Introduction
The West Indian fruit fly, Anastrepha obliqua (Macquart), is a pest widely distributed along tropical areas of the Americas from the Southern United States to Southern Argentina (Norrbom, 2008; Santos et al., 2020). However, it can potentially expand to other tropical regions worldwide (Fu et al., 2014). It is polyphagous and infests fruits of economic importance such as mango (Mangifera indica L.; Sapindales: Anacardiaceae), red or yellow mombin, Mexican apple or “jocote” plums (Spondias purpurea L. and S. mombin L.; Sapindales: Anacardiaceae) and sometimes guava (Psidium guajava L.; Myrtales: Myrtaceae) (Aguirre-Ramírez et al., 2017; Carvalho et al., 1996; Guillén et al., 2022; Hernández-Ortiz & Aluja, 1993). Recently, A. obliqua is considered a cryptic pest species (Ruiz-Arce et al., 2012) and stands out as the most important pest of mango crop in the Neotropics.
The monitoring of adult incidence of A. obliqua has been performed using different hydrolyzed proteins as baits (IAEA, 2018; Lasa y Williams, 2022). Meanwhile, control strategies for this pest in mango orchards include the spraying of a mixture of a hydrolyzed protein bait and an insecticide (Epsky et al., 2014). The most common killing component of these mixtures is malathion, which is a broad-spectrum synthetic organophosphate highly toxic (Díaz-Fleischer et al., 2017). Recent studies suggest that the use of a toxic bait formulated with biorational insecticide spinosad (GF120, Dow Agrosciences) represents a promising alternative to minimize impact against non-target insects and environment (Flores et al., 2011). However, the optimization of any control strategy for this pest requires a comprehensive analysis of population patterns of abundance and frequency of capture at orchard level and spatial distribution at regional scale.
Population properties play a crucial role in the implementation of management strategies for controlling fruit flies in mango crop (Abeijon et al., 2019; Dias et al., 2018; Guillén et al., 2022; Paredes et al., 2021). For instance, spatial fluctuations in local population abundance and frequency of capture may allow growers to identify pest infestation levels and reinforce insecticide application at orchard level to improve control (Pimentel, 2007). On the other hand, pest spatial distribution is useful for the design and development of management programs in an “area wide pest management” approach, which is commonly applied for controlling fruit flies (López et al., 2019; Klassen et al., 2008; Pimentel et al., 2007; Silva et al., 2019). Thus, the analysis of variations in abundance, frequency of capture, and spatial distribution of A. obliqua adult population, and the factors that could potentially modulate them, may be an effective tool to improve agricultural management planning to prevent increased damage and economic impacts in mango crops.
Some studies have suggested that population abundance and frequency of capture of fruit flies are influenced by microclimatic conditions and structural complexity of habitat (i.e., local factors) (Hudiwaku et al., 2021; Krasnov et al., 2019). Particularly, these measures are commonly influenced by several local biotic and abiotic factors of habitat such as ambient temperature, soil moisture, crop type, and host abundance (Bota et al., 2018; Tiring & Satar, 2021). For example, the incidence and abundance of A. obliqua is also dependent of fruit availability for oviposition in mango crop (Aluja & Birke,1993). In fact, fruit fly populations are more abundant and frequent in highly shaded and humid habitats of tropical regions because they have lower fitness costs, meanwhile, in temperate regions they are more abundant and frequent in warmer and sunnier habitats (Navarro-Llopis & Vacas 2014). Due to low-distance dispersion capacity of fruit flies, mainly of the genus Anastrepha (250-300 m), there are reports that their populations are denser in small area plots than in larger ones (Krasnov et al., 2019; Weldon et al., 2014). Although the abundance and frequency of capture of A. obliqua population are firstly shaped by diverse local factors, its spatial distribution is expected to be also influenced by the type of matrix or surrounding non-habitat uses/covers.
The relative influence of the surrounding landscape on spatial distribution of fruit fly populations in agricultural regions is poorly understood. A few studies have suggested that composition (i.e., covered proportion and number of different land uses/land covers) and the configuration (i.e., spatial arrangement of land uses/land covers) of the surrounding landscape presumably modulate this pattern (Aluja & Birke 1993; Clemente & Álvarez, 2019; Krasnov et al., 2019; Ricci et al., 2009). Adult population of A. obliqua is widely distributed within a landscape matrix in lemon orchards (Citrus latifolia Tan.; Sapindales: Rutaceae), meanwhile, it is negatively affected in a sugarcane matrix (Saccharum officinarum L.; Poales: Poaceae) (Pacheco-Morales, personal communication). Moreover, López et al. (2019) reported that shorter distances among mango trees favors the dispersion. Indeed, this population can use different connectivity elements of the landscape (e.g., isolated trees, living fences, or native vegetation remnants), which are composed of plum host species (Spondias spp.), to obtain supplementary and/or complementary resources that may compensate for limited resource availability in mango crop (Schwarzmueller et al., 2019). Therefore, landscape factors may be an important focus for considering in management planning to limit survival, spread and establishment of A. obliqua population in vast agricultural areas.
In Mexico, A. obliqua is one of the main pests that threatens yield and profitability of mango and other economically important crops (Díaz-Fleischer et al., 2017) and, although there is a pest management program in fruit orchards, its populations have not been suppressed effectively. Identification of local and landscape factors that influence adult population of A. obliqua may be useful in decision-making for control strategies establishment that allow producers to mitigate this pest. Therefore, this study analyzes distribution patterns of abundance and capture frequency of A. obliqua adult population and their relation to local and surrounding landscape factors in mango orchards in central Veracruz, Mexico. We hypothesize that variations in abundance and frequency of capture of adult populations depend on at least a local and landscape factor since they are indicators of resource availability and of environmental conditions.
Materials and methods
Study area: Field work was conducted in the municipalities of Jalcomulco and Apazapan, Veracruz, in the middle and lower zone of the La Antigua River basin. The climate in this region is markedly seasonal, with two well-defined seasons, rainy season (June to November) and dry season (December to May). The study area has a seasonally tropical dry or semideciduous forest (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad [CONABIO], 2023; Murphy et al., 1995; Palacios-Wassenaar et al., 2014). The most common land uses/covers are human settlements, water bodies, remnants of native vegetation and agriculture. The main crops are mango, sugarcane, lemon (Citrus limon L.) (Sapindales: Rutaceae) and papaya (Carica papaya L.) (Brassicales: Caricaceae). The soil type is a sandy loam (CONABIO, 2023). Even though this region does not stand out as one of the main mango producers in Mexico, previous studies have shown high population densities of A. obliqua due to the lack of phytosanitary management for this pest (Diaz-Fleischer et al., 2017; Lasa & Williams, 2022).
Sampling plot selection and design: In the study area, we selected a total of 11 permanent sampling plots of 100 × 100 m embedded within “Manila” mango orchards, which were regarded as experimental units in the study (Fig. 1). The selected plots did not have any type of agronomic management and a minimum distance of 500 m among plots was considered to ensure experimental unit independence. Plots were divided in four 50 × 50 m quadrats (0.25 ha), and a mango tree placed in the center of each quadrat was selected randomly as a sampling unit.
Fig. 1 Location of 11 mango orchards (Mangifera indica L.) “Manila” located in the middle part of La Antigua River basin in the municipalities of Jalcomulco and Apazapan, Veracruz. Gray circles indicate the orchard location of the 11 permanent sampling plots.
Sampling of A. obliqua flies: Two traps were set in each sampling unit. These traps were constructed using 0.6 L colorless polyethylene bottles. Four circular holes of 10 mm diameter were made around the circumference at a height of 12 cm from the base (Fig. 2). One trap was completely colorless and other was covered at the bottom with yellow plastic tape (50 mm wide) as a visual stimulus. Both traps were baited with 250 ml of CeraTrap® (Bioiberica, Barcelona, Spain), an enzymatic hydrolyzed protein attractant of animal origin highly attractive to A. obliqua (Lasa & Williams, 2022). CeraTrap® bait is a long-lasting attractant (Lasa et al., 2015), so was not rebaited and only from 10 to 30 ml were added weekly to the trap to maintain a volume of 250 ml. Traps were hung at the upper one-third of the total tree height inside the tree canopy at a central point of lateral branches hung on two different branches (6-8 m distance) in a mango tree placed in the center of each sample point, the trees had a height of 10 to 26.6 m. Traps were monitored weekly for A. obliqua and rotated to the right of their position. Considering the 11 sampling plots and the eight traps per plot, a total of 88 traps were revised weekly, resulting in a total of 264 traps for the entire sampling. Adults of A. obliqua were monitored in the study area for three consecutive weeks, between May 14 and June 4 of 2022, matching with the main period of A. obliqua population increase in that region (Birke et al., 2013; Martínez-Flores et al., 2023). During this period temperature varied from 18.8 to 27.8 ºC and relative humidity from 76 to 81 %.

Fig. 2 Frontal view of the set traps for capturing Anastrepha obliqua in 11 mango orchards in central Veracruz, Mexico. (a) Completely colorless trap. (b) Trap covered at the bottom with yellow plastic tape.
Captured insects were transported to the laboratory in 70 % ethanol, recording the date and type of trap and A. obliqua adults were identified and sorted by sex. The abundance and frequency of capture of A. obliqua were considered as response variables (Ativor et al., 2012; Vayssières et al., 2013). Abundance was considered as the total number of A. obliqua individuals collected during the three-week sampling period on each experimental unit. Availability was expressed as FTD index (flies per trap per day) which is used to know the relative presence of fruit flies in each area and period. This was obtained using the formula:
FTD = F ∕ (T × D)
where F is the number of flies captured, T is the number of traps, and D is the number of days the traps were exposed in the field.
Local scale characterization: The orchards were structurally characterized to identify if the changes present in each of them may directly affect A. obliqua population. Shade cover (%) was recorded for each sampling unit using a convex spherical densitometer (Forestry Suppliers, Jackson, Mississippi). In addition, tree density and tree height (m) were measured in each sampling unit to determine influence of planting density on the levels of infestation by A. obliqua. Also, soil compaction (kg/cm²) in each sampling unit was recorded using a pocket conical penetrometer (Humboldt H-4195, Elgin, Illinois). The proportion of soil cover (%) was estimated by delimiting an area of 1 m2 with a square frame in each sampling unit. All these parameters were quantified in the first day of sampling (14 May 2022), at the beginning of the rainy season.
Landscape scale characterization: To determine landscape configuration, we determined the distance from each individual plot to the nearest edge of other land uses/covers such as: i) native vegetation, ii) other mango orchards, iii) Spondias spp. remnants, iv) sugarcane plantations, v) human settlements and vi) water bodies. Distance measurements were done using the ArcMap Patch Analyst extension version 10.8 (ESRI; 2021).
Data analysis: To identify the predictor variables that significantly influenced the population structure of A. obliqua, single and multiple regression models were performed. As these statistical techniques are sensitive to collinearity between predictor variables, Pearson’s correlation coefficient was used to exclude correlated or redundant variables because all variables are continuous, meanwhile, Spearman’s correlation coefficient was used for numerical discrete variables such abundance (Cohen et al., 2009). Of each set of significantly correlated variables, only the most intuitive and interpretable variable was retained.
We used generalized linear models (GLM) to assess the independent effects of each landscape predictor on abundance and availability of A. obliqua (i.e., a single univariate regression between a response and a predictor variable). We applied a Gaussian error distribution for continuous variables (i.e., availability) and a Poisson error distribution for count-dependent variables (i.e., abundance). For each multiple regression model, variance inflation factor was used to exclude predictor variables that could affect the accuracy of the estimates. We followed an information-theoretic approach and multi-model inference to assess the relative effect of each local and landscape factor on abundance and availability of A. obliqua using the package glmulti for R version 3.5.0 (Calcagno & de Mazancourt, 2010). This function built a set of models representing all possible combinations of landscape predictors for abundance and availability. It also computed the Akaike’s information criterion, corrected for small samples (AICc) for each built model. To correct for the overdispersion associated with count data, abundance was assessed with qAICc instead of AICc values (Burnham & Anderson, 2002). The goodness-of-fit of the models was estimated as the explained deviance for each complete model using the modEvApackage for R version 3.5.0 (Barbosa et al., 2014).
Results
Adult population of A. obliqua: A total of 2 045 individuals of Anastrepha spp. were captured, which 1 869 were A. obliqua (Table 1). The total abundance of this species varied significantly between sexes (F = 54.1, p < 0.05) and plots (F = 18.7, p < 0.05). Female abundance varied from 46 (plot #6) to 318 (plot #5), meanwhile male abundance ranged from 16 (plot #2) to 175 (plot #5). Availability, estimated as FTD, varied significantly between sexes (F = 56. 4, p < 0.05) and plots (F = 18. 5, p < 0.05). Female availability varied from 0.3 flies/trap/day (plot #1) to 2.5 (plot #6), while that of males ranged from 0.11 (plot #2) to 1.15 (plot #5).
Table 1 Variation in adult population measures (mean ± SE) of Anastrepha obliqua studied in mango orchards in central Veracruz, Mexico.
| Adult population measures | Females | Males | Both sexes |
| Abundance (individuals) | 106.36 ± 20.9 | 43.81 ± 13.09 | 150.18 ± 37.11 |
| Availability (flies/trap/day) | 0.55 ± 0.11 | 0.24 ± 0.06 | 0.72 ± 0.13 |
Local factors: In the sampled plots, shade cover ranged from 62.1 % (plot #3) to 92.7 % (plot #2) and was positively correlated with tree availability (R = 0.74, p < 0.05) (Table 2). Tree height ranged from 10.13 (plot #4) to 26.3 m (plot #1) and was positively correlated with soil cover (R = 0.70, P < 0.05) and negatively with soil compaction (R = −0.53, p < 0.05).
Table 2 Local and landscape characteristics of mango orchards in central Veracruz, Mexico.
| a) Local scale factors | Mean ± SE | Range |
|---|---|---|
| Tree height (m) | 18.82 ± 1.33 | 10.1-26.3 |
| Shade cover (%) | 82.63 ± 2.99 | 62.1-92.7 |
| Planting density (trees/ha) | 13.36 ± 0.78 | 9-18 |
| Soil compaction (kg/cm²) | 17.82 ± 3.94 | 4.8-47 |
| Soil cover (%) | 69.54 ± 6 | 19.2-94.8 |
| b) Landscape scale factors | ||
| Distance to nearest native vegetation (m) | 260 ± 50 | 50-530 |
| Distance to nearest mango orchard (m) | 150 ± 20 | 30-350 |
| Distance to nearest Spondias spp. remnant (m) | 1600 ± 460 | 10-4820 |
| Distance to nearest sugarcane plantation (m) | 690 ± 140 | 130-1610 |
| Distance to nearest water body (m) | 630 ± 160 | 20-1450 |
Landscape factors: Distance from each individual plot to the nearest Spondias spp. remnant edge ranged from 10 m (plot #5) to 4820 m (plot #2), and it was positively correlated with distance from each individual plot to the nearest edge of other mango orchards (R = 0.73, p < 0.05), human settlements (R = 0.83, p < 0.05) and water bodies (R = 0.60, p < 0.05) (Table 2). Meanwhile, distance from each individual plot to the nearest edge of Spondias spp. remnants, sugarcane plantations (R = −0.7, p < 0.05) and native vegetation (R = −0.54, p < 0.05) were negatively correlated.
