Pollen is the bee’s major sources of proteins, amino acids, lipids, minerals and vitamins (Marchini, Reis, & Moreti, 2006). It is essential for the growth of larvae and young adult bees (Dietz, 1975; Modro, Silva, Cynthia, Luz, & Message, 2009). Rearing one worker bee from larval to adult stage requires approximately 120-145 mg of pollen (Alfonsus, 1933; Haydak, 1935). Honeybee transports pollen to the hive in specialised structures of its legs (corbicula), in which pollen moistened with nectar and hypopharyngeal and mandibular secretions, is packaged forming pasty pellets called ‘pollen loads’ (Thorp, 2000). The pollen composition in the pollen loads is a reflection of the local flora preferred by the workers of the bee species surrounding hives (DiazLosada, Ricciardelli-d’Albore, & Saa-Otero, 1998). Therefore determination of polleniferous flora in a given area is very important for development of any apiculture industry (Sajwani, Farooq, & Bryant, 2014). In West Bengal some work on polleniferous flora was done by Pal and Karmakar (2013), More, Ghorai, & Bera, 2010. Though few works on pollen analyses of A. mellifera form outside the state as well as from other countries were reported by Suryanarayana, Rao, & Singh, 1992; Noor, Khan, & Camphor, 2009; Lopez, Vives, & Boi, 2013; Saavedra-Carhuatacto, Aguinago-Castro, Rojas-Indrogo, & Delgado-Paredes, 2014 and Freitas, Sattler, Souza, Almeida-Muradian, Sattler, & Barth, 2015.
The present study aims to investigate the botanical origin of pollen loads collected in North 24 Parganas district, West Bengal to provide a guide to the optimal utilization of floral resources by honeybees for sustainable apiculture industries.
Materials and methods
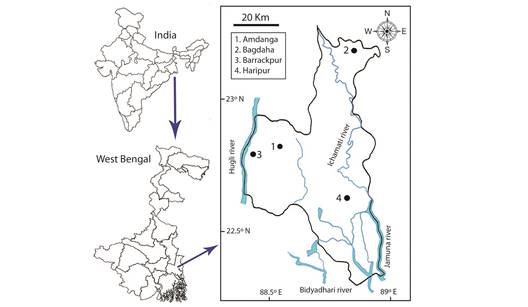
Study site: North 24 Pargana is a district in southern West Bengal of Eastern India. The district situated between (22º11’ - 23º15’ N & 88º20 ‘- 89º5’ E) with a total area of 4 094 km2. The climatic condition is tropical and experiences hot, humid and exhausting summer (mid April-mid June) with daily temperature ranging from 20 ºC to 40 ºC. The next season is monsoon which continued up to August, with an average annual rainfall of 1 579 mm and with high relative humidity level (RH 90 %). The cold and dry winter approach in early December and stay up to mid February with day temperature ranges from 8 to 28 ºC. Major rivers flows through the district are Ganges, Ichhamati, Jamuna and Bidyadhari. The soil of the northern part of the district is sandy, in the central middle part it is sandy and clay loam and in the southern side it is clay loam. The area harbours a diversified flora with a productive agricultural output as the river Ganges flows its long course through the district.
Collection of samples: A total of 2 434 pollen loads were collected from four apiaries viz. Amdanga (634 pollen loads), Bagdaha (586 pollen loads), Barrackpur (610 pollen loads) and Haripur (604 pollen loads) located at four different areas of North 24 Parganas district, West Bengal (Fig. 1). Pollen samples were collected for 12 months (January to December) and three to four sampling days per month. 30 to 64 loads were collected for each sampling day from one apiary. Incoming pollen foragers were captured at the entrance of the hive, and the two corbicular loads were collected. Pollen loads were preserved separately in (5 mL) white glass vials containing FAA (FormalinAceto-Alcohol, 5:5:90) solution.
