Rubiaceae Juss is the fourth largest angiosperm family, consisting of approximately 611 genera, with herbaceous and woody arboreal species that have flowers adapted to different pollinators (The Angiosperm Phylogeny Group et al., 2016). Globally, it is distributed in tropical and subtropical regions, and 30% of its species are found in Latin America (Dutra et al., 2020). Within this family, the genera Borreria G. stands out with approximately 80 species, distributed from the south of the United States to the center of Argentina and Uruguay and some adventitious species in Africa, Asia and Oceania (Miguel et al., 2022). In South America, 30 species are recorded, which grow preferably in open fields withs and y or lateritic soils and less frequently in humidor rocky environments with shrubby or larger elements. (Zuloaga et al., 2019).
Borreria spinosa Cham. & Schltdl. ex. DC., is an herbaceous or sub shrubby plant, 0.1 to 1 meter high. It has a tetragonal, glabrous or pubescent stem. Leaves are pseudo verticillate due to the presence of brachyblasts, sessile to pseudo speciolate. It has elliptic leaves, acute apex, attenuated base, with glabrescent abaxial and adaxial faces, sometimes with papillae of thick base on the abaxial face. It also has terminal and axillary bilateral inflorescences, with one, two or five glomeruli per branch, hemispherical. It is characterized by oblongoid capsules, brown, hairy in the upper third, dehiscent in two valves united at the base. Seeds are oblongoid to subcylindrical and brown (Nepomuceno et al., 2018).
In Argentina, B. spinosa has been reported as a weed of agricultural crops that covers a wide region between the provinces of Salta and Buenos Aires and stands out for its low sensitivity to glyphosate (Luna & Druetta, 2018). Despite this, Scandaliaris et al., (2020) mentioned the apicultural potential of this species in the province of Córdoba. Salgado et al., (2014) reported the presence of traces of pollen of species of the genus Borreria in honeys from the province of Chaco. The presence of pollen of species of this genus has also been observed very frequently in honeys from northeastern Brazil (de Borges, 2020; Silva & Novais, 2020). These reports suggest the apicultural potential of B. spinosa.
In Borreria three ploidy levels 2x, 4x and 6x were recognized (Daviña & Cabral, 1991) and for the genus sporophytic and gametophytic chromosome counts of 2n=8; 40; 64 - 56 and n=14; 26- 27; 28 were recorded, respectively (Goldblatt & Johnson, 1990).
There are no references on the cytogenetic characteristics and palynological studies of B. spinosa. The first aspect contributes to the understanding of its reproductive biology, of great relevance in weed control. Palynology studies have been very useful in issuing allergy risk warnings, in honey production certification and in establishing taxonomic relationships (Marcos et al., 2015).
The aim of this work was to do the cytogenetic characterization of B. spinosa, describe the morphology of its pollen grains and estimate the potential viability of these grains.
Materials and methods
Study area and plant material: The collection of adult plants and seeds of B. spinosa was performed in the locality El Zanjón, province of Santiago del Estero, Argentina (27°55ʹ00ʹʹʹ S, 64°15ʹ00ʹʹʹ W), in May 2017. The village is located in the phytogeographic region of Western Chaco and has subtropical climate with dry season. The average annual rainfall is 579mm, and the average annual temperature is 19,8oC (Landi et al., 2021).
The reference material was deposited in the herbarium of the Facultad de Agronomía y Agroindustrias de la Universidad Nacional de Santiago del Estero (Figura. 1).
Characterization of mitosis: The roots used were obtained from seeds germinated in a humid chamber at 35°C (daytime) and 20°C (nighttime), with a 12-hour photoperiod. The roots were pretreated in 0,002M 8-hydroxyquinoline for 8 h at room temperature. They were then fixed in Farmer's solution (ethyl alcohol-acetic acid, 3:1) for 24 h and preserved in 70° ethanol at 4°C until use. The material was hydrolyzed in 1N HCl at 60°C for 15 min, and transient preparations were made and stained with a drop of 2% propionic hematoxylin. Observations were made under a ZeissAxio LAB.A1 optical microscope, Olympus, and chromosome length measurements were performed using MicroMeasure 3.3 software (Reeves, 2001).
