Introduction
In total, 1 971 bird species are distributed in the Brazilian territory, based on the Brazilian Committee of Ornithological Records, also known as CBRO (Pacheco et al., 2021). The National Research Center for Wild Birds Conservation, also known as CEMAVE, often published the Annual Report of Migrant Birds Routes and Concentration Sites in Brazil. Based on this report, coastal Piauí State (BR) holds two migratory routes; this state is pointed out as concentration site for shorebirds and migrant birds in the country; it also emphasizes the important role played by this site for these species' conservation (CEMAVE, 2020). Oftentimes, these birds get to the wintering sites by late August and early September; they stay in this location until April, when they once more return to their breeding grounds (Cabral et al., 2006; Silva & Rodrigues, 2015; Somenzari et al., 2018).
Several localities along the coast of Piauí State (BR) are wintering sites for migrant birds. Guzzi et al. (2012) reported 17 migrant species in Parnaíba River Delta; 7 species were observed at Parnaíba International Airport - they were identified as Nearctic migrants belonging to families Charadriidae, Scolopacidae and Hirundinidae (Cardoso et al, 2013). Guzzi et al. (2015) reported 16 Nearctic migrant species at Pedra do Sal Beach (in Parnaíba River Delta), as well as three austral migrants and one partially Nearctic migrant species.
Some factors have influenced the distribution and abundance of migrant birds; among them, one finds habitat conditions and the availability or food resources. (Neima et al., 2020; Rodrigues et al., 2015). Studies carried out in neotropic areas, for example, have shown the importance of migrant birds in tropical bird communities, with emphasis on the fact that this group cannot be taken as element external to these communities because they showed domination over and segregation patterns of habitats inside a wintering site (Kelsey, 1992). However, they can be particularly vulnerable to climate changes, either in wintering sites or in breeding grounds (Sanderson et al., 2006).
Climate changes can also influence individual behaviors, breeding success and the population dynamics of migrant birds, a fact that can change the patterns and connections between specific summer and winter populations (Webster & Marra, 2005). Breeding locations are linked to wintering sites by the movements of individuals belonging to any migratory organism (Webster et al., 2002). Many different connectivity patters are possible to happen, in other words, connectivity is strong if most individuals who reproduce in a given site also spend winter together in a given location (Salomonsen, 1995).
The conservation of migrant birds is closely associated with the identification and protection of sites used for resting, feeding and breeding, since the loss of some of these locations can be decisive for some species' survival. Continuity and expansion of monitoring procedures applied to Nearctic migrant birds visiting Brazil are essential for their survival (Graff et al., 2016; Somenzari et al., 2018). Thus, the aim of the present study was to investigate spatiotemporal patterns of migrant bird species richness and composition along the coast of Piauí State, Brazil, by correlating these community features to both local climate and vegetation features.
Materials and methods
Study site: Data were collected at 10 sites along the coast of Piauí State, Brazil (Fig. 1), one site in Ilha Grande County, two in Luis Correia County and seven sites in Parnaíba County. These sites were chosen because of previous knowledge on their occupation by migrant birds. Each site was classified based on its prevalent vegetation type, according to Santos-Filho et al. (2010). The points were classified into two main vegetation types, based on this scheme: ''caatinga'' (typical of dry inland areas) and ''restinga'' (typical of coastal regions). Restinga was divided into four subtypes: flooded fields (2 sites), non-flooded fields (3 sites), transition grasslands (1 site) and orchards (3 sites).
Data collection: Quantitative survey on migrant birds was carried out between April 2009 and February 2016 based on visual observations (with binoculars) and mist net captures. Visual observations were performed in study sites, which were divided into transects that were surveyed at dawn and dusk with the aid of binoculars (10X50) and digital recorder equipped with directional microphone to capture and replay birds' vocalizations. Mist nets (2.5 m × 30 mm × 12 m) were used at dusk and dawn; they were visited every 20 min. The nets were extended near vegetation formations and/or aquatic sites. Stotz et al. (1996), Nunes and Tomas (2008), Pacheco et al. (2021) and Somenzari et al. (2018) were used as reference for birds' identification. Their conservation statuses were determined by following the classification by the International Union for Conservation of Nature (IUCN, 2022).