Palynological analyses: Each pollen load was analyzed separately. The pollen loads were suspended in 50 mL of 95 % ethanol and thoroughly mixed (Jones & Bryant, 2004). The final suspension was then centrifuged at 4 000 rpm for 5 min. After decanting supernatant, the sediment was subjected to acetolysis (Erdtman, 1960) with a 9:1 ratio of acetic anhydride to conc. sulphuric acid. After thoroughly mixing, the mixture containing tube was placed into a water bath (at 80 oC) for 3 min. Then 5 mL of glacial acetic acid was added to each sample. The samples were rinsed twice with distilled water, centrifuging and decanting each time, and then once with 95% ethyl alcohol. After centrifuging, the pellet was taken on a small piece of glycerine jelly and transferred to the centre of a glass slide. Glycerine jelly was used as a mounting medium to prepare the samples for light microscopy (Ohe, Oddo, Piana, Morlot, & Martin, 2004). Then warmed gently to melt the jelly containing pollen sediment and covered by cover glass and sealed with paraffin wax. Identification of pollen types was done (on the basis of 500 grains counted per slide) with the help of reference slides prepared from the local flora as well as from published accounts (Nayar, 1990; Layek & Karmakar, 2016). Analysis was conducted using Leica DM1000 and photomicrographs of suitable magnifications were made with Leica DFC295 Digital camera. The contents of each pollen load were designated as being unifloral (with single pollen type ≥ 90 %), bifloral (with pollen of two types, one 80-90 % and other 20-10 %) and multifloral (with pollen of more than two types ≥ 10 %). Month wise percentages of the pollen types were also determined. Then we classified the obtained pollen types into three groups: very frequent (> 20 %), frequent (1020 %) and less frequent (< 10 %) (Layek & Karmakar, 2018).
Data Analysis: Statistical analyses of the pollen materials were done to get the arithmetic mean and standard deviation. To assess the association between different variables we followed Kearl Pearson’s correlation coefficient method.
Results
The analyzed pollen loads were designated as unifloral, bifloral and multifloral according to their pollen composition. The overall number of unifloral loads was 1 776 (72.97 %), bifloral was 462 (18.98 %) and multifloral was 196 (8.05 %). Among the multifloral loads, the majority had three pollen types and only eight loads were with four pollen types in composition. The highest proportion of unifloral loads was recorded during April (89.8 %), followed by March (86.1 %), November (85.05 %), January (82.30 %), December (80.18 %), February (79.34 %), October (71.4 %), September (64.89 %), May (61.96 %), June (53.33 %), July (50.60 %) and August (49.50 %). The sequences were almost reverse in cases of bifloral and multifloral loads (Table 1, Fig. 2). A total of 43 pollen types belonging to 28 plant families were identified (Table 2). Month wise analysis of loads revealed highest number of pollen types during March (13 types), followed by July (11 types), February, June and August (each of them with ten types), January, April, May and September (each with 8 types), October and December (both of each with seven types), and November with 6 pollen types (Table 2). According to frequency classes very frequent (> 20 %) pollen types were Brassica nigra, Phoenix sylvestris (during January); Coriandrum sativum (in February); Borassus flabellifer (during March and April); Sesamum indicum (in May); Acacia auriculiformis, Cocos nucifera (in September); Cocos nucifera, Poa gangetica (in October) and Brassica nigra during November and December. Besides the above mentioned pollen types, other frequent pollen types were Alangium salviifolium, Citrus × aurantiifolia, Citrus maxima, Croton bonplandianum, Cyanotis axillaris, Luffa cylindrica, Neolamarckia cadamba and Trema orientalis. Plants that accounted for the higher number of pollen loads were Brassica nigra (7.59 %), followed by Cocos nucifera (4.57 %), Poa gangetica (3.34 %), Borassus flabellifer (3.20 %), Sesamum indicum (3.19 %), Coriandrum sativum (3.08 %), Phoenix sylvestris (2.68 %), Trema orientalis (2.37 %) and Acacia auriculiformis (2.16 %). Plant families that accounted for a large number of loads were Arecaceae (21 %), Brassicaceae (16.23 %), Poaceae (6.68 %), Pedaliaceae (6.38 %), Apiaceae (6.16 %) and Fabaceae (5.38 %). The families that represented the greater number of taxa were Asteraceae and Euphorbiaceae (each of them with 4 plant taxa); followed by Arecaceae and Cucurbitaceae (each with 3 plant taxa); each of the family viz. Brassicaceae, Fabaceae, Myrtaceae and Rutaceae represented by 2 plant taxa. The remaining 20 families represented by single plant taxon.
