Plants synthesize a diversity of low molecular weight, structurally complex chemical compounds, commonly known as secondary metabolites (Moore, Andrew, Külheim, & Foley, 2014) These are present mainly under stress conditions, but their absence does not cause harmful effects to the plants (Edreva, 2005; Edreva et al., 2008; Khan, Aliabbas, Kumar, & Rajkumar, 2009). Among them phenolic compounds are one of the most pronounced secondary metabolites found in plants, play an important role in plant interactions as defense chemicals and their distribution is shown throughout the entire metabolic process (Kurmukov, 2013). These can act as antioxidants, structural polymers (lignin), attractants for pollinators insects (flavonoids and carotenoids), UV screens (flavonoids), signal compounds (salicylic acid and flavonoids) and defense response chemicals (tannins and phytoalexins) (Brglez Mojzer, Knez Hrnčič, Škerget, Knez, & Bren, 2016). From a human physiological standpoint, phenolic compounds are vital in defense responses such as antiaging, anti-inflammatory, antioxidant and antiproliferative activities (Irchhaiya et al., 2015; Khan et al., 2011; Lin et al., 2016).
In the search for alternatives to produce desirable medicinal compounds from plants, biotechnological approaches, specifically plant tissue cultures, have the potential to act as supplemental alternatives to traditional agriculture in the industrial production of bioactive plant metabolites. Therefore, some advantages of in vitro culture plant systems over the conventional cultivation of whole plants are: useful compounds can be produced under controlled conditions independent of climatic changes or soil conditions; the cells of any plants, tropical or alpine, could easily be multiplied to yield their specific metabolites; automated control of cell growth and regulation of metabolite processes would reduce labor costs and improve productivity among other advantage (Bourgaud, Gravot, Milesi, & Gontier, 2001; Giri & Zaheer, 2016). Moreover, the evaluation of many secondary plant metabolites for bioprospecting should be a priority towards sustainable conservation and rational utilization of biodiversity in order to be applied in health, cosmetic and pharmaceutical industry.
Radiation induces damage to living cells mediated by the generation of free radicals and related reactive oxygen species (ROS) that damage cellular targets such as DNA, membrane lipids and proteins. Naturally occurring antioxidants are effective photoprotectors due to their ability to scavenge free radicals or neutralize their reactions (Londhe, Devasagayam, Foo, & Ghaskadbi, 2009). Some species of Baccharis, belonging to the Asteraceae family of the tropical and subtropical areas of America and, particularly, Colombia, (Cuatrecasas, 1967, 1981; Díaz-Piedraita & Cuatrecasas, 1991) have shown anti-inflammatory, antioxidant, antimicrobial and antifungal activities (Abad et al., 2006; Gené et al., 1996). Baccharis antioquensis has been described as a potential source of new photoprotective compounds with antioxidant capacities (MejíaGiraldo, Henao-Zuluaga, Gallardo, Atehortúa, & Puertas-Mejía, 2016a; Mejía-Giraldo, Winkler, Gallardo, Sánchez-Zapata, & PuertasMejía, 2016b). The advantages of polyphenols are their high accessibility, specificity of their response and low toxicity; whereas the main problems of these compounds are their rapid metabolism and low bioavailability. Recent studies have proposed the nanoformulation of polyphenols in order to prevent their rapid degradation and, consequently, enable delivery of increased concentrations to the target cells (Moga et al., 2016).
Conversely, biotic and abiotic elicitors, which are classified on their origin, have been considered as an effective way to stimulate secondary metabolites due to the fact that both plant defense mechanism and metabolite production are interrelated via secondary metabolism (Manaf, Rabie, & Abd El-Aal, 2016). Among them, Ultraviolet-B radiation is an important abiotic factor that improves the production of secondary metabolites (Cetin, 2014) and low UVB doses increase the production of secondary metabolites (Gil, Pontin, Berli, Bottini, & Piccoli, 2012). Therefore, the aim of the present work was to develop a micropropagation protocol of B. antioquensis and evaluate the production of polyphenols by tissue culturee after UV-B treatment.
Materials and methods
Plant material: Branches in juvenile stage of B. antioquensis, were collected from wild individuals in July 2014, in Llanos de Cuivá, Yarumal, Antioquia, Colombia, at an altitude of 2 730 m.a.s.l. (6°49’50.6” N - 75°29’29.9” W). A voucher specimen was deposited in the Herbarium of the University of Antioquia, Colombia (HUA 194796).
