Introduction
Pinalia Lindl. with the type species, Pinalia alba Buch.-Ham. ex D.Don, belongs to subtribe Eriinae Benth. whithin the tribe Podochileae Pfitzer (Cribb & Ng in Pridgeon et al. 2005). It comprises about 21 species in Peninsular Malaysia (POWO 2023). Pinalia was previously classified within polyphyletic genus Eria Lindl., comprising taxa formerly treated under Eria subgenus Pinalia and Eria sections Hymeneria, Polyura, Secundae, and Urostachya. The most recent phylogenetic study by Ng (2002), cited in Pridgeon et al. (2005) and Wood et al. (2011) resurrected Pinalia as a distinct genus. Among its sections, only sect. Urostachya, sect. Hymeneria, and sect. Pinalia exhibit a trilobed lip. In general, Pinalia is characterized by fleshy stems with enlarged internodes covered by a semi-transparent leaf sheath, with a few leaves near the apex (O'Byrne et al. 2018). Pinalia is morphologically similar to Bryobium Lindl., but it can be distinguished by its plurifoliate pseudobulbs or stems (2-6 leaves in the upper part) and either resupinate or non-resupinate flowers (only non-resupination has been observed in Bryobium). Most of its species, previously under sect. Urostachya have long-tapering labellum with basal lobes or a densely lanate inflorescence rachis. Pseudobulbs are clustered, generally about half the length of the leaves or longer, and not sequentially borne on the rhizomes (Pridgeon et al. 2005). The flowers are usually small, mostly white, or greenish yellow, with not spreading tepals, but they are attractive in various sorts of forms and arranged in a large cluster on one peduncle, which is why at least two species of Eria was commonly called ''Lily of the Valley Orchid' (Pinalia bicolor (Lindl.) Kuntze (Internet Orchid Species Encyclopedia 2023); Pinalia spicata (D.Don) S.C.Chen & J.J.Wood (Lindley 1841)). While easy to grow, these species are not as popular as other species and orchid growers seldom cultivate them compared to other orchid species, such as the slipper orchids of genus Paphiopedilum, or butterfly orchids of Phalaenopsis, due to their less showy features. Their gross floral and vegetative morphology is important for distinguishing sections and species. The shapes of floral parts (especially the labellum) are critical in sectional delimitation, while the pollinia shapes are useful for species differentiation (Go & Tang 2007). However, the sectional treatment is as subjective and insufficient for both Pinalia and Eria (O'Byrne et al. 2018). Nevertheless, assessing diversity, geographic distribution, and conservation status of a species requires the taxonomic expertise of the assessor.
Apart from the unclear taxonomic circumscription, species of Pinalia are rare, endemic, and confined to the highland areas, and their conservation should be a high priority both globally and regionally (Besi et al. 2020a, Brundu et al. 2017, Juiling et al. 2020, Schmeller et al. 2008). Orchids with narrow and limited distribution ranges can be severely threatened, even if they are locally abundant, especially in this era of prevalent anthropogenic disturbance (Dixon et al. 2003). Therefore, this article focuses on Pinalia elata, an endemic species, recorded in only six localities in Peninsular Malaysia. Unfortunately, previous descriptions of P. elata in Hooker's protologue of Eria elata (the homotypic synonym of P. elata) and in Ridley's monograph were brief and lacked a detailed description of the floral colour variation and size. A major problem with endemic species occurring in very few populations is the very low chance of finding individuals with a complete floral structure. The lack of fertile individuals, combined with declining habitat quality results in a lack of knowledge about this species. In the case of Pinalia elata, for example, only six populations are known, with four occurring on the summit of Genting Highlands. This area is the most accessible cloud forest habitat in Peninsular Malaysia and nourishes high abundance of orchid species and endemism. Unfortunately, the summit area has been seriously disturbed since the development of Genting Highlands began in 1967. The development, which includes hotels, casinos, and a theme park, has led to habitat destruction (Besi et al. 2020b, Go et al. 2015, Ng et al. 2012, Stone 1981). Fragmentation of the montane forests can have a damaging impact on biodiversity, which is a key indicator of ecosystem stability (Chua & Saw 2001, Korner 2002). Recognising the role of experts under the IUCN Species Survival Commission (SSC), and with assistance from the Awana Genting Highland's Conservation Management Group, this paper provides taxonomic notes of the less-known Pinalia elata, as well as primary in situ data on its local distribution, habitat ecology, phenology, and microclimate. These data are relevant for a conservation assessment of this endemic species.