Table 1 Pollen loads of A. mellifera collected in North 24-Parganas, West Bengal (India)
| - | - | Percentages of different types of pollen load (mean ± std. deviation) | ||
|---|---|---|---|---|
| Months | No. of loads analyzed | Unifloral | Bifloral | Multifloral |
| January | 226 | 82.3 ± 4.2 | 13.2 ± 2.7 | 4.4 ± 1.5 |
| February | 242 | 79.3 ± 8.9 | 14.9 ± 3.4 | 5.8 ± 5.7 |
| March | 244 | 86.0 ± 4.3 | 11.5 ± 2.1 | 2.4 ± 3.1 |
| April | 216 | 89.4 ± 4.7 | 5.5 ± 2.1 | 5.0 ± 4.1 |
| May | 184 | 61.3 ± 7.6 | 28.9 ± 4.9 | 9.8 ± 4.1 |
| June | 120 | 53.3 ± 9.4 | 35.0 ± 6.4 | 11.6 ± 6.4 |
| July | 166 | 50.5 ± 4.2 | 39.9 ± 7.1 | 9.5 ± 3.3 |
| August | 202 | 50.1 ± 5.6 | 31.7 ± 2.9 | 18.1 ± 4.3 |
| September | 188 | 65.0 ± 4.3 | 20.2 ± 0.4 | 14.8 ± 4.4 |
| October | 210 | 71.0 ± 5.4 | 17.1 ± 3.4 | 11.9 ± 5.9 |
| November | 214 | 84.1 ± 7.8 | 12.0 ± 6.5 | 3.9 ± 2.9 |
| December | 222 | 80.4 ± 7.0 | 15.2 ± 3.7 | 4.3 ± 4.1 |
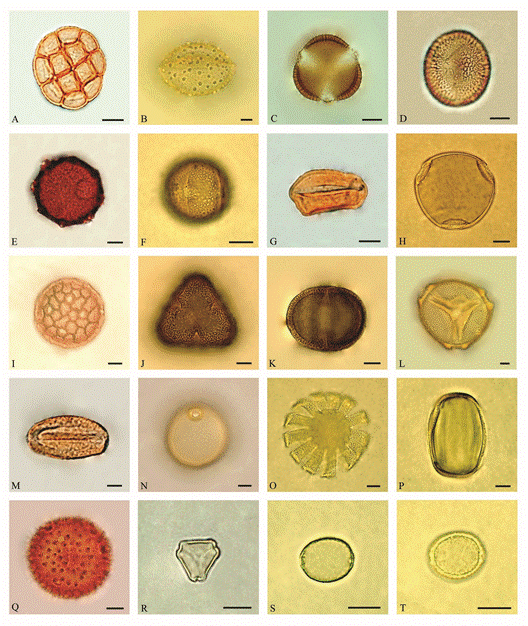
Fig. 2 Some pollen types obtained from pollen loads of Apis mellifera: A) Acacia auriculiformis; B) Borassus flabellifer; C, D) Brassica nigra; E) Chrozophora rottleri; F) Citrus x aurantiifolia; G) Cocos nucifera; H) Cucumis sativus; I) Delonix regia; J, K) Euphorbia tithymaloides; L) Luffa cylindrical; M) Monochoria hastate; N) Poa gangetica; O, P) Sesamum indicum; Q) Sida acuta; R) Syzygium cumini; S, T) Trema orientalis. Scale bars = 10 µm.
