems were performed by alternative reciprocal movement using the X Smart plus electric motor (Dentsply Maillefer, Switzerland). For Reciproc system was employed #15 file up to work length with diameter D1 of 0,25mm and 8% conicity and for Wave One Gold system the root canals were permeabilized up to apical zone by using #10 file with diameter D1 of 0,25mm and 7% conicity and later the glide path was performed with ProGlyder file (Denstply) up to work length. Later of instrumentation with each system, the root canals were irrigated with two milliliters of 2.25% NaOCl using the Endoeze syringe (Ultradent Products Inc, South Jordan, Utah) with the simultaneous aspiration of the content of the root canal. The apical permeability was maintai- ned using the #10 K file, and the final irrigation was made with passive ultrasonic irrigation with 17% EDTA and 5.25% NaOCl. Immediately after the root canals instrumentations, the post-instrumentation samples were taken with #35 K file inside the root canal and placed in BHI/DS culture medium for 24h at 37°C. Controls were roots without instrumenta- tion and roots without biofilm formation. In addition, distilled water was used as irrigation control.
Count of colony-forming units
After 24h of incubation of the pre-and post- instrumentation samples, the McFarland scale was measured for all samples, and serial dilutions were made with sterile distilled water and spread on BHI/DS agar plates through a massive spread with an ''L''shape loop. Then, the plates were incuba- ted for 24h at 37°C, and the CFU was counted in a semiautomatic counter (Felisa, Jalisco, Mexico). The total CFU count was made with this formula: CFU= (number of CFU) (dilution factor)/milliliters of culture. In addition, the percent of elimination of microbial load was calculated for the 3 employed systems, using distilled water as control and sodium hypochlorite as irrigant at 2.25% and 5.25%.
Scanning electron microscope
After post-instrumentation samples were taken, all teeth were submitted to the evaluation of the cleanliness of the apical zone of root canals using the different instrumentation systems through the Scanning Electron Microscope (SEM) (Jeol JSM-6610LV, Peabody MA). First, the mesial roots were longitudinally cut in sense lingual-vestibular and were dehydrated with alcohol at different concentrations (10% until absolute alcohol). Then, the roots were totally dehydrated in a critical point dryer (Leica EM CPD030, Wien, Austria). Later, gold coating was added to all the dental roots using the JEOL JFC-1100 equipment (Jeol), and the samples were processed until 10,000 magnifications using SEM. Data of cleanliness of the apical zone was reported as permeability of denti- nal tubules by using the next formula: percent of permeability=100-percent of microbial presence. The presence of microbial load in root canals was evaluated by 2 observers to simple blind using the modified scale Rome, at 1 and 3 millimeters in apical zone.
Statistical analysis
The data regarding the decrease of microbial load and cleanliness of apical zone are expressed as the mean±standard deviation or as the median and interquartile range, according to their normal or non-normal distribution, respectively. The Kolmo- gorov-Smirnov test was employed for calculating the normality of data. Comparisons among the groups were performed by using Student's t-test, the U Mann-Whitney test, the Kruskal-Wallis test, or one-way analysis of variance (ANOVA), as required. All data were analyzed by using GraphPad Prism software v5.0 (GraphPad, San Diego, CA), and p<0.05 was considered significant.