Materials and methods
The morphological description was based on the specimens collected from a cloud forest habitat of the summit area in Genting Highlands. An existing trail was used in the forest to access the orchids comprehensively. The specimens were deposited in the Herbarium of the Department of Biology, Faculty of Science, Universiti Putra Malaysia (UPM). The measurement and morphological examination of spirit-preserved floral specimens were performed using the AM4113ZT Dino-Lite Digital Microscope (AnMo Electronics Corporation, Taiwan). The identification of specimens was based on their macro-morphological characteristics: (1) vegetative characters: life form, growth type, rhizome, stem or pseudobulb, leaf number, leaf arrangement, leaf marking, leaf shape, leaf size, leaf surface appearance; (2) floral characters: inflorescence type, inflorescence position, flowers arrangement, flower size, flower colour, perianth appearance, labellum shape, column shape. Morphology and distribution studies were done by examining the type specimens, monograph, and protologue. The specimen details were compared in detail with original drawings and descriptions provided in the protologue of Eria elata (homotypic synonym of P. elata in Hooker 1889), and description of the species in the monograph of Seidenfaden & Wood (1992), and several type specimens deposited in both local and international herbaria. The second author examined materials in KEP and KLU herbaria. Meanwhile, the herbarium study for specimens deposited in the international herbaria, CAL, K, NHN, and SING, was limited to the study of digital images. Herbarium collections, botanical drawings, and records deposited in the National Herbarium of the Netherlands (NHN) were accessed through Browse Dutch Natural History Collections: BioPortal (Naturalis) (http://bioportal.naturalis.nl/), Herbarium of Singapore Botanic Gardens (SING) were accessed through BRAHMS Online managed by University of Oxford (http://herbaria.plants.ox.ac.uk/bol/sing), Botanical Survey of India, Indian Virtual Herbarium (https://ivh. bsi.gov.in/), Kew Herbarium Catalogue (http://apps.kew.org/herbcat/gotoSearchPage.do), and Plants of the World Online (POWO) (http://www.plantsoftheworldonline.org/) were studied and cited in the taxonomic treatment. In some cases, unavailable digitised images in the online portals were assessed by image sharing via email with the curators. Orchid species have undergone nomenclatural changes over time. Therefore, the accepted name was based on POWO.
The conservation assessment was based on information collected during the field survey and herbarium collections. The endemism of the species was also checked via the POWO (2023). The conservation status was assessed following the IUCN Red List of Threatened Species Version 2021-3 (IUCN 2022). The range of distributions and distribution maps of the species were assessed using Geospatial Conservation Assessment Tool (GeoCAT) (http://geocat.kew.org/) (Bachman et al. 2011). The extinction risk for the species was determined based on Criteria A, C and D. To allow the underlying data to be analysed, a set of standard terms (Classification Schemes) (https://www.iucnredlist.org/resources/classification-schemes) developed by IUCN for documenting taxa on The IUCN Red List was followed. Additionally, a heatmap for the mean temperature trend of Peninsular Malaysia from January 2012 to April 2022 was designed using Inverse Distance Weighting (IDW) interpolation method di QGIS 3.10 (Copyright(c) 2004-2020 QGIS Development Team, Website: https://www.qgis.org) with distance coefficient of 2 and pixel size 0.001, and interpolation of 11 climate stations or temperature stations data (Sitiawan, Lubok Merbau, Ipoh, Cameron Highlands, Batu Embun, Muadzam Shah, Temerloh, Kuantan, Subang, Petaling Jaya, and KLIA Sepang) from Malaysia Meteorology Department. It is important to note that these weather stations were placed under artificial shade, not within vegetation cover.
Taxonomic treatment
Pinalia elata (Hook.f.) Kuntze, Revis. Gen. Pl. 2: 679 (1891). ≡ Eria elata Hook.f., Hooker's Icon. Pl. 19: t. 1848 (1889) (Hooker 1889, Ridley 1924). Type: PENINSULAR MALAYSIA. Perak: B. Scortechini 569b (lectotype designated here: K-photo!; barcode: K000827353; isolectotypes designated here: CAL-photos!; barcodes: CAL0000000280, CAL0000000281).
Specimen examined: PENINSULAR MALAYSIA. Pahang: Mount Ulu Kali, ca. 1700 m elev, 24 October 2018, E.E. Besi, D. Sandin, P.J. Satap & R. Go EDW147 (UPM!) (Fig. 1-2).