The following variables were evaluated: total chromosome length (c), long arm length (l), short arm length (s), centromeric index (ci), total complement length (TCL), mean chromosome length (cme), maximum and minimum chromosome length (c max, c min). Chromosomes were classified according to the methodology proposed by Levan et al., (1964), inter- and intrachromosomal asymmetries were calculated according to Romero (1986), and the criterion of Bataglia (1955) was used for the description of satellites. For the elaboration of the karyotype and idiogram, seven metaphase plates with well extended chromosomes were considered.
Characterization of meiosis and pollen viability: Flower buds were immersed in Farmer's solution (ethanol-acetic acid 3:1) for 24 h and subsequently preserved in 70° ethanol at 4°C. Anthers were stained with a drop of 2% acetic orcein acetic acid and observed under a ZeissAxio LAB.A1 light microscope, Olympus.
Viability estimation was performed using the Müntzing, with a count of at least 1000 pollen grains (Maryam et al., 2015).
Pollen morphology: The material was prepared following the techniques for nonacetolysed and acetolysed pollen proposed by Wodehouse (1935) and Erdtman (1960), respectively. Preparations were mounted in glycerol gelatin. For scanning electron microscopy (SEM) analysis, acetolysed pollen grains were metallized with a gold-palladium bath. Pollen grains were described taking into account the number, type and position of apertures. The following parameters proposed by Faegri & Iversen (1966) were measured in 20 pollen grains: polar axis (P), equatorial diameter (E), shape (P/E), width and length of the colpi and thickness of the exine. Grain size was defined according to the size classes of Erdtman (1952). The terminology used in the description of pollen type corresponds to Punt et al., (1994).
Observations were made with a ZeissAxio LAB.A1, Olympus, optical microscope and a Zeiss Supra 55VP scanning electron microscope.
Results
The study evidenced that B. spinosa has a sporophytic number 2n = 56 chromosomes and karyotypic formula 46m + 10sm (Table 1).
Table 1 Morphometric parameters of Borreria spinosa chromosomes
| Chromosome pair | c(µm) x ± SD | l±(µm) x ± SD | s±(µm) x ± SD | Ci% | Chromosome type |
| 1 | 2,70±0,63 | 1,43±0,26 | 1,27±0,22 | 47,14 | m-sat |
| 2 | 2,61±0,54 | 1,44±0,27 | 1,17±0,12 | 44,75 | m |
| 3 | 2,47±0,40 | 1,32±0,15 | 1,15±0,10 | 46,54 | m |
| 4 | 2,38±0,31 | 1,32±0,15 | 1,05±0,01 | 44,33 | m |
| 5 | 2,32±0,25 | 1,26±0,09 | 1,06±0,01 | 45,63 | m |
| 6 | 2,27±0,20 | 1,24±0,07 | 1,04±0,01 | 45,62 | m |
| 7 | 2,24±0,17 | 1,20±0,03 | 1,05±0,01 | 46,67 | m |
| 8 | 2,18±0,11 | 1,23±0,06 | 0,94±0,11 | 43,36 | m |
| 9 | 2,14±0,07 | 1,17±0,01 | 1,00±0,05 | 46,94 | m |
| 10 | 2,12±0,05 | 1,14±0,03 | 0,98±0,07 | 46,36 | m |
| 11 | 2,06±0,01 | 1,10±0,07 | 0,96±0,14 | 46,76 | m |
| 12 | 2,02±0,05 | 1,11±0,06 | 0,91±0,14 | 44,86 | m |
| 13 | 1,98±0,09 | 1,08±0,09 | 0,90±0,15 | 45,24 | m |
| 14 | 1,91±0,16 | 1,06±0,11 | 0,85±0,20 | 44,31 | m |
| 15 | 1,87±0.20 | 1,05±0,12 | 0,82±0,23 | 43,78 | m |
| 16 | 1,79±0,28 | 0,96±0,21 | 0,82±0,23 | 46,08 | m |
| 17 | 1,76±0,31 | 0,97±0,20 | 0,79±0,26 | 44,98 | m |
| 18 | 1,73±0,34 | 0,92±0,25 | 0,81±0,24 | 46,79 | m |
| 19 | 1,68±0,39 | 0,92±0.25 | 0,77±0,28 | 45,60 | m |
| 20 | 1,59±0,48 | 0,91±0,26 | 0,68±0,37 | 42,85 | m |
| 21 | 1,50±0,57 | 0,81±0,36 | 0,69±0,36 | 45,74 | m |
| 22 | 1,41±0,66 | 0,76±0,41 | 0,64±0,41 | 45,72 | m |
| 23 | 2,49±0,42 | 1,55±0,38 | 0,94±0,11 | 37,99 | m |
| 24 | 2,45±0,38 | 1,69±0,52 | 0,76±0,29 | 31,17 | sm |
| 25 | 2,46±0,39 | 1,59±0,42 | 0,86±0,19 | 35,12 | sm |
| 26 | 2,26±0,19 | 1,47±0,30 | 0,79±0,26 | 35,03 | sm |
| 27 | 1,93±0,14 | 1,29±0,12 | 0,64±0,41 | 33,01 | sm |
| 28 | 1,81±0,26 | 1,14±0,03 | 0,67±0,38 | 37,19 | sm |
Mean total chromosome length (c), mean long arm length (l), mean short arm length (s), mean centromeric index (ci), metacentric (m), submetacentric (sm), satellite (sat) and standard deviation (SD).