Table 2 Pollen types obtained from loads of Apis mellifera in North 24 Parganas, West Bengal (India)
| - | - | - | - | - | - | Months | - | - | - | - | - | - | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pollen types | - | - | - | - | - | - | - | - | - | - | - | No. of loads | |||||
| - | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | - | ||||
| Amaranthaceae | - | - | - | - | - | - | - | - | - | - | - | - | 7 | ||||
| Amaranthus spinosus | - | - | - | - | - | - | LF | LF | - | - | - | - | 7 | ||||
| Apiaceae | - | - | - | - | - | - | - | - | - | - | - | - | 75 | ||||
| Coriandrum sativum | F | VF | - | - | - | - | - | - | - | - | - | F | 75 | ||||
| Arecaceae | - | - | - | - | - | - | - | - | - | - | - | - | 254.49 | ||||
| Borassus flabellifer | - | - | VF | VF | - | - | - | - | - | - | - | - | 78 | ||||
| Cocos nucifera | LF | LF | LF | - | - | LF | F | F | VF | VF | - | LF | 111.32 | ||||
| Phoenix sylvestris | VF | F | - | - | - | - | - | - | - | - | - | F | 65.17 | ||||
| Asparagaceae | - | - | - | - | - | - | - | - | - | - | - | - | 11.50 | ||||
| Polianthes tuberosa | - | - | - | - | - | - | LF | LF | - | - | - | - | 11.50 | ||||
| Asteraceae | - | - | - | - | - | -- | - | - | - | - | - | - | 36.83 | ||||
| Helianthus annuus | - | LF | LF | - | - | - | - | - | - | - | - | - | 8 | ||||
| Mikania scandens | LF | - | - | - | - | - | - | - | - | - | LF | LF | 16.33 | ||||
| Tagetes erecta | LF | - | - | - | - | - | - | - | - | - | - | LF | 8 | ||||
| Tridax procumbens | - | - | - | - | LF | - | - | - | - | - | - | 4.50 | |||||
| Brassicaceae | - | - | - | - | - | - | - | - | - | - | - | - | 197.50 | ||||
| Brassica nigra | VF | F | LF | - | - | - | - | - | - | - | VF | VF | 184.67 | ||||
| Raphanus sativus | LF | - | - | - | - | - | - | - | - | - | - | LF | 12.83 | ||||
| Cannabaceae | - | - | - | - | - | - | - | - | - | - | - | - | 57.67 | ||||
| Trema orientalis | - | - | - | LF | LF | F | F | F | LF | - | - | - | 57.67 | ||||
| Capparaceae | - | - | - | - | - | - | - | - | - | - | - | - | 11 | ||||
| Capparis zeylanica | - | - | LF | LF | - | - | - | - | - | - | - | - | 11 | ||||
| Cleomaceae | - | - | - | - | - | - | - | - | - | - | - | - | 7.33 | ||||
| Cleome viscosa | - | - | - | - | - | LF | LF | - | - | - | - | - | 7.33 | ||||
| Combretaceae | - | - | - | - | - | - | - | - | - | - | - | - | 10 | ||||
| Terminalia arjuna | - | - | - | LF | - | - | - | - | - | - | - | - | 10 | ||||
| Commelinaceae | - | - | - | - | - | - | - | - | - | - | - | - | 17.67 | ||||
| Cyanotis axillaris | - | - | - | - | - | LF | LF | - | LF | F | LF | - | 17.67 | ||||
| Cornaceae | - | - | - | - | - | - | - | - | - | - | - | - | 27.67 | ||||
| Alangium salviifolium | - | - | F | LF | - | - | - | - | - | - | - | - | 27.67 | ||||
| Cucurbitaceae | - | - | - | - | - | - | - | - | - | - | - | - | 61.67 | ||||
| Cucumis sativus | - | - | - | - | - | - | LF | LF | LF | - | - | - | 21 | ||||
| Cucurbita maxima | - | - | LF | - | - | - | - | - | - | - | - | - | 5.50 | ||||
| Luffa cylindrica | - | - | LF | - | LF | F | LF | LF | LF | - | - | - | 35.17 | ||||
| Euphorbiaceae | - | - | - | - | - | - | - | - | - | - | - | - | 45.17 | ||||