Additional specimens examined: PENINSULAR MALAYSIA. Pahang: Ulu Kali, 6 November 1969, E.F. Anderson (GH-photo!; barcode: 02126402); Ulu Kali, 12 October 1974, M.M.J. Balgooy 2141 (NY-photo!; barcode: 04088049); Bentong, Genting Highlands, Mount Ulu Kali, 24 August 1971, J.B. Lowry 450 (KLU!); Bentong, Genting Highlands, Mount Ulu Kali, mossy forest, 29 January 1978, B.C. Stone BCS13553 (KLU!); Bentong, Genting Highlands, Mount Ulu Kali, montane elfinwood thickets, 21 December 1982, B.C. Stone BCS15362 (KLU!); Bentong, Genting Highlands, Mount Lari Tembakau, trail to the summit, 1,720 m a.s.l., 15 January 1994, A. Noorsiha, S. Kamarudin, B. Baya FRI41043 (KEP!); Bentong, Genting Highlands, trail to Batu Tunggul, 17 January 1994, B. Perumal, C.L. Gan, Angan, Bedul FRI41658 (KEP!); Bentong, Genting Highlands, Mount Ulu Kali, roadside near telekom tower, 24 March 2008, S.N. Phoon FRI60489 (KEP!); Bentong, Genting Highlands, Mount Chin Chin, 14 November 2009, A. Faiz FA007 (UPM!); Bentong, Genting Highlands, Mount Chin Chin, 15 December 2009, A. Faiz, FA019 (UPM!); Bentong, Genting Highlands, Mount Lari Tembakau, 12 June 2009, Shahrudin SHA007 (UPM!); Bentong, Genting Highlands, Mount Lari Tembakau, 21 October 2009, Shahrudin SHA058 (UPM!); Bentong, Genting Highlands, Mount Lari Tembakau, 18 December 2009, Shahrudin SHA064 (UPM!); Bentong, Genting Highlands, Mount Lari Tembakau, 21 October 2009, Y.J. Ng NYJ039 (UPM!); Bentong, Genting Highlands, Mount Lari Tembakau, 29 June 2010, Y.J. Ng NYJ169 (UPM!); Bentong, Genting Highlands, Mount Ulu Kali, 3 September 2010, Y.J. Ng NYJ200 (UPM!); Cameron Highlands, Mount Irau, 12 March 2023, Q.A. Mad Jabar, E.E. Besi, R. Go, I. Mat Esa, S.H. Tan, M.N. Ghazalli EDW148 (UPM!); SELANGOR. Mount Mengkuang Lebar, 20 January 1913, H.C. Robinson s.n. (SING-photo!; barcode: 0138302).
Left,
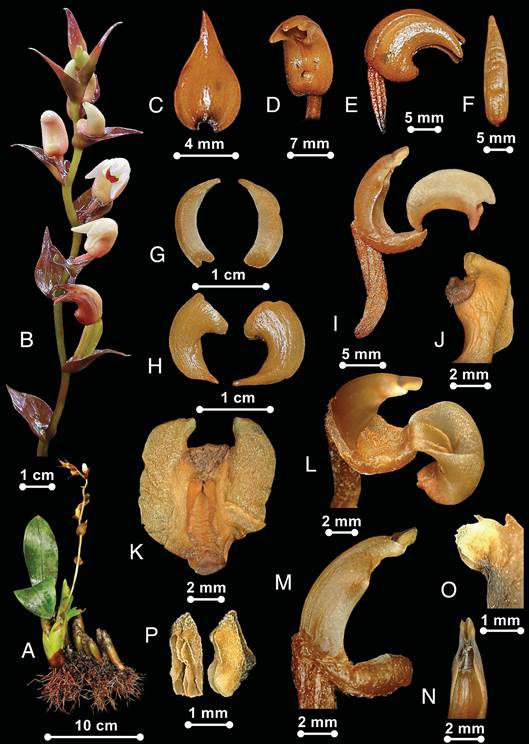
A. Plant. B. Raceme showing floral bracts. C. Floral bracts. D. Flower, front view. E. Flowers, lateral view. F. Dorsal sepal. G. Petals. H. Lateral sepals. I. Column + labellum, lateral view, showing non-saccate base. J. Labellum, lateral view. K. Labellum, flattened. L. Column + labellum, top view, showing labellum base hinged to the shortly pubescent column foot. M. Column with anther attached. N. Column apex, front view, showing concave with two wing-like stelids. O. Stellidia, lateral view, showing dentate outer margin. P. Flat anther-cap, front, and lateral views. Images by Edward Entalai Besi.
Figure 1 Pinalia elata.

A. A plant growing on Leptospermum branch. B. A plant showing an erect inflorescence. C. Front view of the flower. D. Lateral view of the flower.
Images by Edward Entalai Besi and Rusea Go.
Figure 2 Plant of Pinalia elata growing in-situ, in a montane habitat that is always cloaked by mist, cold, and experiencing high rainfall is an ideal habitat for P. elata.
Etymology: According to Pridgeon et al. (2005) and Schultes & Peace (1963), Pinalia was named after Chevalier Pinal, a French botanist who collected plants in the 19h century and paid particular attention to the smaller species. However, the neotropical genus Pinelia was named after Chevalier Pinel, not Pinal. Naive & Ormerod (2018) discussed the origin of Pinalia in their description of Pinalia jimcootesii, where we find: ''The genus Pinalia was proposed by John Lindley in Orchidearum Sceletos in 1826, based on an unpublished name coined by Francis Buchanan-Hamilton that was derived from a local Nepalese term for an orchid species called 'Bun Pinali.' 'Bun' meaning forest, and 'Pinalu' or 'Pinali' meaning a type of forest yam (Raskoti, pers. comm.).'' The specific epithet derives from the Latin 'elatus', meaning tall. Although Hooker did not specify what 'elata' refers to, it likely denotes the plant's combined height of pseudobulb and inflorescence.