Data processing and analyses: The sampling effort among sites and months was first equalized through rarefaction analysis to determine the spatial and temporal patterns of migrant bird species' richness. The expected richness (i.e., the rarefied number of species) plus or minus twice its standard deviation was the calculation used to make the comparisons. Expected (rarefied) richness per month was regressed against monthly values recorded for five climatic variables to help explaining the temporal patterns, namely: mean temperature (ºC), total rainfall (mm), mean wind speed (m/s), mean insolation (W/m2) and mean atmospheric pressure (Pa), which were collected from the Agrometeorological Bulletin of EMBRAPA Meio-Norte. An elastic net regression was used, since these five variables can be highly correlated and, consequently, hinder model interpretation and applicability. It was done by assuming the Gaussian error distribution and by estimating penalization (a) and shrinkage (l) parameters via leave-one-out cross-validation, in order to select variables and regulate model coefficients (Zou & Hastie, 2005). Finally, spatial patterns were investigated through PERMANOVA by using a Bray-Curtis dissimilarity matrix of field campaigns grouped by vegetation type. Therefore, we investigated vegetation's likely influence on bird assemblies' composition in each sampling site. The IndVal test was used to identify potential indicator species for each vegetation type, among the migratory birds, in order to qualify these differences among communities. All statistical analyses were run in R software (R Core Team, 2019), in packages vegan 2.4-6 and glmnet 2.0-16 - values were considered statistically significant when P < 0.05. The trophic categories followed the classification proposed by Motta-Júnior (1990): granivores (GRAN), with – or more grains; frugivores (FRU), with – or more fruits; insectivores (INSET), with – or more insects and other arthropods in the diet; omnivores (ONIV), with more than – of insects, other arthropods and fruits, in similar proportions; aquatic invertebrates (INVAQ), diet with more than – of aquatic invertebrates; carnivores (CARN) and scavengers (NECRO), living and dead vertebrates, respectively, at least in – of the diet; malacophagi (MAL), with – or more of mollusks and piscivores (PISC), with – or more of fish.
Results
Eighty-two migrant bird species were recorded along the coast of Piauí State. They belonged to 13 orders and 28 families, and represented 41 intracontinental migrant species (50 %) in South America, 26 species of Northern visitors (31.7 %), 14 nomad species (17.07 %) and 1 vagrant species (1.21 %). These species were distributed into 10 feeding guilds, with the prevalence of trophic guilds, namely: 22 species (26.8 %) feeding on aquatic arthropods; 19 species (23.2 %) of insectivorous birds; 16 omnivore species (19.5 %); 10 piscivorous species (12.2 %); 6 granivore species (7.3 %); three nectarivore species (3.7 %); 2 carnivore species (2.4 %); 2 molluscivorous species (2.4 %); one frugivore species (1.2 %); and one insectivore/granivore species (1.2 %). With respect to sensitivity to environmental changes, 47 bird species (57.3 %) were classified as low-sensitivity species, 24 (29.3 %) as medium-sensitivity species, 6 (7.3 %) as high-sensitivity species; 5 (6.1 %) species did not record any sensitivity at all. As for dependence on forest environments, 61 species (74.4 %) were independent, 9 (10.1 %) were semi-dependent, 7 (8.5 %) were dependent, and 5 species (6.1 %) did not record any dependence. Finally, when it comes to conservation status, 80 species (97.56 %) were of least concern, whereas 2 species (2.44 %) were considered as close to endangering (Calidris canutus and C. pusilla) (Table 1).
Table 1 Migrant birds recorded along the coast of Piauí State, Brazil.