Sterilization of plant material: Segments of 2-3 internodes and apical sprouts of 2 cm in length were washed with disinfectant soap Quirucidal and rinsed with running water. Subsequently, in laminar flow chamber, segments were immersed in Benomil fungicidal solution (2 g/L) and antibiotic Streptomycin (1 g/L) for 3 hours. Then were immersed in solution of sodium hypochlorite (NaClO 0.5, 1 or 2 % (v/v)) or mercuric chloride (HgCl2 0.1 % (w/v)), for 15 minutes, for a total of three (3) treatments. Finally, explants of each treatment were washed 3 times with sterile distilled water.
Development of micropropagation protocols: Explants were cultured on half strength Murashige and Skoog (1/2 MS) medium (Veraplakorn, 2016), supplemented with citric acid (AC) 200 ppm, ascorbic acid (AA) 200 ppm, polyvinylpyrrolidone (PVP) 500 ppm and sucrose 2 % (w/v). All culture media were prepared with distilled water and homogenized with constant stirring. The initial pH was adjusted to 5.75 with NaOH 1 N and/or HCl 1 N, before autoclaving and sterilized for 20 minutes at 121 °C and 15 psi. Treatment with growth regulators 6- benzylaminopurine (BA), gibberellic acid (GA3), thidiazuron (TDZ) were evaluated both in liquid and solid media, as well as two different lighting conditions (light/darkness), for a total of 16 treatments, including control (Murashige and Skoog basal medium without growth regulators; treatments 13-16), at 23 ± 1 °C (Table 1). Treatments in liquid medium were kept in continuous agitation at 100 rpm and the light intensity was 20 µmol/m2s. After which periodic subcultures were performed every 10 days during two months and then every 20 days.
Table 1 Concentration of growth regulators used in each treatment to assess the establishment phase and in vitro multiplication of apexes and node of B. antioquensis
| - | Growth regulators (mg/L) | Media type | Light conditions | ||||
|---|---|---|---|---|---|---|---|
| Treatment | BA | GA 3 | TDZ | Solid | Liquid | Light | Darkness |
| 1 | 1 | 0.5 | - | X | - | X | - |
| 2 | 1 | 0.5 | - | X | - | - | X |
| 3 | 1 | 0.5 | - | - | X | X | - |
| 4 | 1 | 0.5 | - | - | X | - | X |
| 5 | 2 | 0.5 | - | X | - | X | - |
| 6 | 2 | 0.5 | - | X | - | - | X |
| 7 | 2 | 0.5 | - | - | X | X | - |
| 8 | 2 | 0.5 | - | - | X | - | X |
| 9 | - | - | 0.5 | X | - | X | - |
| 10 | - | - | 0.5 | X | - | - | X |
| 11 | - | - | 0.5 | - | X | X | - |
| 12 | - | - | 0.5 | - | X- | - | X |
| 13 control | - | - | - | X | - | X | - |
| 14 control | - | - | - | X | - | - | X |
| 15 control | - | - | - | - | X | X | - |
| 16 control | - | - | - | - | X | - | X |
BA: 6- benzylaminopurine, GA3: gibberellic acid, TDZ: thidiazuron.
Elicitation of in vitro plants by UVR: Groups of in vitro plants were exposed to different UVR treatments, (UV light produced by Philips UVC light, 200 - 280 nm), evaluating different times and distances of exposure to this radiation, as well as two post treatment collection times (Table 2). The leaves of the plants were collected and the absorption coefficient in the UVA-UVB range, the antiradical capacity and the total phenol content (TPC) in all in vitro plants and the wild plant extracts were evaluated.