Plant epiphyte and sympodial. Rhizome present but not clear and aggregate. Stems to 3-6 cm tall, 1-2 cm wide, oblong, clustered, 2 to 3-leaved, slightly tapering towards apex, greenish brown, covered by greenish-brown, triangular and overlapping sheath, sheath 2-4 cm long; internodes present about 1.5 cm long. Leaves 8-10 × 3.4-3.7 cm, elliptic to oblanceolate, apex obtuse, green, venation prominent at both abaxial and adaxial surfaces, sometimes shortly petiolated or subsessile. Inflorescence 20 cm long, 1-2 per stem, raceme, half of the length flowerless with sterile bracts, emerging laterally near the base of the stem, weakly flexuous, twice longer than the vegetative part, porrect to erect, glabrous, 5-6-flowered, peduncle yellowish green with pedicel brownish and pubescent, basal part covered by 2-5 cm long, rather thick sheaths; floral bracts 1.7-2.0 × 1-1.4 cm, ovate to triangular, apex attenuate to acute, purplish brown, base reniform, clasping to the peduncle. Flowers 11 × 8 mm, resupinate, always closed, dorsal surface keeled, yellowish brown with the apex of the petals and sepals white, petals and sepals pointy with basal parts fused to the column foot, the apex of the lateral sepals resemble a tusk, labellum enclosed by the petals and sepals, hardly appear, except the protruding, reddish and hairy apex, scentless. Mentum 3 mm long, sessile, shortly blunt. Pedicel-with-ovary 1 cm long, clavate, greenish brown, pubescent, reddish indumentum, ribbed. Dorsal sepal 19 × 4 mm, hooded, lanceolate, apex acute, mostly pointing downward. Lateral sepals 12 × 6 mm, 6 mm wide at base, oblong, apex acute to acuminate, concave, subfalcate. Petals 12 × 3 mm, oblong, apex acute, subfalcate, concave, dorsal surface keeled. Labellum 7 × 4 mm, trilobed, distally trilobed, curved in general outline, when flat, almost rhombic (looks like a bat spreading its wings when flattened), 8 × 7 mm when flat, enclosed by lateral sepals, immobile; claw 2 mm long, decurved, rectangular, hinged to the column foot; side lobes 2.5 × 1.4 mm, large, rounded to oblong, apex free, separated from triangular mid lobe by a distinct sinus, indumentum along the upper margins, free portion 1.5 mm long; mid lobe 1.7 mm long, rounded, apex decurved, thick, papillose, papillose callus 1.5 mm wide, margin revolute, base hinged to column foot, base not saccate, with three raised keels or ridges (middle keel shorter and indistinct) continuous from the base to 2/3 length of the blade, 3 mm long, deeply furrowed on the basal half; claw 1.7 mm long, more or less rhomboid. Column 8 mm long (including foot), somewhat fusiform, narrower and flat towards both ends; apex 2.9 × 1.7 mm, stiff, concave with two wing-like stellidia that create the shape of the trap and dentate outer margins, resembles a venus fly trap when anther-cap removed; stellidia rounded, apex margin dentate, 1 mm long; flap 0.4 mm, triangular; foot 6 mm long, 1 mm wide at the middle, curved halfway, 3 mm wide gutter-like structure with sides raised, swollen at the basal end, basal end shortly pubescent; anther-cap 1.7 × 0.6 mm, flattened, 1.7 × 2.0 mm, orbicular when flat; pollinia not seen. Capsules 2 cm long, winged.