Results
Decreasing of microbial load by using the different instrumentation systems. After 31 days of biofilm formation inside root canals, the samples were instrumented and irrigated, as indicated in the material and methods section. There was a significant diminution of CFU at the three different files systems after instrumentation in comparison to that before instrumentation (Wave One Gold=8.31x107±4.37x107 vs 71.50±213.5, mean±standard deviation, before and after instrumentation, respectively. Reciproc=3.98x107±3.64x107 vs 255.0±710.0,mean±standard deviation, before and after instrumentation, respectively. TFA=5.35x107±6.42x107, vs 351.0±776.5, mean±standard deviation, before and after instrumentation, respectively) (P<0.0001) (Figure 1. A-C); in addition, after comparing the diminution of the microbial load in the three systems after instrumentation of a single file using Wave One Gold or Reciproc and multiple files by TFA, there were no statistically significant differen- ces among the three files systems (71.50±213.5, 255.0±710.0, 351.0±776.5, mean±standard deviation, respectively) (P=0.54) (Figure 1. D). The percent of elimination of the microbial load was calculated for each instrumentation system using different concentrations of NaOCl and distilled water as a control, and there was a significant difference in the percent elimination of microbial load with NaOCl in comparison to distilled water (WaveOne Gold=0.6±0.54 vs 99.9±0.04, mean±standard deviation, Reciproc=0.6±0.0547 vs 99.98±0.044, mean±standard deviation, TFA=0.06±0.54, vs 99.9±0.047, mean±standard deviation (P<0.0001) in all instrumentation systems (Figure 2. A-C), but there were no significant differences emplo- ying 2.25% NaOCl in comparison with 5.25% in all cases (100±0.00015, 100±0.01, 100±0.002, mean±standard deviation) (P>0.05) (Figure 2. A-C). When comparing the elimination percent of the microbial load with the three different systems using 2.25% NaOCl as an irrigant, there was no statistically significant difference (p=0.34) (Figure 2.D). In controls without instrumentation, in vitro formation of E. faecalis and C. albicans biofilms was observed; cocci and yeast are observed at different magnifications along the root canal (Figure 3. A-B).
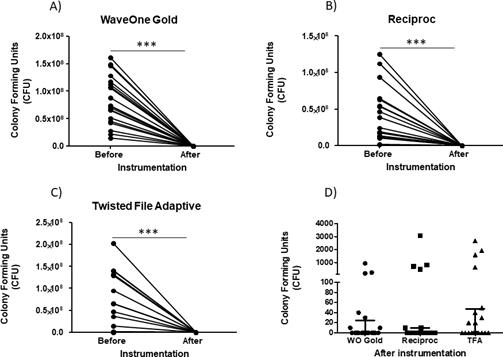
Figure 1 Diminution of colony forming units after instrumentation. The counts for the diminution of the bacterial load of the mixed biofilm was performed as indicated in the methods for each different instrument system employed. A) Number of colonies forming units (CFU) before and after instrumentation with the WaveOne Gold system. B) Number of colonies forming units (CFU) before and after instrumenta- tion with the Reciproc system. C) Number of colonies forming units (CFU) before and after instrumentation with the Twisted File Adaptive (TFA) system. D) Number of colonies forming units (CFU) after instrumentation with WaveOne Gold, Reciproc and Twisted File Adaptive (TFA) systems. Horizontal lines (D) correspond to the median and the first and third quartiles. P=0.05; P=0.01; P=0.005.
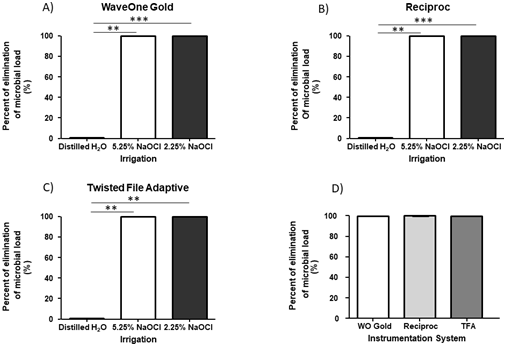
Figure 2 Percent of elimination of the microbial load. The percentage of elimination of the microbial load was calculated for each instrumentation system employed. A) Percent of elimination of the microbial load for the Wave One Gold system. B) Percent of elimination of the microbial load for the Reciproc system. C) Percent of elimination of the microbial load for the Twisted File Adaptive (TFA) system. D) Percent of elimination of the microbial load for the WaveOne Gold, Reciproc and Twisted File Adaptive (TFA) systems. Horizontal lines (A-D) correspond to the mean. P=0.05; P=0.01; P=0.005.

Figure 3 In vitro formation of the biofilm of E. faecalis and C. albicans. Controls without instrumentation were employed to evaluate the in vitro formation of the biofilm on root canals and root canals without biofilm formation was employed as negative controls. A) Root canals without biofilm formation, 400X, 1000X and 2000X of magnification. B) Cocci and yeast corresponding to E. faecalis and C. albicans inside root canals at 2500X, 7500X and 10000X of magnification. C) Inside of a root canal with a biofilm at 55X, 4000X and 10000X.