| TAXON NAME | STATUS | GUILD | SE | UH | SC | EN | FR |
| ANSERIFORMES Linnaeus, 1758 | |||||||
| ANATIDAE Leach, 1820 | |||||||
| DENDROCYGNINAE Reichenbach, 1850 | |||||||
| Dendrocygna viduata (Linnaeus, 1766) | INTRA | ONI | BAI | IN | LC | OS | 17 |
| ANATINAE Leach, 1820 | |||||||
| Amazonetta brasiliensis (Gmelin, 1789) | NO | PIS | BAI | IN | LC | OS | 2 |
| PODICIPEDIFORMES Fürbringer, 1888 | |||||||
| PODICIPEDIDAE Bonaparte, 1831 | |||||||
| Podilymbus podiceps (Linnaeus, 1758) | INTRA | PIS | MED | IN | LC | ||
| Tachybaptus dominicus (Linnaeus, 1766) | INTRA | PIS | MED | IN | LC | ||
| SULIFORMES Sharpe, 1891 | |||||||
| PHALACROCORACIDAE Reichenbach, 1849 | |||||||
| Nannopterum brasilianum (Gmelin, 1789) | INTRA | PIS | BAI | IN | LC | TG | 98 |
| PELECANIFORMES Sharpe, 1891 | |||||||
| ARDEIDAE Leach, 1820 | |||||||
| Ardea alba Linnaeus, 1758 | INTRA | ONI | BAI | IN | LC | FF | 89 |
| Ardea cocoi Linnaeus, 1766 | NO | ONI | BAI | IN | LC | FF | 4 |
| Bubulcus ibis (Linnaeus, 1758) | NO | INS | BAI | IN | LC | NF | 152 |
| Egretta caerulea (Linnaeus, 1758) | INTRA | ONI | MED | IN | LC | FF | 30 |
| ACCIPITRIFORMES Bonaparte, 1831 | |||||||
| PANDIONIDAE Bonaparte, 1854 | |||||||
| Pandion haliaetus (Linnaeus, 1758) | VN | PIS | MED | IN | LC | FF | 16 |
| ACCIPITRIDAE Vigors, 1824 | |||||||
| Elanus leucurus (Vieillot, 1818) | INTRA | CAR | BAI | IN | LC | ||
| Rostrhamus sociabilis (Vieillot, 1817) | INTRA | MAL | BAI | IN | LC | TG | 148 |
| GRUIFORMES Bonaparte, 1854 | |||||||
| ARAMIDAE Bonaparte, 1852 | |||||||
| Aramus guarauna (Linnaeus, 1766) | INTRA | MAL | MED | IN | LC | TG | 88 |
| RALLIDAE Rafinesque, 1815 | |||||||
| Laterallus melanophaius (Vieillot, 1819) | NO | ONI | BAI | SD | LC | OS | 2 |
| Porphyrio martinica (Linnaeus, 1766) | INTRA | ONI | BAI | IN | LC | NF | 4 |
| CHARADRIIFORMES Huxley, 1867 | |||||||
| CHARADRII Huxley, 1867 | |||||||
| CHARADRIIDAE Leach, 1820 | |||||||
| Charadrius collaris Vieillot, 1818 | INTRA | INVAQ | ALT | IN | LC | NF | 150 |
| Charadrius semipalmatus Bonaparte, 1825 | VN | INVAQ | MED | IN | LC | NF | 68 |
| Pluvialis dominica (Statius Muller, 1776) | VN | INVAQ | ND | ND | LC | NF | 6 |
| Pluvialis squatarola (Linnaeus, 1758) | VN | INVAQ | BAI | IN | LC | FF | 26 |
| RECURVIROSTRIDAE Bonaparte, 1831 | |||||||
| Himantopus melanurus Vieillot, 1817 | VN | INVAQ | MED | IN | LC | NF | 25 |
| Himantopus mexicanus (Statius Muller, 1776) | VN | INVAQ | MED | IN | LC | FF | 7 |
| SCOLOPACI Steijneger, 1885 | |||||||
| SCOLOPACIDAE Rafinesque, 1815 | |||||||
| Actitis macularius (Linnaeus, 1766) | VN | INVAQ | BAI | IN | LC | NF | 27 |
| Arenaria interpres (Linnaeus, 1758) | VN | INVAQ | ALT | IN | LC | FF | 25 |
| Calidris alba (Pallas, 1764) | VN | INVAQ | ND | ND | LC | ||
| Calidris bairdii (Coues, 1861) | VN | INVAQ | ND | ND | LC | ||
| Calidris canutus (Linnaeus, 1758) | VN | INVAQ | ALT | IN | AT | FF | 13 |
| Calidris fuscicollis (Vieillot, 1819) | VN | INVAQ | MED | IN | LC | FF | 4 |
| Calidris minutilla (Vieillot, 1819) | VN | INVAQ | MED | IN | LC | NF | 8 |