Diagnostic characters: Fortunately, our collection of a healthy fertile individual with good-condition floral structure enabled a detailed morphological description of the species to be made. The morphological description is accompanied with additional notes on vegetative and floral parts, which are not available in the protologue. Although the sectional circumscription of Pinalia is still at an early stage with unresolved delineation, based on the traditional classification, P. elata belongs to the former Eria sect. Hymeneria by having the labellum claw attached by a hinge to the column foot and non-saccate labellum base. The basal margins of the labellum are not fused to the column margins as observed in some Pinalia species placed in the Eria sect. Urostachya. The lateral lobes of the labellum are not tapering and are rather widely rounded or rectangular in the middle of the labellum. The inflorescence, floral bracts, petals, sepals, and labellum were used to distinguish the taxon from others. The fertile plant is easily comparable to the other Pinalia species in Peninsular Malaysia by having an inflorescence with flowers well-spaced, subtended by rather large and brownish bracts, and always closed with labellum is completely enclosed by the petals and sepals. Moreover, the flowers are very distinctive due to the lateral sepals being incurved with pointy apices that somehow the form resemble a sorceress wearing a horned headdress and bat wing-like cloak with a pointed collar. Also, the labellum of the plant that we discovered is very similar to the Hooker's description of the holotype, especially on the morphology of the labellum ''the orbicular lip is very peculiar, the side lips being directed inwards towards the small mid lobe forming a large sinus, and embracing the latter'', as illustrated in Plate 1848, in Hooker (1889). In addition, the flower and labellum morphologies are also similar to the illustration given on one of the type specimens deposited in the Kew Herbarium (K), B. Scortechini 569b (barcode: K000827353). Although there is no specific designation on which type specimen, it was collected by Father Scortechini, the same collector of the holotype, in 1897, several years after the species was published. Therefore, this specimen in K is here designated as the lectotype of the name. It is clearly the basis for the name, because the specimen matches the plate in Hooker's Icones Plantarum and has Hooker's original sketches. Also, the two herbarium sheets in CAL (barcodes: CAL0000000280, CAL0000000281) would become isolectotypes. The image of holotype specimen (barcode: K000827475) used in POWO for P. elata is wrongly assigned and must be rectified, which it is the holotype of another different species of a different section, Cylindrolobus elatus J.J.Wood.
Habitat and ecology: Pinalia elata grows on thick moss pads on boughs of an intermediate tree in cloud forest or mossy upper montane forest and elfin forest, at an elevation of 1600-1800 m of altitude (Fig. 2). In Genting Highlands, based on genus and family dissimilarities, the forest at an elevation of 1100-1700 m can be classified as an upper montane forest (Nakashizuka et al. 1992). The upper montane and elfin forests are often referred to as 'cloud forest', since they are constantly intercepted with clouds and are often drizzling, even in the afternoon, contributing to the high moisture in the area. The elfin forest is characterised by having stunted, twisted trees with its canopy of 5-7 m tall, dense, flattened crown with coriaceous leaves (Stone 1981), such as Dacrydium comosum Corner, Leptospermum javanicum Blume, and L. polygalifolium Salisb., where the latter Leptospermum species or gelam bukit are the common phorophytes for P. elata. Leptospermum amboinense Blume is also seen growing within the same summit area. All three of these previous of Leptospermum were often misidentified as L. flavescens Sm.
Studies conducted on P. elata in the wild have shown that plants typically flower in October-November. Despite observations made during the visits, no pollinators were observed in its natural habitat. Information on pollinator visitation in the natural habitat for many orchid species remains limited as it is often accidental. However, crab spiders from the family Thomisidae were sometimes seen probing and feeding on plant materials or exudates on the floral parts of orchids, including P. elata in its natural habitat. However, no pollinarium removal or deposition by the crab spider was observed in Pinalia flowers (Fig. 3). Crab spiders are known to be insectivorous predators that prey on insects visiting the flowers. So, their presence on the flowers is not surprising. They may catch pollinating insects or discourage them from visiting the flowers. But spiders can benefit plants as they also eliminate plant-eating insects and their larvae that feed on the floral parts and damage the plant (Knauer et al. 2018, Vasconcellos-Neto et al. 2017). Information on plant-spider relationships is scattered throughout the literature, but a question remains: are crab spiders or spiders capable of cross-pollinating the 'almost' unopened flowers of P. elata? While unopened flowers such as those of P. elata are capable of autogamy resulting in fruit production, further study is needed to verify if crab spiders can remove the pollinia by fitting through the small opening of the P. elata flowers. The flat-shaped anther can be easily removed from the column apex; however, the narrow openings of the thecae may hinder pollinia removal. Also, the flowers did not emit any notable scent; suggesting that pollinator visitation was mostly triggered by the contrast of the bright reddish labellum. It can be supposed that the pollination strategy of P. elata is by both deception and reward.
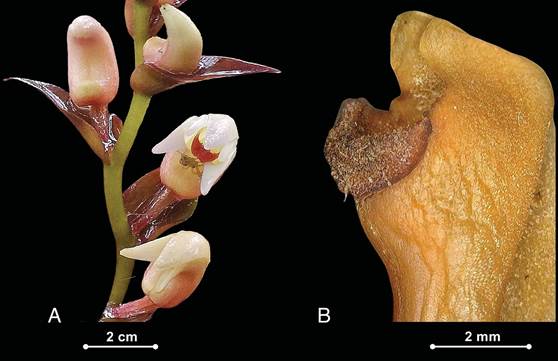
A. Rachis showing an opened flower with a prominent reddish and papillose labellum midlobe, and a crab spider can be seen feeding on exudate on the lower surface of the labellum. B. Lateral view of the spirit-preserved labellum showing papillose midlobe.
Images by Edward Entalai Besi.
Figure 3 Flower of Pinalia elata.