Evaluation of dentinal permeability. The dentinal permeability of the apical third was evaluated by scanning electron microscope as indicated in material and methods. There were no significant differences in the percent permeability of the dentinal tubules evaluated at 1mm in the different instrumentation systems, WaveOne Gold, Reciproc and Twisted File Adaptive (35.71±28.35, 17.86±27.82 and 46.43±39.34, mean±standard deviation) (p=0.285) (Figure 4. A). Similar results were observed at 3mm, where no significant differences in the percent of dentinal permeability in the different instrumentation systems was found (35.71±31.81, 46.43±39.34 and 50.0±40.82, mean±standard deviation) (P=0.753) (Figure 4. B).
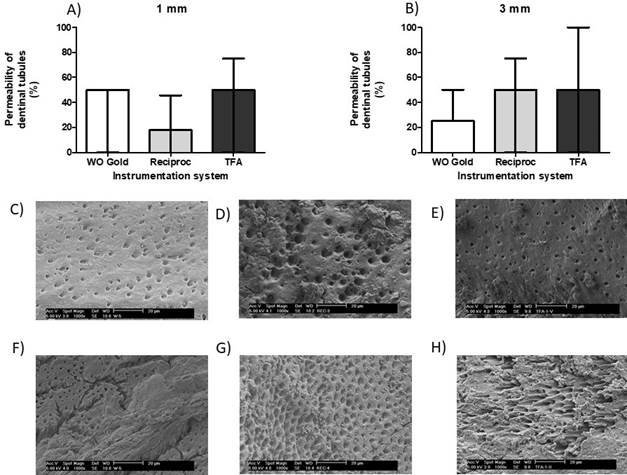
Figure 4 Permeability of the dentinal tubules of the apical third. The permeability of the dentinal tubules was evaluated at 1 and 3 milli- meters by scanning electron microscopy for the evaluation of the cleaning of the apical third of the dental roots. A) Percent of permeability of the dentinal tubules at 1 millimeter (mm) for the WaveOne Gold, Reciproc and TFA systems. B) Percent of permeability of the dentinal tubules at 3 millimeters (mm) for the WaveOne Gold, Reciproc and TFA systems. C) Scanning electron microscope image of a root canal at 1mm instrumented with WaveOne Gold. D) Scanning electron microscope image of a root canal at 1mm instrumented with Reciproc. E) Scanning electron microscope of a root canal at 1mm instrumented with Twisted File Adaptive. F) Scanning electron microscope image of a root canal at 3mm instrumented with WaveOne Gold. G) Scanning electron microscope image of a root canal at 3mm instrumented with Reciproc. H) Scanning electron microscope image of a root canal at 3mm instrumented with Twisted File Adaptive. Horizontal lines (A, B) correspond to the median and the first and third quartiles.
Discussion
There are several endodontic pathogenic microorganisms capable of causing pulp pathology; one of the aims of root canal treatment is to decrease these opportunistic pathogens and maintain the ideal shape and anatomy of the root canals (15). Indeed, there are many instrumentation systems that allow the reduction of the microbial load in the minimal amount of time and maintain the root canals in the best possible conditions (16). However, in some cases, it is not possible to eliminate all microorganisms present inside root canals, driving to an endodontic failure or endodontic secondary infections. The microorganisms commonly found in secondary infections are E. faecalis, with an incidence of 24-77%, which is associated more frequently with asymptomatic cases (17). Nevertheless, there are many studies that have demonstrated the presence of fungi in endodontic infections, not only bacteria. Thus, fungi are also of interest in the development of periradicular infections since they can form biofilms alone or with other bacterial species (18). The most common fungus that causes infection in root canals is the yeast C. albicans, as well as its pseudohyphae, which can penetrate dentinal tubules (19). Thus, we decided to employ these two microorganisms in this study. In addition, there are some studies indicating that E. faecalis has the capacity to invade the dentinal tubules and survive endodontic treatment, staying viable ex vivo in root canals for at least 12 months (20). Sen et al. (7) have demonstrated that the presence of the smear layer increases the adherence of C. albicans in the dentine. They raised the hypothesis that an increase in adhesion is due to the disintegrated organic structure of dentine and the availability of calcium ions as sources for growth and adherence. These microorganisms form biofilms, which make it difficult to eliminate microbes from root canals. In this study, the formation of a mixed biofilm was performed using a static system over 31 days, as has been reported in other studies where maturation of the biofilms of these microorganisms is carried out over at least 20-30 days (7).