| Chrozophora rottleri | - | - | - | - | LF | LF | - | - | - | - | - | - | 9.17 | ||||
| Croton bonplandianum | - | - | - | LF | LF | F | - | - | - | - | - | - | 22 | ||||
| Euphorbia tithymaloides | - | - | - | - | LF | - | - | - | - | - | - | - | 2 | ||||
| Ricinus communis | - | - | - | - | - | - | - | - | LF | LF | - | - | 12 | ||||
| Fabaceae | - | - | - | - | - | - | - | - | - | - | - | - | 65.50 | ||||
| Acacia auriculiformis | - | - | - | - | - | - | LF | LF | VF | LF | - | - | 52.50 | ||||
| Delonix regia | - | - | - | LF | LF | - | - | - | - | - | - | - | 13 | ||||
| Malvaceae | - | - | - | - | - | - | - | - | - | - | - | - | 10.17 | ||||
| Bombax ceiba | - | - | LF | - | - | - | - | - | - | - | - | - | 3.17 | ||||
| Sida acuta | - | - | - | - | - | - | - | - | - | LF | LF | - | 7 | ||||
| - | -- | - | - | - | - | Months | - | - | - | - | - | - | |||||
| Pollen types | - | - | - | - | - | - | - | - | -- | - | - | No. of loads | |||||
| - | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | - | ||||
| Moringaceae | - | - | - | - | - | - | - | - | - | - | - | - | 6.33 | ||||
| Moringa oleifera | LF | LF | - | - | - | - | - | -- | - | - | - | - | 6.33 | ||||
| Myrtaceae | - | - | - | - | - | - | - | - | - | - | - | - | 28.83 | ||||
| Eucalyptus globulus | - | - | - | - | - | - | - | - | - | LF | LF | LF | 22.83 | ||||
| Syzygium cumini | - | - | LF | - | - | - | - | - | - | - | - | - | 6 | ||||
| Nelumbonaceae | - | - | - | - | - | - | - | - | - | - | - | - | 2.50 | ||||
| Nelumbo nucifera | - | - | - | - | LF | - | - | - | - | - | - | - | 2.50 | ||||
| Pedaliaceae | - | - | - | - | - | - | - | - | - | - | - | - | 77.67 | ||||
| Sesamum indicum | - | - | - | F | VF | - | - | - | - | - | - | - | 77.67 | ||||
| Poaceae | - | - | - | - | - | - | - | - | - | - | - | - | 81.33 | ||||
| Poa gangetica | - | - | - | - | - | - | - | F | LF | VF | F | - | 81.33 | ||||
| Pontederiaceae | - | - | - | - | - | - | - | - | - | - | - | - | 7.67 | ||||
| Monochoria hastata | - | - | - | - | - | - | - | LF | - | - | - | - | 7.67 | ||||
| Ranunculaceae | - | - | - | - | - | - | - | - | - | - | - | - | 8.33 | ||||
| Nigella sativa | - | LF | - | - | - | - | - | - | - | - | - | - | 8.33 | ||||
| Rubiaceae | - | - | - | - | - | - | - | - | - | - | - | - | 21.84 | ||||
| Neolamarckia cadamba | - | - | - | - | - | LF | F | LF | - | - | - | - | 21.84 | ||||
| Rutaceae | - | - | - | - | - | - | - | - | - | - | - | - | 32.17 | ||||
| Citrus × aurantiifolia | - | - | - | - | - | LF | F | - | - | - | - | - | 11 | ||||
| Citrus maxima | - | LF | F | - | - | - | - | - | - | - | - | - | 21.17 | ||||
| Salicaceae | - | - | - | - | - | - | - | - | - | - | - | - | 5 | ||||
| Flacourtia jangomas | - | - | LF | - | - | - | - | - | - | - | - | - | 5 | ||||
| Sapindaceae | - | - | - | - | - | - | - | - | - | - | - | - | 1.50 | ||||
| Litchi chinensis | - | LF | - | - | - | - | - | - | - | - | - | - | 1.50 | ||||
| Ulmaceae | - | - | - | - | - | - | - | - | - | - | - | - | 18.50 | ||||
| Holoptelea integrifolia | - | LF | LF | - | - | - | - | - | - | - | - | - | 18.50 | ||||
| No. of pollen types | 8 | 10 | 13 | 8 | 8 | 10 | 11 | 10 | 8 | 7 | 6 | 8 | - | ||||
VF: very frequent, F: frequent, LF: less frequent