Table 2 UVR in vitro elicitation of B. antioquensis plants
| Treatment | Light intensity (mW/m 2 ) | Exposure distance (cm) | Exposure time (min) | Collection time (hour) |
|---|---|---|---|---|
| T1 | 28-29 | 49 | 5 | 24 |
| T2 | - | - | - | 48 |
| T3 | - | - | 10 | 24 |
| T4 | - | - | - | 48 |
| T5 | 32-33 | 39 | 5 | 24 |
| T6 | - | - | - | 48 |
| T7 | - | - | 10 | 24 |
| T8 | - | - | - | 48 |
| Control | - | - | - | 0 |
Extraction procedure: The extraction was performed according to the method described previously (Mejía-Giraldo et al., 2016b) with some modifications. Fresh vegetal material (leaves) was dried at room temperature protected from light. Then, dry vegetal material (DVM) was crushed using an electric grinder (IKA, A11 basic S1). Briefly, 100 mg of DVM crushed was subjected to extraction with 10 mL of methanol at room temperature (c.a. 25 °C) with magnetic stirring for 24 hours, and then extract was filtered. Finally, the crude extracts obtained were concentrated to dryness in a rotary evaporator (IKA, RV10 basic) at 40 ± 2 °C. The extraction yield (percentage of dry extract), TPC, antiradical capacity (DPPH assay) and absorptive capacity in UVA-UVB range, were evaluated in eight treatments of the elicitation procedure, in vitro plants control, and wild plant.
Antiradical capacity - DPPH Assay: For each extract, different concentrations were estimated according to the method described previously (Mejía-Giraldo, Gallardo, & Puertas-Mejía, 2015) with some modifications, and the effective relative concentration (EC50) at which 50 % of DPPH has been removed was expressed as mg of dry extract/mmole DPPH radical, based on equation: Efficient Concentration (EC50) = concentration of test at steady state/concentration of DPPHt=0. The initial concentration of DPPH (100 µmol/L) in the reaction system was calculated according to a calibration curve (y = 1.146E-2x- 4.192E-3; r = 0.9999) at 514 nm, where y= absorbance and x= concentration of DPPH. All spectrophotometric data were obtained using a Thermo Scientific Evolution 60S UV-Visible Spectrophotometer. Disposable cuvettes (1 cm step length) were used for visible absorbance measurements for analyses. BHT (butylated hydroxy toluene) was used for comparison purposes.
Total phenol contents: TPC of samples were measured using a modified colorimetric Folin-Ciocalteu method (Puertas-Mejía, Rincón-Valencia, & Mejía-Giraldo, 2015). Briefly, 10 μL of extract solution and 615 μL of deionized water were added to a test tube. Then, 125 μL of Folin-Ciocalteu reagent was added to the solution and allowed to react for 5 minutes. After that, 1 250 μL of 20 % sodium carbonate solution was added into the test tubes and mixed. The absorbance was read at 760 nm using an Evolution 60S Spectrophotometer (Thermo Fisher Scientific, Inc., Shanghai, China). The results are expressed as milligrams of galic acid equivalents per g dry extract (GAE/g DE) (y = 0.125627X + 0.0312029; r = 0.99864)
UVA-UVB absorption coefficient: Briefly, an adequate dilution of the extracts was added in a quartz cuvette (1 cm step length) and their absorption spectrum (wavelengths 200 - 400 nm) was recorded on a Thermo Scientific Evolution 60S UV-Visible Spectrophotometer, against a blank containing methanol. The absorption coefficient (absorbance/(mg dry extract/mL)) was calculated at 290, 310, 340 and 380 nm (Mejía-Giraldo et al., 2015).
The results were expressed as the means ± SD. All data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey tests when appropriate, using R Development Core Team (2013). P values less than 0.05 (P < 0.05) were considered significant (R Development Core Team, 2013).
Results
After 25 days, explants were transferred to continuous light conditions to avoid the etiolation process, preserving state and composition of culture medium, and allowing their complete development. After 5 days, buds developed foliar primordia and de novo buds, each bud had between 3 and 4 buds which were individualized under the same conditions (Fig. 2). After 40 days of individualization, developed explants were transferred to culture medium conditions. In summary, only treatments 8 and 12 (Table 1) were successful for development of buds in explants (Fig. 1).
Micropropagation: Disinfection protocols required a combined disinfection protocol of NaClO 0.5 % (v/v) and HgCl2 0.1 % (w/v), resulting in a disinfection rate greater than 70 %. Regarding to the establishment of explants, treatments containing BA + GA3 regulators (2 and 0.5 mg/L respectively) and TDZ (0.5 mg/L) showed positive results. In addition to growth regulators, light conditions and type of culture medium (solid or liquid), had a favorable effect on the response of explants, since there was only bud growth with these growth regulators in liquid medium and dark free of growth regulators, and observed for 60 days until complete plant development. The material obtained was used for multiplication from internodes, obtaining a multiplication coefficient of 4 in vitro plant/explant/ month (Fig. 3).