Distribution and endemism: It is a narrow endemic species confined to montane forests in Titiwangsa Range in Peninsular Malaysia. The primary specimen was found in the summit area of Genting Highlands, and the exact locality is withheld in this paper to protect the population from illegal collections. The summit area in Genting Highlands is located within Bukit Tinggi Forest Reserve to the east and Batang Kali Forest Reserve to the west. Bukit Tinggi Forest Reserve covers an area of 27,791.72 ha in Bentong district, Pahang, with most of its forest with elevations of 700 m to 1000 m. However, Batang Kali Forest Reserve covers a very large area located in Hulu Selangor District, Selangor, with most of its forest above 1000 m in elevation (Ng 2012). The geographical distribution of P. elata in Peninsular Malaysia is as shown in Fig. 4.
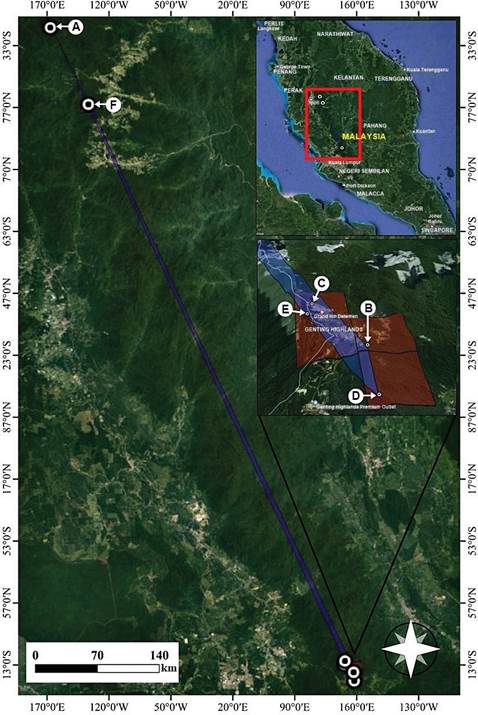
A. Mount Korbu, Perak. B. Mount Mengkuang Lebar, Pahang. C. Mount Chin Chin, Pahang. D. Mount Lari Tembakau, Pahang. E. Mount Ulu Kali, Selangor. F. Mount Irau, Pahang.
Map was designed by Edward Entalai Besi and Aqlima Amiri.
Figure 4 Distribution of the endangered Pinalia elata in Titiwangsa Range in Peninsular Malaysia.
Conservation assessment: Assessing the conservation status of an individual species, especially the narrow endemic ones, is often inadequate. Once the species is identified, it is necessary to relocate them in the field. Pinalia elata is assessed as Critically Endangered (CR), as four out of the only six known localities are now highly disturbed. The estimated extent of occurrence (EOO) and area of occupancy (AOO) are narrow (Table 1) and fall below the threshold for threatened IUCN category. Additionally, there is a continuing decline in the extent and quality of habitat.
Table 1 IUCN Red List Assessments of Pinalia elata (at regional and global levels) follow the IUCN protocol. Notes: CR A2acd C2a(i) D: Critically endangered with population size estimated to number fewer than 250 mature individuals and continuingly decline with no subpopulation estimated to contain more than 50 mature individuals.
| Classification Criteria | Assessments |
|---|---|
| Occurring localities (Global) | 6 (Peninsular Malaysia - endemic) |
| Occurring localities (Regional/Malaysia) | 6 |
| Plant growth forms | Epiphyte |
| EOO | 112.252 km2 (EN) |
| AOO | 24 km2 (EN) |
| Threats | 1.1 (Tourism and recreation areas); 6.1 (Recreational activities) |
| Severely fragmented | Yes, so far it has only been recorded in six localities within a mountain range in Peninsular Malaysia, four of which are highly fragmented due to the development of a highland tourism spot |
| Extreme fluctuations | No |
| Continuing decline | Yes (quality of habitat and number of mature individuals) |
| CITES Appendix | II |
| Previous assessment (IUCN) | NE |
| New assessment (IUCN criteria A, C, D) | CR A2acd C2a(i) D |
The imminent threat faced by this endemic species is the loss of habitat due to tourism development. The development in Genting Highlands has significantly affected the cloud forest in the summit area of Genting Highlands, which comprises Mount Ulu Kali, Mount Chin Chin, Mount Mengkuang Lebar, and Mount Lari Tembakau. These four of six localities for P. elata are under threat. For instance, the summit of Mount Mengkuang Lebar has now been entirely developed into a theme park. The impact of development on this region is clear when we consider the findings of Chua & Saw (2001), who found that at least four out of nine plots established by Stone had been replaced with buildings after 25 years. Chua & Saw (2001) observed changes in species composition and identified severe environmental changes in the summit area in Genting Highlands. Similarly, Bedawi et al. (2009) reported that 80.5% of the terrestrial pteridophytes previously recorded by Piggott (1977), were no longer found in the area. This indicates a significant loss of original biodiversity in the region. Fragmentation of forests may have negative impacts on some plant species, while others may not be affected (Chua & Saw 2001). The hypothesis is that climate change did occur in the summit area of Genting Highlands. This assumption is based on the fact that climate change is a global and regional phenomenon, even in Peninsular Malaysia. Globally, the average temperature has increased by approximately 0.6°C (Root et al. 2003). Data from the Malaysian Meteorology Department (2022) shows that Peninsular Malaysia has experienced a mean temperature increase of 0.01°C to more than 0.04°C per decade between 2012 and 2022, based on 11 stations (Fig. 5). The map illustrates that the mean temperature of the six localities of P. elata has increased by 0.01-0.02°C in their respective areas (Fig. 5).