| Calidris pusilla (Linnaeus, 1766) | VN | INVAQ | MED | DP | AT | NF | 30 |
| Gallinago paraguaiae (Vieillot, 1816) | INTRA | INVAQ | BAI | IN | LC | TG | 41 |
| Limnodromus griseus (Gmelin, 1789) | VN | INVAQ | ALT | IN | LC | FF | 12 |
| Numenius hudsonicus Latham, 1790 | VN | INVAQ | ND | ND | LC | FF | 14 |
| Numenius phaeopus (Linnaeus, 1758) | VA (N) | INVAQ | MED | DP | LC | FF | 38 |
| Tringa flavipes (Gmelin, 1789) | VN | INVAQ | BAI | IN | LC | NF | 26 |
| Tringa melanoleuca (Gmelin, 1789) | VN | INVAQ | BAI | IN | LC | NF | 31 |
| Tringa semipalmata (Gmelin, 1789) | VN | INVAQ | ND | ND | LC | ||
| Tringa solitaria Wilson, 1813 | VN | INVAQ | BAI | IN | LC | NF | 24 |
| LARI Sharpe, 1891 | |||||||
| LARIDAE Rafinesque, 1815 | |||||||
| Leucophaeus atricilla (Linnaeus, 1758) | VN | PIS | MED | IN | LC | ||
| STERNIDAE Vigors, 1825 | |||||||
| Phaetusa simplex (Gmelin, 1789) | INTRA | PIS | ALT | IN | LC | ||
| Sterna hirundo Linnaeus, 1758 | VN | PIS | MED | SD | LC | NF | 2 |
| Sternula superciliaris (Vieillot, 1819) | INTRA | PIS | BAI | IN | LC | NF | 23 |
| RYNCHOPIDAE Bonaparte, 1838 | |||||||
| Rynchops Níger Linnaeus, 1758 | INTRA | PIS | ALT | IN | LC | NF | 5 |
| COLUMBIFORMES Latham, 1790 | |||||||
| COLUMBIDAE Leach, 1820 | |||||||
| Columbina picui (Temminck, 1813) | NO | GRA | BAI | IN | LC | OS | 128 |
| Patagioenas picazuro (Temminck, 1813) | INTRA | FRU | MED | SD | LC | FF | 14 |
| CUCULIFORMES Wagler, 1830 | |||||||
| CUCULIDAE Leach, 1820 | |||||||
| CUCULINAE Leach, 1820 | |||||||
| Coccyzus americanus (Linnaeus, 1758) | VN | INS | MED | SD | LC | ||
| Coccyzus euleri Cabanis, 1873 | INTRA | INS | MED | SD | LC | CA | 4 |
| CAPRIMULGIFORMES Ridgway, 1881 | |||||||
| CAPRIMULGIDAE Vigors, 1825 | |||||||
| Chordeiles acutipennis (Hermann, 1783) | INTRA | INS | BAI | IN | LC | CA | 9 |
| Podager nacunda (Vieillot, 1817) | INTRA | INS | BAI | IN | LC | NF | 12 |
| APODIFORMES Peters, 1940 | |||||||
| APODIDAE Olphe-Galliard, 1887 | |||||||
| Tachornis squamata (Cassin, 1853) | NO | INS | BAI | IN | LC | NF | 3 |
| TROCHILIDAE Vigors, 1825 | |||||||
| POLYTMINAE Reichenbach, 1849 | |||||||
| Anthracothorax nigricollis (Vieillot, 1817) | INTRA | NEC | BAI | SD | LC | ||
| Chrysolampis mosquitus (Linnaeus, 1758) | NO | NEC | BAI | IN | LC | CA | 10 |
| TROCHILINAE Vigors, 1825 | |||||||
| Thalurania furcata (Gmelin, 1788) | NO | NEC | MED | SD | LC | CA | 4 |
| FALCONIFORMES Bonaparte, 1831 | |||||||
| FALCONIDAE Leach, 1820 | |||||||
| Falco peregrinus Tunstall, 1771 | VN | CAR | MED | IN | LC | ||
| PASSERIFORMES Linnaeus, 1758 | |||||||
| TYRANNIDA Wetmore & Miller, 1926 | |||||||
| TITYRIDAE Gray, 1840 | |||||||
| Xenopsaris albinucha (Burmeister, 1869) | INTRA | INS | MED | IN | LC | CA | 1 |
| TYRANNIDAE Vigors, 1825 | |||||||
| ELAENIINAE Cabanis & Heine, 1860 | |||||||
| Elaenia cristata Pelzeln, 1868 | INTRA | ONI | MED | IN | LC | CA | 7 |
| Elaenia mesoleuca (Deppe, 1830) | NO | INS | BAI | DP | LC | CA | 2 |
| Elaenia spectabilis Pelzeln, 1868 | INTRA | ONI | BAI | DP | LC | CA | 1 |
| Phaeomyias murina (Spix, 1825) | INTRA | ONI | BAI | IN | LC | ||