Elicitation: Exposure time and distances increase the absorption coefficient in the UVAUVB range and the antioxidant capacity. On the other hand, absorption capacity in UVA-UVB, TPC and antiradical capacity of extracts from wild plant of B. antioquensis exhibited higher values than extracts in each treatment of in vitro plants exposed to different conditions of UVB irradiation (Fig. 4, Table 2 and Table 3).
Table 3 Extraction yield and antioxidant capacity of B. antioquensis in vitro plants
| Sample | % Yield (% DE*) | TPC ¥ (mg EAG/g MVS) | Antiradical capacity EC 50 † |
|---|---|---|---|
| Wild plant | 27.65 ± 1.28 | 76.74 ± 5.61 | 0.13 ± 0.01 |
| Control | 38.74 ± 0.80 a | 8.64 ± 0.31 | 1.78 ± 0.07 |
| T1** | 35.92 ± 0.55 b | 13.25 ± 1.14 a | 1.21 ± 0.09 a |
| T2 | 38.80 ± 0.33 a | 15.85 ± 0.20 a | 0.98 ± 0.01 b |
| T3 | 41.19 ± 1.43 | 15.81 ± 1.65 a | 0.99 ± 0.01 b |
| T4 | 37.27 ± 0.12 a,b | 15.10 ± 0.25 a | 0.92 ± 0.02 b |
| T5 | 36.64 ± 0.38 b | 12.87 ± 0.20 a | 1.25 ± 0.21 a |
| T6 | 37.44 ± 0.32 a,b | 15.48 ± 0.21 a | 0.98 ± 0.01 b |
| T7 | 37.81 ± 0.18 a,b | 12.52 ± 0.30 a | 1.25 ± 0.06 a |
| T8 | 38.75 ± 1.13 a | 15.18 ± 0.50 a | 1.16 ± 0.02 a |
*See Table 2 for details. ** DE: Dry Extract. ¥ TPC: Total Phenol Content (mg gallic equivalents/g dry extract). †Effective Concentration at 50 % (g dry extract/mmol DPPH). Results are expressed as the mean value ± standard deviation (n = 3). Values in the same column followed by different letters are significantly different at the 5 % level, according one-way analysis of variance (ANOVA) and Tukey tests.
Discussion
Because phenolization reactions were observed in tissue plant, antioxidant compounds such as ascorbic acid (AA), citric acid (CA) and PVP were added to the culture in order to stop the reaction. PVP addition help to phenolization control, which may be associated with its polymeric characteristics, that confer specific adsorptive functions for certain organic compounds such as phenols, preventing the oxidation and polymerization (Concepción et al., 2005). Similar reactions were reported in the culture of B. tridentate, but using a mixture of CA+AA as antioxidant to stop the phenolization process (Kajiki & Shepherd, 2006). Nevertheless, a combination of CA+AA+PVP was necessary to stop such reaction in the B. antioquensis culture, similar to M. esculenta reports (Bhatt & Dhar, 2004). The best response to in vitro establishment of B. antioquensis was obtained from apexes, using GA3+BAP and TDZ as supplemental agent. However, during the multiplication stage these growing regulators promoted an accelerated production of sprouts without differences between them. Therefore, the multiplication process was carried out in a medium free of growth regulators, obtaining the development of complete plants (Fig. 3 and Fig. 4). These results were similar to those reported previously (Kajiki & Shepherd, 2006) for B. tridentata, whose also reported that the apices were the explants with the best response in the establishment process, in addition to the cultivation of apices allow the maintenance of the identity of the genotype, which stimulate the process of multiplication of the species.

Fig. 3 A. in vitro plants with fully developed stem, leaf and radicular system. B. in vitro plants multiplied.