In the first metacentric chromosome pair, the presence of a satellite in a terminal position on the short arm was observed (Fig. 2A).
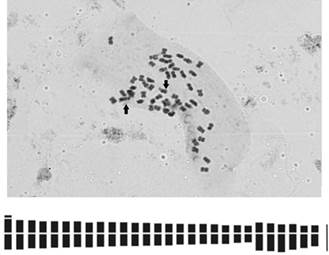
Figura. 2 Mitotic metaphase and idiogram of Borreria spinosa. A) Metaphase 2n = 56 chromosomes. B) Idiogram (46m + 10sm). Arrows indicate the chromosome pair with satellite. Scale 10µm
The total length of the haploid chromosome complement was 58,12µm, the mean chromosome length was 2,07µm and the range of variation in mean chromosome lengths was 1,81- 2,70 µm (Table 2).
Table 2 Karyotypic parameters of Borreria spinosa
| Parameter | B. spinosa |
| 2n | 56 |
| Karyotype formula | 46m + 10sm |
| TCL (µm) | 58,12µm |
| cme (µm) | 2,07µm |
| cmax. (µm) | 2,70µm |
| c min. (µm) | 1,41µm |
| A1 | 0,20 |
| A2 | 0,18 |
Total complement length (TCL), mean chromosome length (cme), maximum and minimum chromosome length (c max, c min), mean centromeric index (ci), intrachromosomal asymmetry index (A1), interchromosomal asymmetry index (A2). m: metacentric, sm: submetacentric.
Chromosomes showed a gradual size decrease (Fig. 2B). The karyotype is unimodal, since the chromosome size was similar; this is evidenced by the value of the interchromosomal asymmetry index A2 = 0,18. The intrachromosomal asymmetry index indicates a symmetrical karyotype with A1 = 0,20.
B. spinosa has a gametophytic number of n =28 II (Fig. 3A). Numerous irregularities could be observed at both stages of meiotic division. Pollen mother cells (PMCs) in MI exhibited chromosomes outside the equatorial plate in 10% of them (Fig. 3B), AI with lagging chromosomes in 17% of them (Figura. 3C) and multiple spindle formation in about 90% of them (Fig. 3D). Cells in TI were regular, and lagging chromosomes were occasionally observed in 2% of them. During the first stage of meiotic division, cytomixis was observed with the formation of cytoplasmic channels between two or more cells in the stages of diakinesis (Fig. 3E), MI and TI (Figura. 3F).
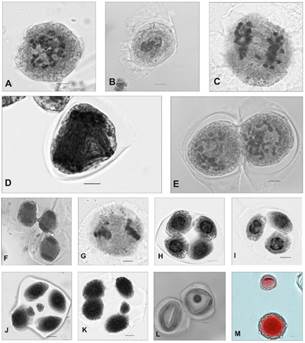
Figura. 3 Meiosis in Borreria spinosa. A) Diakinesis n = 28. B) MI. C) AI with lags. D) Multiple polar spindles in AII. E) Diakinesis with cytoplasmic channels. F) PMCs in AI with formation of cytoplasmic channels. G) MII with lagging chromosome. H) Tetrad. I) Triad. J) Hexad. K) Pentad. L) Cells without cellular content. M) Viable and nonviable pollen grain. Scale 5 µm.