Discussion
Major proportion of unifloral loads implies the floral fidelity behaviour of the bee species. Bifloral and multifloral loads could be due to the variety of flowering plants that were blooming simultaneously. This multifloral availability appears to divert the attention of some foraging bees (Alves & Santos 2014). Greater proportion of unifloral loads were obtained during March-April (spring-summer) and NovemberJanuary (winter) derived from Borassus flabellifer and Brassica nigra, Phoenix sylvestris respectively. The importance of these plant taxa as polleniferous plant were also reported by Layek, Nandi, & Karmakar (2016) form West Bengal and Suryanarayana et al. (1992) from Bihar in India. In addition to the above mentioned taxa, other important polleniferous plants were: Acacia auriculiformis, Cocos nucifera, Coriandrum sativum, Poa gangetica, Sesamum indicum and Trema orientalis. The significance of some the above taxa as pollen contributor were also recorded in India as well as from other countries (by Noor et al., 2009; Karmakar, Layek, & Pal, 2011; Layek, Bhakat, & Karmakar, 2015). But the significance of Trema orientalis as polleniferous plant was not previously recorded by any author in West Bengal, India. The small percentage of pollen from: Bombax ceiba, Euphorbia tithymaloides, Flacourtia jangomas, Litchi chinensis, Nelumbo nucifera, Tridax procumbens indicate that these plants are either sporadic in distribution, fairly rare in abundance, do not produce sufficient amount of pollen for bee foraging, or produce pollen that is not preferred by honeybees due to low nutritional value (Dórea, Novais & Santos, 2010). Among the major pollen supplied families, Apiaceae, Pedaliaceae and Poaceae comprises of single plant taxon. On the other hand, largest represented families Asteraceae, Cucurbitaceae and Euphorbiaceae contributed moderate pollen to the bee species. Hence, the correlation between represented plant taxa of a family and their contribution as pollen supplier is low to medium (r = 0.40, n = 28).
The present study was undertaken to identify the polleniferous flora of the study area as well as floral fidelity behaviour of the bee species. Regarding pollen composition of each load, it was found that majority were unifloral type which signifies the floral fidelity behaviour of the bee species. Floral constancy level increases with the presence of excellent and huge polleniferous plants during March-April and November-January, whereas decreases during dearth period from June-August due to absence of very frequent pollen types in these regions. However, Trema orientalis play a major role to sustain the pollen flow for the bee colony during the dearth period in the studied area. The present work provides us with a considerable knowledge about plant diversity and the annual flowering pattern. By knowing the flowering pattern and the preferred plant species for pollen collection by A. mellifera, the apiculturists will get a proper knowledge regarding successful establishment of apiaries in that region.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. A signed document has been filed in the journal archives.












 uBio
uBio