Previous studies have described that UV light radiation induced gene transcription process of enzymes involved in biosynthesis of secondary metabolites (Logemann, Tavernaro, Schulz, Somssich, & Hahlbrock, 2000); in addition, excessive exposure to UVB radiation increases the synthesis of flavonoids (Agati, Galardi, Gravano, Romani, & Tattini, 2002; Kotilainen, Tegelberg, Julkunen-Tiitto, Lindfors, & Aphalo, 2008). Thus, the synthesis of polyphenols induced by UVR supposes that the characteristic photoprotection is related to the absorption of this solar radiation to increase the production of polyphenols (Agati et al., 2013; Agati & Tattini, 2010; Harborne & Williams, 2000). According to this premise, the in vitro plants showed important TPC values, scavenging capacity and absorption of UV radiation, but were lower than that observed on wild plants (Fig. 4, Table 3). These results are to be expected and reasonable because of the physiological aspect, principally the age of in vitro plants and environmental conditions when compared to wild plants.
Nevertheless, compare to control plant, the UVR showed a substantial effect on antioxidant and photoprotection properties in vitro plants. Phenolic composition of the foliar tissue samples changed significantly with irradiation (P < 0.05). This result agrees with Zu reports (Zu et al., 2010) that found UVB radiation induced significant flavonoid accumulation that was able to protect the Taxus chinensis plant from radiation damage, although with some morphologic changes. Other researchers reported higher content of caffeic acid and its derivatives in callus culture and cell suspension of Echinacea purpurea after UVB irradiation (Manaf et al., 2016). Antognoni reported (Antognoni et al., 2007) that UVB irradiation of callus of P. incarnata was able to increase the production of all four glycosyl flavonoids and callus cultures treated with the UVB dose that enhanced flavonoid production showed a higher antioxidant activity compared to untreated callus. When Gil et al. (2012) exposed in vitro cultured plants of Vitis vinifera to two different UVB doses, the low UVB treatment increased the levels of the membrane-related triterpenes, being more notable in young leaves. Our results showed that the capacity of B. antioquensis in vitro plants to biosynthesize secondary metabolites can be enhanced by appropriate forms of elicitation with UVR. This effect has been reported in many others studies carried out in different species of plants such as Passiflora sp (Antognoni et al, 2007), Rosmarinus officinalis (Luis, Pérez, & González, 2007), Catharanthus roseus (Ramani & Jayabaskaran, 2008), Hypericum perforatum (Germ, Stibilj, Kreft, Gaberščik, & Kreft, 2010) and Ginkgo biloba (Sun et al., 2010).
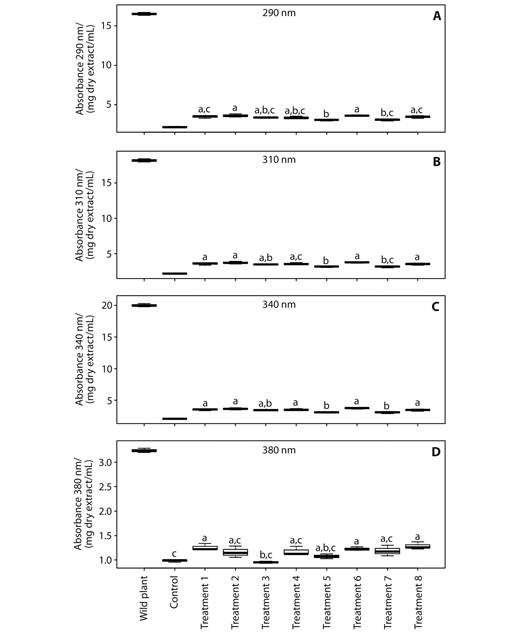
Fig. 4 UVA-UVB absorption coefficient assays of wild plant and in vitro plants extracts of B. antioquensis. The results are presented as the mean ± SD (n = 3). The boxes with different letters have a statistically significant difference with a confidence level of 5 %, according one-way analysis of variance (ANOVA) and Tukey tests. Absorption coefficients at (a) 290 nm, (b) 310 nm, (c) 340 nm, (d) 380 nm.
Finally, a protocol for in vitro introduction of B. antioquensis was first established using UVR, with a potential aspect related to future applications on genetic adaptations, elicitation and in vitro culture conditions in order to produce a large quantity of photoprotective compounds by sustainable and conservation practices of the species. The use of UVR as an eliciting agent of B. antioquensis was able to increase the content of polyphenolic compounds with UVA-UVB radiation absorption and antioxidant capacity, as compared to the control plant.












 uBio
uBio 