The decrease of endodontic microorganisms can be achieved by indirect methods, for example, by not eliminating the endodontic microorganisms but rather by only altering their environment. It has been demonstrated that a change in the internal environment where the infection is produced can destabilize bacterial metabolism and thus reduce the number of bacteria and the severity of pathology (21,22). This is an important factor that should be considered in future in vitro evaluations.
Recently, it has been confirmed that single file instrumentation systems can prepare and completely clean the root canals (23) and decrease the global time of work by 40% in comparison with traditional rotatory techniques using continuous movement3, but the downside to using these NiTi instruments is fracture risk associated with an increase of fatigue caused by repetitive use and the possibility of cross contamination (24). There- fore, it is recommended to use multiple file instru- mentation systems of different diameters with the aim of gradually widening the root canal. For this reason, in the present study, the evaluation of the cleaning and disinfection efficiency of this system was performed.
Bürklei et al. (25) studied curved root canals in vitro using a single file instrumentation system such as WaveOne Gold and Reciproc in comparison to serial instrumentation systems such as Mtwo and Protaper, and they compared the time of work and the cleaning using 2.25% NaOCl as an irrigant; in addition, the evaluation of the cleaning was performed using a numeric scale, and the presence of a smear layer was evaluated by SEM of the coronal, medium and apical third of the root canals. In the four systems, they found similar results with partially no instrumented areas. In this study, no significant differences in the presence or absence of the smear layer or dentinal permeability among the different instrumentation systems studied were found; similar results have been observed in other studies (16,26,27,28). Thus, the single file instrumentation systems are as effective in the cleaning of root canals as the multiple files instrumentation systems. In addition, these single file systems (WaveOne Gold and Reciproc) showed shaping ability in severely curved canals (29). However, opposite results have been observed in other studies, which mention that multiple file systems (as Hyflex EDM and XP-endo shaper) show significantly greater bacterial reduction in comparison to single file systems such as WaveOne Gold (30). These authors concluded that the limited shaping ability of single file systems in wider canals could compromise disinfection.
Other authors have reported that a reduction in the bacterial inner root canals does not depend on the instrumentation system employed; they conducted a study comparing different instruments and concluded that the key factors for bacterial reduction are the extension and irriga- tion of root canals, in addition to the total time of instrumentation and irrigation (31,32,33).
In this study, we evaluated the elimination of the intraradicular biofilm of three different instrumentation systems in vitro; however, in vivo studies evaluating the microbial load before and after the instrumentation process in patients who underwent endodontic treatment and comparing different single and multiple file systems are necessary to support these results. In addition, it would also be interesting to perform in vitro studies evaluating the reduction of biofilm in teeth with major curvatures, since the decrease represents a greater challenge by the endodontist, and more technical ability is required in those cases.
The relevance of this study relies in the fact that in endodontic practice, one of the main objectives is the cleansing of root canals; this objective is achieved, among others, by using the instrumentation systems. The single file systems have the advantage of reducing work time and eliminating the endodontic microorganisms in the same form as the multiple file systems; in addition, the shown simplicity and reduction of crossed contamination, and the fact that every time there is a better acceptance of the single files instrumentation by Endodontists. Although there are several studies focusing on the evaluation of the ability of cleansing and disinfecting different instrumentation systems, this study has data with a mixed biofilm of endodontic treatment resistant microorganisms, since biofilm usually consists of different species in root canals.
Conclusions
The use of single file instrumentation systems is comparable in the reduction of bacterial concentration and the cleaning of the apical third with multiple file instrumentation systems.
Author contribution statement
Conceptualization and design: V.M.G. and C.C.C.
Literature review: M.V.N. and A.H.D.L.
Methodology and validation: V.M.G., A.M.G.A. and A.A.P.
Formal analysis: M.V.N.
Investigation and data collection: A.P.G., M.V.N. and D.L.A.H.
Resources: C.C.C.
Data analysis and interpretation: A.A.P. and M.V.N.
Writing-original draft preparation: A.P.G., M.V.N. and D.L.A.H.
Writing-review & editing: A.P.G.
Supervision: A.P.G.
Project administration: V.M.G.
Funding acquisition: V.M.G.