| Suiriri suiriri (Vieillot, 1818) | INTRA | INS | MED | IN | LC | OS | 4 |
| TYRANNINAE Vigors, 1825 | |||||||
| Myiarchus swainsoni Cabanis & Heine, 1859 | INTRA | INS | BAI | IN | LC | FF | 4 |
| Myiodynastes maculatus (Statius Muller, 1776) | INTRA | ONI | BAI | DP | LC | ||
| Myiozetetes cayanensis (Linnaeus, 1766) | INTRA | INS | BAI | DP | LC | ||
| Myiozetetes similis (Spix, 1825) | INTRA | ONI | BAI | SD | LC | CA | 4 |
| Tyrannus melancholicus Vieillot, 1819 | INTRA | INS | BAI | IN | LC | FF | 100 |
| FLUVICOLINAE Swainson, 1832 | |||||||
| Arundinicola leucocephala (Linnaeus, 1764) | NO | INS | MED | IN | LC | ||
| Fluvicola albiventer (Spix, 1825) | NO | INS | MED | IN | LC | NF | 6 |
| Fluvicola nengeta (Linnaeus, 1766) | NO | INS | BAI | IN | LC | OS | 11 |
| PASSERI Linnaeus, 1758 | |||||||
| CORVIDA Wagler 1830 | |||||||
| VIREONIDAE Swainson, 1837 | |||||||
| Vireo olivaceus (Linnaeus, 1766) | VN | ONI | BAI | DP | LC | CA | 5 |
| PASSERIDA Linnaeus, 1758 | |||||||
| HIRUNDINIDAE Rafinesque, 1815 | |||||||
| Hirundo rústica Linnaeus, 1758 | VN | INS | BAI | IN | LC | NF | 10 |
| Progne chalybea (Gmelin, 1789) | INTRA | INS | BAI | IN | LC | ||
| Progne tapera (Linnaeus, 1766) | INTRA | INS | BAI | IN | LC | ||
| Tachycineta albiventer (Boddaert, 1783) | INTRA | INS | BAI | IN | LC | ||
| TURDIDAE Rafinesque, 1815 | |||||||
| Turdus amaurochalinus Cabanis, 1850 | INTRA | ONI | BAI | SD | LC | CA | 21 |
| MOTACILLIDAE Horsfield, 1821 | |||||||
| Anthus chii Vieillot, 1818 | INTRA | INS/GRA | BAI | IN | LC | NF | 76 |
| PASSERELLIDAE Cabanis & Heine, 1850 | |||||||
| Ammodramus humeralis (Bosc, 1792) | NO | GRA | BAI | IN | LC | CA | 12 |
| ICTERIDAE Vigors, 1825 | |||||||
| Chrysomus ruficapillus (Vieillot, 1819) | INTRA | ONI | BAI | IN | LC | OS | 10 |
| Molothrus bonariensis (Gmelin, 1789) | NO | ONI | BAI | IN | LC | ||
| Leistes superciliaris (Bonaparte, 1850) | INTRA | ONI | BAI | IN | LC | OS | 74 |
| THRAUPIDAE Cabanis, 1847 | |||||||
| Sporophila caerulescens (Vieillot, 1823) | INTRA | GRA | BAI | IN | LC | CA | 1 |
| Sporophila lineola (Linnaeus, 1758) | INTRA | GRA | BAI | IN | LC | ||
| Sporophila nigricollis (Vieillot, 1823) | INTRA | GRA | BAI | IN | LC | CA | 2 |
| Volatinia jacarina (Linnaeus, 1766) | INTRA | GRA | BAI | IN | LC | OS | 9 |
STATUS: INTRA (intracontinental migrants); NO (nomads); VN (Northern visitors); VA(N) (vagrant species originating from the Northern hemisphere). GUILDS: ONI (omnivores); PIS (piscivores); INS (insectivores); CAR (carnivores); MAL (molluscivores); ONI (omnivores); INVAQ (aquatic invertebrates); GRA (granivores); FRU (frugivores); NEC (necrophagic); INS/GRA (insectivore and granivore). SE (sensitivity): ALT (high); BAI (low); MED (medium); ND (not determined). UH (habitat use): IN (independent); SD (semi-dependent); DP (dependent); ND (not determined). SC (conservation status): LC (little concern); AT (almost endangered). EN (Environment): CA (Caatinga); FF (Flooding Fields); NF (Non-Flooding Fields); TG (TRANSITION GRASSLANDS); OS (Orchard Sites). FR (frequency).
