Introduction
Juglans neotropica (Diels), commonly known as walnut, is a tropical species native to South America and is distributed between 1800 and 2800 masl, in dry and low humid montane forests (1). In Costa Rica, this species is suggested to be planted in the future due to its high-value timber (2). Some research in areas producing species of the genus Juglans has shown that fungal species of the Botryosphaeriaceae family produce cortical lesions on stems, shoots and twigs and fruit rots and leaf spots (3).
In the rainy season, in the province of Cartago, Costa Rica, plants of the species J. neotropica with dieback were observed for the first time, producing death of leaves and shoots and cankers in the wood. Therefore, the objective of this study was to identify the pathogen causing apical dieback of Juglans neotropica.
Materials and methods
In an 8 months old plantation of J. neotropica, , located in the province of Cartago, Agua Caliente district (9°49' 50'' N and 83° 54' 01'' W) Ciudad de los Niños, a total of 178 J. neotropica trees showed apical death (Fig. 1.a). Affected trees were cut and were transferred to the Forest Pathology Laboratory of the Tecnológico de Costa Rica. Samples were surface disinfected using liquid antibacterial soap and distilled water. Sections of 1.5 cm were cut from the pathogen advance zone (between healthy and stained wood) and superficially disinfected with 70% alcohol for 30 s, 5% sodium hypochlorite for 2 min and rinsed three times with sterile distilled water, finally placed on an absorbent paper napkin and transferred to a petri dish containing Potato Dextrose Agar (PDA) with antibiotic (Chloramphenicol 0.2 g L-1 + Streptomycin 0.2 g L-1 + Penicillin 0.2 g L-1) and incubated at 25°C for seven days. After incubation, individual colonies were obtained by transferring hyphae to the PDA culture medium with antibiotic.
Mycelial discs of the isolates obtained and sterilized pine needles were placed in Petri dishes with 2% agar-water culture medium (AA) to induce sporulation and to obtain the reproductive structures of the fungi; these plates were incubated under UV light near 25°C. For morphological identification 30 measurements of conidia length and width were performed and the mean maximum, minimum, standard deviation and 95 % confidence intervals were calculated. Molecular identification was performed by DNA extraction, followed by PCR with universal fungal primers (ITS4 and ITS5) which were sequenced by Macrogen in South Korea. Consensus sequences were compared with GenBank accessions using the NCBI Blast tool and neighbor-joining analysis was conducted by MEGA version 11 (4).
Pathogenicity tests were carried out on five-month-old J. neotropica plants with an average height of 29.19 cm and an average diameter of 8.66 mm. The surface of the stems to be inoculated was disinfected with 70 % alcohol, a wound with a n°2 laboratory punch (to expose the cambium) was made, and a mycelial plug was inserted in that area, the wound was covered with Parafilm for five days to avoid dehydration. For the control treatment, the plants were inoculated with sterile PDA. Wound length was assessed 30 days after inoculation. For statistical analysis, a t-test for independent samples was performed with Infostat software. Subsequently a re-isolation of the inoculated plants was carried out, to comply with Koch's Postulates.
Results and discussion
The presence of diseased trees in the plantation of this study represents an incidence of 30%.
Seventy percent of the fungal isolates were obtained that initially formed white colonies that turned olive-gray on the top and black on the back of the plate over the days (Fig. 1.b).
On Pinus sp. needles, solitary black globular conidiomata (pycnidia), covered with grey mycelium, were formed (Fig. 1.c). Hyaline conidiogenous cells (Fig. 1d) and unicellular, hyaline, fusiform conidia, some with truncated base, average size 16.6 µm ±1 µm x 5.8 µm ± 0.5 µm (LxW); ranges (14.9 µm-18.3 µm)x (4.8 µm-6.9 µm) (Fig. 1.e), were observed. All these morphological characteristics coincide with those reported by (5) and correspond to the fungus Neofusicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillip.

Figure 1 Symptoms and fungal structures of Neofusicoccum parvum on Juglans neotropica: a) walnut plant with apical dieback, b) colony colour on PDA on top and reverse side of plate, c) conidiomata (pycnidia) on pine needles, d) conidiogenous cells, e) conidia. Scale: 10 µm (d and e).
In addition, in the ITS gene sequencing results (ITS4-ITS5) the isolates obtained were also identified as N. parvum (GenBank Accession MN634050.1) with 100% similarity. Based on Neighbor-Joining method, phylogenetic inference of the ITS DNA sequences Neofusicoccum parvum_J.neotropica CR isolate clustered together within other species of Neofusicoccum parvum (Fig. 2). Evolutionary analyses were conducted in MEGA 11 (4).
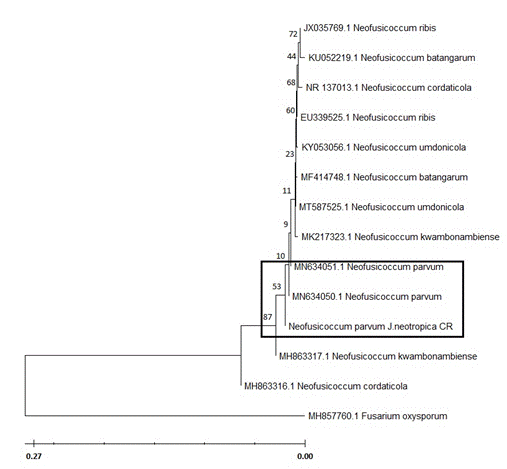
Figure 2 Phylogenetic tree based on ITS DNA sequences of representative isolates of Neofusicoccum parvum and some other species of Neofusicoccum, generated by Neighbor-Joining method.
In pathogenicity tests, the inoculated N. parvum isolate caused larger lesions, with a mean length of 51.5 mm, while the control had lesions of 7.8 mm in length showing significant difference (α≤0,05) and normal distribution among the variables. This confirms the pathogenic capacity of the fungus on J. neotropica plants. The same inoculated organism was reisolated from these plants, thus completing Koch's postulates.
Based on the Bootstrap test of 500 replicates, the percentage of clustering of trees by the associated taxa was obtained, which is shown above the phylogenetic tree’s branches. Using the Kimura 2 parameter model, the evolutionary distance was calculated and the variation of the rate between sites with a shape parameter of 0.21 was determined according to a gamma distribution. The tree was rooted to Fusarium oxysporum, Neofusicoccum parvum_J.neotropica CR is highlighted in the tree.
In Juglans regia (L.), symptoms induced by N. parvum have been observed in diverse geographic regions. Notably, in Turkey, this pathogen has been linked to branch dieback (6), while in Australia, it has been identified as a contributing factor to fruiting spur dieback (7). In China, the pathogen was detected causing cankers and plant mortality (8). In Italy, the fungi N. mediterraneum and N. parvum were isolated from cankers, leading to symptoms such as branch dieback, wood discoloration, and gummosis (3). To our knowledge, this is the first formal report of N. parvum causing J. neotropica dieback in Costa Rica.
Conclusions
Dieback symptoms were observed in 30 % of the trees established in an 8-month-old walnut plantation in Ciudad de Los Niños, Cartago, Costa Rica.
Neofusicoccum parvum has been identified as the causal agent responsible for the dieback disease in Juglans neotropica.
To our knowledge, this is the first report of N. parvum causing the death of J. neotropica in Costa Rica.