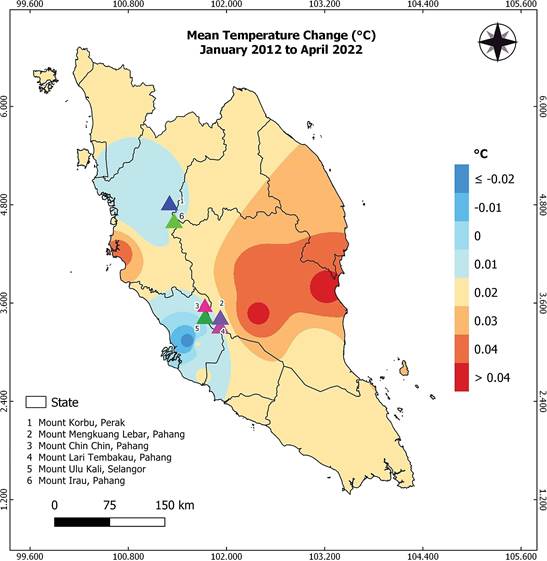
Data validation and map design were by Edward Entalai Besi and Suzika Juiling.
Figure 5 Map showing the mean temperature trend of Peninsular Malaysia from 2012 to 2022 (Source of the raw data: Malaysia Meteorology Department), with particular focus on the current localities of Pinalia elata.
Similar to other mountains in central Peninsular Malaysia, such as those in the Titiwangsa Range, Cameron Highlands has also experienced a slight increase in its temperature. The mean daily temperatures at Cameron Highland Station have increased by 0.7°C over the past ten years (Malaysian Meteorology Department 2022). Furthermore, the mean minimum temperature has shown a warming trend of 0.2°C over that period when compared to the last 25 years. At Habu Station, daily temperatures have increased by 1.5oC and 0.6oC at Tanah Rata Station over the last 25 years. Moreover, the mean minimum temperature has shown a warming trend of about 2oC over this period (Barrow et al. 2010). All these supported the claim that the summit area in Genting Highlands and Cameron Highlands most likely have undergone climate change since the areas do not stand on their own. Forest fragments in the upper montane forest are susceptible to drought and lack the resilience to withstand high temperatures and increased exposure to dry winds (Smith 1979). As a result, many of the epiphytic orchids and bryophytes in the summit area turn yellowish or brownish because of sudden drops in moisture levels. Highland epiphytic orchids require high-moisture environments and often colonize the constantly moist trunks and branches covered by mosses and lichens. The change signifies their sensitivity to atmospheric changes (Foster 2001). Also, trees in the montane forest are susceptible to decay due to the sudden fluctuations in temperature and moisture stress at anthropogenically created forest edges. For instance, one of the very few known phorophyte trees of Hymenorchis javanica (Teijsm. & Binn.) Schltr. in Malaysia died and collapsed, and its occurrence in the country has only been recorded in Genting Highlands.
Even worse, the construction of a private helipad and public car park near Chin Swee Caves Temple in Genting has caused the extermination of the only known population of critically endangered Paphiopedilum lowii (Lindl.) Stein. in the Genting Highlands area (Fig. 6). While many orchids are successful colonisers throughout the elevation gradient, hyperendemic and highland species are much more specialized in their habitat requirements. Consequently, the failure to take conservation action both in situ and ex situ to protect the habitat and population of these rare species has put them at grave risk of extinction. Habitat destruction and fragmentation, combined with climate change, threaten to extirpate these endemic species locally or drive them to extinction altogether. It is therefore urgent to prioritize the conservation of the summit area in Genting Highlands, as many species can only thrive in this unique environment with its distinctive climate conditions.

Here, for example, clearance of the Paphiopedilum lowii habitat for constructing a helipad and car park area in Genting Highlands.
A-C. A flowering plant of P. lowii captured in its natural habitat at the Chin Swee Caves Temple area in 2009. D. 12 years later, the natural habitat of P. lowii is cleared for a helipad and car park area.
Images by Rusea Go.
Figure 6 Previously known habitat of endangered wild orchids in Genting Highlands, showing the degraded montane forest in Peninsular Malaysia over a period.