Migrant birds were recorded over the year. However, richness peaks, which were estimated by rarefying the sampling effort of each month, were mainly observed during season transitions after major changes in weather conditions in the assessed region, mainly at the beginning of the rainy season, or in the beginning and at the end of the dry season - this pattern was observed in all three classes of migrants, i.e., intracontinental, Northern visitors and nomads (Fig. 2).
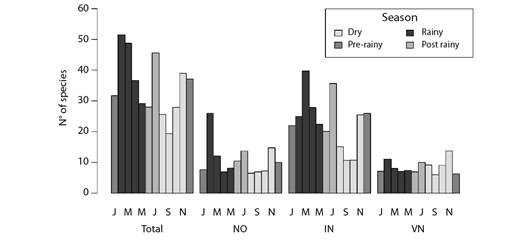
Fig. 2 Barplot of the expected (i.e., rarefied) number of migrant bird species per month at the coast of Piauí State, Brazil. Groups of bars indicate migratory status: NO (nomad species); IN (intracontinental migrants); VN (Northern visitor species). Bar colors indicate different seasons often observed in the site.
The elastic net regression led to a model capable of explaining 30.6 % of species richness variance based on only two of the five original climatic variables, namely: insolation and atmospheric pressure. These two variables had negative and positive influence on the number of migrant bird species at the coast of Piauí State, respectively. This finding suggested the preference of some species for the easy weather conditions observed in the region, namely: little rain and low wind speed (as reflected by the barometric trend), without excessive solar radiation - represented by insolation. (Fig. 3).
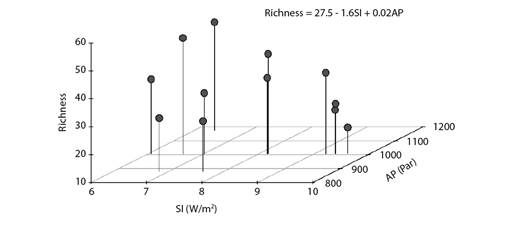
Fig. 3 Scatterplot depicting the association among monthly migrant bird species richness, mean insolation (SI) and mean atmospheric pressure (AP) at the coast of Piauí State, Brazil. The model's equation derived from an elastic net regression.
Vegetation type was a good predictor of migrant birds' spatial distribution, since the habitats were occupied by each species (PERMANOVAF4,472 = 16.5, R2 = 0.12, P = 0.001). Thus, there were significant differences in bird assemblies' composition among different vegetation types observed in coastal Piauí State (Fig. 4). Despite such a statistical significance, based on many superpositions observed in Fig. 4, some species seem to be found in more than one habitat, or at least in transition sites among them.
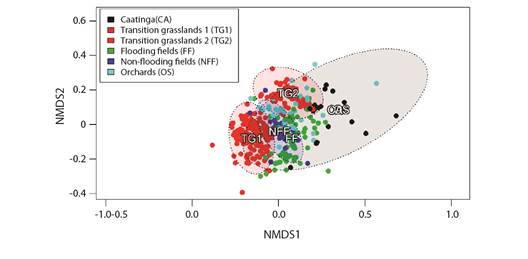
Fig. 4 Biplot of Non-metric Multidimentional Scalling (NMDS) generated from the Bray-Curtis dissimilarity of sampling campaigns; it depicts differences in species composition of migrant bird assemblies from different vegetation types at the coast of Piauí State.
These differences in avifauna can be represented by different bird species that can work as indicators for each vegetation type; i.e., some species were more closely associated with certain habitats, such as the ruby-topaz humming bird (Chrysolampis mosquitus), which was associated with caatinga sites; the whimbrel (Numenius phaeopus) species, which was associated with flooded fields; the collared plover (Charadrius collaris) species, which was correlated to non-flooded fields; the snail kite (Rostrhamus sociabilis) species, which was associated with transition grasslands; and the Picui ground dove (Columbina picui) species, which was mostly related to orchards (Table 1).
It is noteworthy that transition grasslands were the only vegetation type sampled in 2 discontinuous periods (between 2009-2011 and 2015-2016); they presented differences in bird assemblies between these two-time intervals: TG1 and TG2, respectively (Fig. 4). However, both intervals seemed consistently different from those of other vegetation types. Thus, snail kite was the main indicator-species between 2009-2011, whereas white-browed meadowlark (Sturnella superciliaris) became more abundant between 2015 and 2016. This finding suggests that other factors, besides vegetation - although associated with it -, may influence the wintering site of migrant birds at the coast of Piauí State.