In the second stage of meiotic division, PMCs in MII presented chromosomes outside the equatorial plate in 8% of them (Fig. 3G) and the formation of perpendicular spindles in 2% of them, whereas in AII and TII lagging chromosomes were observed in 2% of them. During spore formation, 1,47% triads (Fig. 3I), 81,54% tetrads (Fig. 3H), 15,86% pentads (Fig. 3K), 1,10% hexads with microspores and micronuclei (Fig. 3J) were recorded. Cytoplasmic channel formation was observed between spores. PMCs without cellular content were recorded throughout the division (Fig. 3L). Seventy percent of the pollen grains were viable; the rest were smaller and nonviable (Fig. 3M).
The nonacetolysed pollen grain (Fig. 4A-D) is small to medium-sized, suboblate to oblate spheroidal, with P 21 (23±1,58) 25μm and E 24 (26±1,32) 28μm and colpi5 (5,92±0,67) 6,60µm long to 1,70 (2,35±0,47) 3µm wide.
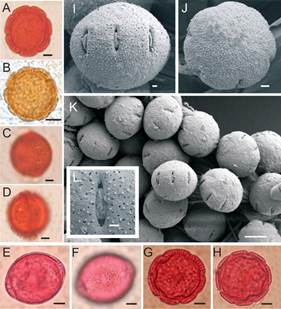
Figura. 4. Pollen grains on Borreria spinosa. A-D Nonacetolysed pollen. A) Equatorial view in optical section. B) Equatorial view on surface. C) Polar view in 8-colporate optical section. D) Polar view in 7-colporate surface. E-H Acetolysed pollen. E) Polar view in 9-colporate optical section. F) Polar view in 7-colporate optical section. G and H) Equatorial view on surface. I-L MEB. I) Equatorial view. J) Polar view. K) General view of the grains. L) Detail of aperture and surface. Scale 5µm
Acetolysed pollen grains (Fig. 4 E-H) are medium-sized, suboblate to oblate spheroidal, with P 24 (25,40±1,26) 27µm, E 27 (28,81±1,38) 31µm, circular in scope, 7-8-9 zonocolporate (52% 8- zonocolporate, 35% 7-zonocolporate, 13% 9-zonocolporate). Colpi are short (5,5-6µm long), and their width varies from one line, when fully folded, to 1,5µm, if expanded. The lalongate endo aperture is difficult to determine if it forms an endocingulum. The mesocolpium is 8,50 (10,19±1,07) 11,50µm, and the apocolpium is 21 (24,31±1,87) 26µm. Perforations are observed in the tectum. The exine, 3 to 4µm thick, decreases towards the colpi. In the sexine, thicker than the nexine, simple columellae are observed.
Scanning electron microscopic observation corroborates that the grains are 7-8-9 zonocolporate (Fig. 4I-J), with microperforations of various shapes, from 0,20 to 0,50 µm in diameter (Fig. 4K-L). The positive elements observed with optical microscopy are nano-spinules ranging from 0,25 to 0,30 µm in height with a sharp apex. Perforations, as well as nanospinules, are densely and regularly arranged.
Discussion
The basic chromosome number proposed for the tribe Spermacoceae is x= 14; thus, B. spinosa would be a tetraploid species with 2n = 56.
In accordance with the results obtained for B. spinosa, Selvaraj (1987) performed chromosome counts and karyotypes in B. articularis and B. pusilla and reported the same chromosome number (2n = 56) with ranges of variation from 1 to 3,5 and 0,5 to 3,5 µ, respectively. The chromosome variation recorded in B. spinosa is more closely related to that of B. articularis. In addition, this author reported karyotypic formulas 30m + 18sm + 4st+ 2B (B chromosomes) for B. articularis and 30m + 22m + 4st for B. pusilla that do not coincide with those recorded in the species under study (46m + 10sm). Selvaraj (1987) observed the presence of satellites in several chromosomes, which agrees with what was observed in B. spinosa, although in the latter satellites were only present in the largest chromosome pair.