Influence of local factors on A. obliqua population: Abundance was significantly and negatively influenced by shade cover produced by mango trees. When the percentage of shade increased the number of individuals decreased (Z = −19.4, d.f. = 10, p < 0.05) (Fig. 3A). The remaining structural variables did not influence abundance (p > 0.05). Availability was significantly and negatively influenced by soil compaction (t = −3.5, d.f. = 10, p < 0.05) (Fig. 3B). Plots with compacted soils presented a lower availability of individuals. Remaining structural variables did not influence availability (p > 0.05).
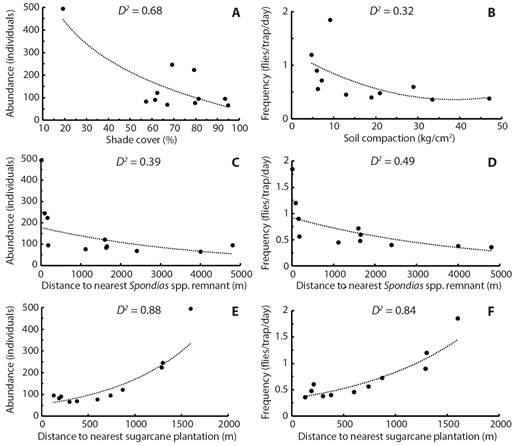
Fig. 3 Significant regression models of local and landscape factors and the abundance and availability of adult population of A. obliqua associated with mango orchards in central Veracruz, Mexico. The goodness-of-fit of each model is indicated in each panel as the percentage of deviance explained by each model (D 2 ).
Influence of landscape factors on A. obliqua population: Abundance (Z = −17.07, d.f. = 10, p < 0.05) and availability (t = −2.5, d.f. = 10, p < 0.05) were significantly and negatively influenced by distance from each individual plot to the nearest Spondias spp. remnant edge (Fig. 3C, Fig. 3D). In addition, abundance (Z = 26.4, d.f. = 10, p < 0.05) and availability (t = 5.6, d.f. = 10, p < 0.05) were significantly and positively explained by distance from each individual plot to the nearest sugarcane plantation edge (Fig. 3E, Fig. 3F). The A. obliqua populations increased with shorter distances between orchards and Spondias spp. trees, while populations decreased with shorter distances between orchards and sugarcane crops.
Discussion
Local and surrounding landscape characteristics of diverse economically important crops are key factors, which modulate insect pest populations (Carrière et al., 2012). The present study provides insights into the importance of variables, indicators of resources and conditions, at local and landscape level and their influence on adult population A. obliqua associated with mango orchards. These results also improve the understanding of the main drivers, which commonly are not consider in the management of A. obliqua distribution among agricultural settings devoted to mango production. Overall, abundance and availability of this fruit fly in mango orchards are mainly modulated by the shade cover, soil compaction, distance to plum trees and distance to sugarcane plantations.
The shade cover is a characteristic of crop biophysical structure, which is directly related to physiognomy vegetation and leaf litter in soil. This local factor generates habitat partitions where a vast diversity of insects is distributed according to specific requirements. Our results indicate that total abundance of males and females decreased as function of shade cover in mango orchard. This pattern is due to in more shaded orchards there are insects that patrol mango trees and protect or defends food resources against competitors (Cuautle et al., 2005). In these orchards there is a thicker leaf litter layer, which is a microhabitat of a specious arthropods assemblage with highly specialized feeding habits, including predation (García-Martínez et al., 2015). Then, A. obliqua larvae are more susceptible to be predated by spiders, ants and beetles when infested mango fruits fall to ground (Cruz-Miralles et al., 2022; García et al., 2020). Thus, although our results indicate a significant negative relation between shade cover in mango orchards and A. obliqua adult population, it should be taken as a hypothesis for testing on the ecology of this pest.