Besides the composition of assemblies, the species richness of migrant birds also differed among vegetation types - orchards and non-flooded fields were the richest ones, and transition grasslands were the poorest categories (Fig. 5).
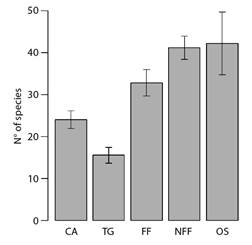
Fig. 5 Barplots depicting the expected (i.e., rarefied to equalize sampling effort) number of migrant bird species in each vegetation type along the coast of Piauí State, Brazil. Lines represent 2x the standard deviation of the expected richness. CA = Caatinga; TG = Transition grasslands; FF = Flooding Fields; NFF = Non-flooding Fields; OS = Orchards.
Despite differences in number and composition of species among vegetation types, the temporal pattern recorded for species richness was relatively similar among flooded fields, non-flooded fields and transition grassland categories - which were the only categories with samples collected in all assessed months (Fig. 6).
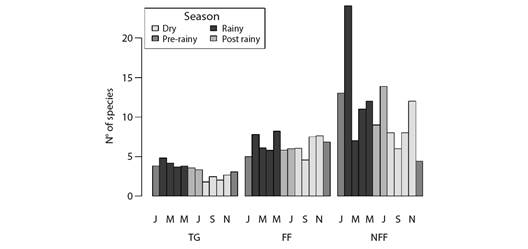
Fig. 6 Barplots depicting monthly variation in the expected (i.e., rarefied to equalize sampling effort) number of migrant bird species in each vegetation type along the coast of Piauí State, Brazil. TG = transition grasslands, FF = flooding fields, NFF = non-flooding fields. Bar colors represent different seasons.
This temporal pattern was also similar to the one observed when the whole site was taken into account, including the different types of migrant species, namely: intracontinental, Northern visitors and nomads.
Discussion
In order for shorebirds to survive the annual migration from the Southern hemisphere to breeding sites in the Artic, they count on a series of locations throughout their route. These places provide them with food for them to replace the energy lost during the flight (Clark, 2015). The coastal region of Piauí State has the potential to house a large number of migrant shorebirds because it is an estuarine environment (Putra et al., 2017). Muddy habitats in these locations provide a broader source of food, and it can be observed from the large number of bird species foraging on them (Silva & Rodrigues, 2015).
It is essential highlighting that sites are influenced by tides and habitat physiognomy at different times of the year, a fact that makes food available for birds throughout the whole seasonal cycle. This feature reduces the need of these species to move to other physiognomies in order to find food (Cabrera-Cruz et al., 2020; Palacín et al., 2017). Species may not finish their trajectory if only one of these sites is compromised (Clark, 2015).
Migrant species are notably consistent in terms of their wintering sites, since they systematically visit habitats known for their food-resource availability to satisfy their feeding needs and foraging strategies (Nunes & Tomas, 2008). These requirements help explaining the significant differences observed in species compositions between different analyzed sites, as well as why some species are found in very specific sites - therefore, they are considered indicator species. Different sites present different resources, and they determine species compositions among migrant birds. On the other hand, despite the generally observed differences among environments, the coast of Piauí State is a dense mosaic of different landscapes (Santos-Filho et al., 2010); thus, it is not surprising that several superpositions take place in it. Therefore, at least some species can be distributed in transition zones, or even in more than one habitat; eventually, it results in complex patterns at species level.
According to the classification system by Santos-Filho et al. (2010), the study site includes non-flooding, flooding and grassland fields, as well as orchards. The first two site types are basically covered by herbaceous vegetation, and they mainly differ from each other because of their water accumulation during the rainy season. These differences in vegetation are followed by differences in avifauna, as expressed by the indicator-species. Wet environments seasonally received more water birds (such as Numenius phaeopus and Rostrhamus sociabilis) than dry environments, for example. Although birds always return to the same wintering sites, severe changes in these environments can induce species to search for other, and more favorable, locations. Assumingly, it happened in sites TG1 and TG2, which recorded significant differences in their species compositions overtime. These observations corroborated Sick (1997), who emphasized the importance of habitat conservation for species conservation and noticed that birds are totally dependent on their environments; therefore, habitat losses have negative impact on migrant bird populations (Howard et al., 2018).