Meiosis is a decisive process for sexual reproduction in plants. Abnormalities are occasionally observed that can lead to sterility of gametes as well as variation in their genetic constitution (Thangavel et al., 2023; Wang et al., 2023). Extensive chromosomal rearrangement and the formation of a single, transient spindle during mitosis and meiosis ensure proper chromosome segregation (Felismino et al., 2015). In plants, the formation of a bipolar spindle is the rule and allows for viable gametes and balanced genetic constitution. However, the formation of multipolar spindles can lead to meiotic irregularities (Shamina et al., 2003). Borreria spinosa exhibited multipolar spindle formation consisting of three or more poles; its chromosomes, according to Sidorchuk & Deineko (2014), would be randomly positioned in metaphases and then distributed in three or more directions during anaphase. This irregular spindle activity would result in the random misorientation of chromosomes in PMCs and subsequent formation of independently oriented chromosome subgroups. In the taxon studied, irregular spindle activity could be responsible for dyads, triads, polyads and micronuclei during microsporogenesis and subsequently result in pollen grains with reduced viability, as observed by Shamina et al., 2003.
During the first stage of meiotic division, a little-known phenomenon was recorded: cytomixis. It involves the transfer of chromatin or nuclei among plant cells through intercellular channels, frequently observed during microsporogenesis (Mursalimov et al., 2014; Mursalimov & Deineko, 2017). Whether cytomixis is a spontaneous or induced process, it can have severe genetic consequences, which combined with other meiotic abnormalities significantly affects pollen fertility (Mursalimov & Deineko, 2017; Mandal & Nandi, 2017).
The anomalies observed during meiosis are related to pollen sterility and low seed production characteristic of this species, which has the ability to reproduce sexually and asexually (Luna & Druetta, 2018). Thus, the presence of meiotic irregularities may imply that the process of sexual reproduction is not fully effective for its propagation.
According to the literature, the pollen morphology of B. spinosa has not yet been studied; on the contrary, different authors have found considerable variations in the pollen characters of this genus, thus defining a Euro-pollen genus. Gonçalves-Esteves et al., (2020) described the morphology of pollen grains of Rubiaceae from the Atlantic Forest of the state of Rio de Janeiro (Brazil), including
B. brachystemoides, B. capitata, B. latifolia, B. palustris, B. scabioisoides and B. verticillata. In these species, the pollen grains were small to medium-sized, oblate-spheroid and suboblate, colporate, with varying numbers of openings (5-12). Polar diameter was highly variable among the species studied, with a minimum value of 24,1±0,2 μm in B. brachystemoides and a maximum of 43,6 ±0,3 in B. latifolia. The equatorial diameter ranged between values of 25,7±0,1 in B. brachystemoides and 48,7 ± 0,3 μm in B. latifolia. Dutra et al., (2020) described B. verticillata (L.) G. Mey pollen grains collected in the Brazilian cerrado as medium-sized, prolate spheroidal, with a polar diameter ranging from 27,5 to 32,5 μm and an equatorial diameter ranging from 25,0 to 27,5 μm, 7-colpate and tectate-microechinate exine.
Borreria spinosa is a tetraploid, and most of its chromosomes are metacentric or submetacentric. It exhibits irregular meiotic behavior and cytomixis, with concomitant low viability in pollen grains. In this taxon, palynology showed that pollen grains have similar characteristics to other Borreria species.
Ethical, conflict of interest and financial statements
The authors declare that they have fully complied with all pertinent ethical and legal requirements, both during the study and in the production of the manuscript; that there are no conflicts of interest of any kind; that all financial sources are fully and clearly stated in the acknowledgements section; and that they fully agree with the final edited version of the article. A signed document has been filed in the journal archives.
The statement of each author’s contribution to the manuscript is as follows: P.B.P, V. de los A.P, and D.A.M.: Study design, data collection, analysis, result interpretation and manuscript writing.
R.A.A. and M.L.E.: Data collection and final approval of the manuscript. All co-authors: preparation and final approval of the manuscript.












 uBio
uBio 