Availability and abundance of males and females was affected by increasing soil compaction. This is physical property of any type of soil, which is associated with soil composition and structure and is influenced by the size of the interacting particles allowing the presence of water and air. Compacted soils have greater difficulty in wetting and generally have fewer pores to allow aeration which hinders the viability of organisms, such as fruit flies. This local characteristic points to a microhabitat where A. obliqua pupal stage takes places. For example, when the larvae reach the last stage of development, the mango fruits fall to the ground, subsequently, they leave the fallen fruits and begin to quickly excavate the most superficial layer of soil to start the pupation phase. This high compaction prevents or hinder soil penetration. In addition, those larvae which can bury and pupate, face the same obstacle imposed by compaction at the time of emerging as adults (Abeijon et al., 2019; Fernandes et al., 2012; Ismail & Ahmed, 2020). Although soil compaction is correlated with soil moisture and both are critical for adult emergence (Ismail & Ahmed, 2020), its importance stands out as a mechanical resistance to digging in that severely impacts larvae survival in shadier orchards. (Fernandes et al., 2012; Ismail & Ahmed, 2020; Khan et al., 2020). Soil compaction represents an easy management practice which may severely impacts on larvae survival and adult emergence however, its implementation should be carefully taken due to implications on soil biodiversity associated with mango orchards (Ishak et al., 2014).
Regarding landscape factors, abundance, and availability of A. obliqua adults increased significantly in mango orchards near to plum trees of S. mombin and S. purpurea. This result is concordant with a previous study carried out in Central Veracruz, which reports a high level of A. obliqua infestation (435 pupae/kg) in Spondias fruits when compared with a low level (14.55 pupae/kg) in mango fruits (Aluja et al., 2001). This is due to, Spondias spp. fruits have an optimal development for being infested about 3 to 5 weeks earlier than mango so one fruit fly generation precedes the beginning of mango harvest in this region (Lasa, 2016; Tinoco-Dominguez, 2019). In addition, the increase in plum fruit number significantly increases infestation levels of this pest in mango orchards (López et al., 2019). For this reason, plum trees in surrounding landscape might be acting as reservoirs for A. obliqua adult population associated with mango orchards. Even though these trees are also considered as reservoirs of fruit fly parasitoid species (de Sousa et al., 2021), parasitoid abundance is relatively low (usually < 10 %) compared to adult emergence of A. obliqua (Sivinski et al., 2000). Unfortunately, relictual Spondias spp. trees are commonly isolated, scattered and used in live fences throughout the study area (Díaz-Fleischer et al., 2017). Given this context, collection, and destruction of plum fruits and/or trees in the surrounding landscape, or even spraying insecticides, is necessary to mitigate adult population growth A. obliqua in mango production regions.
The abundance and availability of A. obliqua decreased in mango orchards near to sugarcane crop land cover. This result is not surprising since there is a previous report that a landscape matrix composed of such a crop negatively impacts this species (Pacheco-Morales, 2022). In Mexico, this monoculture is intensively managed, which includes a biophysical structure simplification and a disproportionate agrochemical use (Meza-Palacios et al., 2019). These crop fields are deeply tilled before planting, have a homogeneous surface highly exposed to sun radiation and during harvest sugarcane plants are burned to facilitate cutting. For this conditions, A. obliqua population associated with mango orchards near or surrounded by this crop is adversely affected and decreased. Therefore, sugarcane crop plays a key role as a barrier for A. obliqua distribution and movement at regional scale (Vermunt et al., 2019). However, broader planning instruments of control strategies should take into consideration the environmental cost of this crop in tropical regions devoted to mango production.
This study demonstrates empirically that adult population of A. obliqua is dependent on factors inside (i.e., local level) and outside (i.e., landscape level) of mango orchards. We demonstrate the significance and intimately relations among shade cover, soils compaction and Spondias spp. and sugarcane land covers for shaping abundance, availability, and distribution of adult population of A. obliqua. These findings may allow optimization for selecting and applying agronomic management practices against A. obliqua in mango crop. However, to highlight the importance of local and landscape factors to regulate A. obliqua populations, this study should be replicated in other mango producing regions along the Gulf of Mexico such as Actopan or Cosamaloapan, Veracruz. Finally, effective outcomes will only be achieved if the scope of this study may be included as an efficient tool in the integrated management of this pest to promote protection of mango crop and improve its fruit quality in this producing region.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio 