To support the IUCN Resolutions and Recommendations, and to meet the goals of the Convention on Biological Diversity (CBD) Aichi Biodiversity Targets, it is crucial for management or advisory groups, as well as developers, to recognize the significant risks associated to unrestrained and poorly governed downgrading, downsizing, and degazettement of the protected areas. Such actions can have a detrimental impact on biodiversity and exacerbate the effects of climate change. To combat habitat loss, targeted approaches should be taken, including: (1) assessing of the population size and structure of a species to determine whether the populations can sustain themselves; (2) identifying and assessing the current and future threats to a particular site; and (3) collecting seeds or cuttings for germplasm conservation purposes. Mapping the distribution of endangered species is an essential step in identifying areas that require protection. These can be put forward as areas for protection. The detailed assessments following the IUCN Classification Schemes are shown in Table 1. The best protects endangered plants, it is necessary to safeguard their habitat, especially those habitats that are rich in endemic species, such as Gunung Chin Chin and Gunung Ulu Kali in Genting Highlands (Ashton 2008). The Forest Department of Peninsular Malaysia (FDPM) has implemented various measures to conserve highly endangered populations, including the establishment of Virgin Jungle Reserves (VJRs) and Key Biodiversity Areas (KBAs). VJRs are particularly effective for protecting small areas that contain rare species (Laidlaw 1998). The long-term conservation goal is to promote the biodiverse mountain areas as KBAs. VJR are ideally suited to protecting small areas, particularly those containing rare species (Laidlaw 1998). The long-term conservation goal is to promote the biodiverse mountain areas as KBA. These conservation approaches could play a significant role in supporting the rapid socio-economic development of ecotourism in Genting Highland. In line with the IUCN One Plan Approach, in situ conservation should be prioritized, with ex situ conservation serving as a supplementary or alternative option when in situ conservation is not possible. Many ecotourism areas in Peninsular Malaysia lack professionally organized and accredited ex situ conservatories, botanical gardens, and/or orchards as sources for germplasm. Given the World Conservation Congress (WCC) 2020 Resolution 079, which emphasizes the importance of linking in situ and ex situ efforts to save threatened species, ex situ conservation is becoming more crucial as back-up for conserving plants in the wild. This is especially important when many plants are facing threats in their natural habitats (Heywood 1990). Ex situ conservation involves not only taking whole plants to be planted in a botanical garden, but can also include certain plant parts, such as seeds, tissues, or genetic materials, including field gene banks, clonal collections, and germplasm banks (Ashton 1987) in order to secure the genetic materials. In the case of orchid species found in the summit area in Genting Highlands, ex situ conservation may be particularly relevant for narrow endemic species or populations that are vulnerable to extinction in their natural habitat. Some parts of the summit area in Genting Highlands are highly accessible to the public for recreational purposes. In addition to the summit area, the lowland and hill forest areas within the Batang Kali have become hotspots for illegal collection. However, this claim lacks substantial evidence and is based only on casual and personal observation. Coupled with habitat destruction, the illegal harvesting of orchids from the wild, especially the attractive and rare species with high commercial demand, has put these species under extreme pressure and led to significant reductions of their populations. Recently, one of the entrances to the mossy forest, which is also one of the known habitats of P. elata, but was previously a popular hiking spot, has been gazetted as privately managed conservation area by Environmental Services Department, Resorts World Genting Awana. The Environmental Services Department has stepped up its commitment to nature tourism and the conservation and sustainable use of this pioneering discovery area of biodiversity and is now introducing and consolidating conservation practices. Since 2015, they have started conducting proper botanical inventories and research to locate the flagship species and understand population fluctuations prior to in situ and ex situ conservation.
Conclusions
The identification of the lesser-known P. elata is complemented here by a detailed morphological description and clear photographic evidence. This information is useful for subsequent taxonomic studies of this taxonomically complex genus. Additionally, the photographic evidence of the plant in its natural habitat can serve as a basis for subsequent phenological studies. Pinalia elata represents a notable example of local endemism and a threatened species in Malaysian flora, with a narrow distribution and habitat niche confined to montane forest area. Therefore, protection of the montane forest area should be considered as a matter of the highest priority. Although the current conservation status assessment was partly based on an herbarium study, an approximation of the extent of occurrence, area of occupancy, and threats can be obtained. As one of the most famous tourist sites in Peninsular Malaysia, the development of Genting Highlands is inevitable, and the consequence is that its natural habitat will be affected. Nevertheless, conservation can still be carried out in the area. Conservation actions are crucial ensure the sustainability of this ecosystem and its biodiversity. This study provides the information needed and suggestions for conservation efforts in the summit area in Genting Highlands, especially towards orchid species. The current information is essential to further inform knowledge of biodiversity and conservation through its application in assessment for the IUCN Red List, IUCN Green List, and proposal for Key Biodiversity Area (KBA).












 uBio
uBio 