Other determining factors regarding migratory movements lie on climatic influences. Certain environmental variables are related to the arrival and departure of intercontinental migrants, mainly to insolation and atmospheric pressure. At first, both variables can emerge as uncommon biodiversity pattern drivers in tropical regions; however, Romero et al. (2000); Panuccio et al. (2010) and Ben-Hamo et al. (2013) considered climatic factors as determinant for migrant birds' flight paths. They emphasized that weak winds and high barometric pressure represented ideal climatic conditions for migrant birds' high-altitude flights. On the other hand, de Carvalho Melo (2017) reported that the number of adult marine birds in their focal species was positively related to mean wind velocity and negatively associated with atmospheric pressure. Results in the current study seem to be following those recorded by the aforementioned authors, when it comes to atmospheric pressure. According to the positive association with atmospheric pressure and to the negative correlation to solar radiation, at least some species prefer milder weather conditions when they get to Piauí State. The Brazilian equatorial coast is notoriously energetic due to its high temperatures over the year, intense (but highly variable) rainfall during the wet season and strong winds in the dry season (Soares et al., 2021). Accordingly, some species may adjust their arrival timing to transition times between seasons, and it forms the herein observed richness peaks.
It is noteworthy that although insolation and atmospheric pressure were selected by the model-building algorithm, they only explained a small portion of variations in migrant bird species' richness. This finding reflects the complexity of the probable species-specific processes driving the arrival of migrant bird species in the Brazilian equatorial coast. Therefore, caution is advisable at the time to take into consideration the model presented in Fig. 3. It should be regarded as no more than preliminary assessment on how weather clues can be important to migrant bird species' arrival in Piauí State - this subject should be more thoroughly investigated in future studies. Howard et al. (2018) commented that climatic changes would most likely increase the effective migration distances crossed by many migrant species, a fact that would force them to travel longer distances, to increase the numbers of stopovers and, consequently, the total duration of their long-distance migrations - this factor is not commonly considered in studies on climate change. Furthermore, lack of correlation between migratory movements and climatic factors, other than insolation and atmospheric pressure, can be related to the presence of some migrant individuals throughout the year. Sick (1997) noticed that birds who are not able to accumulate sufficient energy resources to return to their breeding grounds may remain in the wintering sites and only return during the next migratory cycle.
Additional factors also need to be considered; for example, there was remarkable coincidence of bird richness peaks and plant fructification peaks nearby restinga vegetation sites (Ribeiro, 2011). This coincidence is noteworthy because only one of the bird species recorded in the present study was classified as frugivore. Thus, increased fructification cannot be the direct cause of higher avifauna diversity. Therefore, other mechanisms should be investigated in order to clarify the association between food availability and bird migration in coastal Piauí State, such as the case of plant-insect phenological synchrony or spatiotemporal variations in benthic fauna composition.
Only two species were classified as nearly endangered: Calidris canutus and C. pusilla. Rosenberg et al. (2017) noticed that resources available for conservation purposes are usually quite limited, and that the recognition of vulnerable species (or of endangered ones) is an essential component for effective conservation planning. Surveys can identify and compare population trends recorded for focal species during different seasons of the year; these results can be used to identify proximal factors accountable for population changes. Information gathered during long-term bird populations' monitoring can be used to guide and optimize management activities critical to maintain or reestablish viable avian populations (Lynn et al., 2017). Up-to-date knowledge about the origin and destination sites of migrant birds, as well as environmental details of each wintering site, will be essential to maintain viable populations.
It was never so urgent taking conservation actions, and actions to avoid climate changes given the large volume of migrant bird species suffering with the drastic decline in their populations in the last few years, since these changes can have impact on migrant shorebirds who breed in the Artic, and change their breeding locations and conditions (Wauchope et al., 2017). Some evidence suggest that the magnitude of migratory movements taken by these species can influence their vulnerability to environmental changes (Webster & Marra, 2005). Although it is not fully understood, the decline in migrant species is a constant concern (Hardesty-Moore et al., 2017). However, a study on migratory movements has shown that greater population variability within these movements leads to higher resilience towards environmental changes (Gilroy et al., 2016). Data in the present study reinforced the observation that migrant birds occupy specific environments during their permanence along the coast of Piauí State and that these birds are seen at higher concentrations during the most humid months of the year.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio