Introduction
Globally, oral cancer is reported to be the eighth most commonly diagnosed cancer with an annual incidence of >300,000 cases (1). This projection emphasizes the urgent need for new diagnostic, preventive, and treatment modalities. Phytochemicals often display properties that are useful in countering carcinogenic changes in human cells. The advantage of plant extracts is their low toxicity compared with other treatment modalities, such as chemo and radiotherapy. Resveratrol and lycopene are components of such extracts (2).
Resveratrol is a natural polyphenol used in medicinal preparations for more than 2000 years. The chemical was first described in 1940 when extracted from the plant, Veratrum grandiflorum. The chemical is present in high concentrations in various fruits and vegetables, chiefly red grapes (3). The richest content of resveratrol is usually present in grape seeds, skin, and woody parts. Resveratrol shows a wide spectrum of biological activities, including anticancer, antioxidant, antiallergic, and phytoestrogenic (4,5,6).
Lycopene is a carotenoid, a family of pigments that gives color to different fruits and vegetables. The chemical is present in high concentrations in red/orange fruits and vegetables, with the highest content found in fresh tomatoes (30%-40%) (7). Lycopene in tomatoes naturally occurs in the trans-form, which is poorly absorbed. However, heat processing of tomatoes enhances isomerization, which improves absorption (8). Lycopene is well known for anticancer, antioxidant, anti-inflammatory, and anti-osteoporotic effects (9,10,11).
Autophagy is a mechanism for removal and degradation of intra- and extracellular substances by lysosomes. The process helps maintain cellular homeostasis, generates amino acids during periods of starvation, and protects against pathogens (12,13,14). Cells undergo autophagy in response to stress, but progressive and uncontrolled autophagy can cause cell death (15).
Autophagy can either promote or inhibit tumor growth. Accordingly, it may aid tumor cells endure stress caused by hypoxia and nutrient deficiency, or it can remove damaged cells and protein aggregates, preserving genetic stability (16). Autophagy-related genes (ATGs) were first recognized in the early 1990s in yeast. The mechanism of autophagy in mammalian cells is morphologically similar (17). ATG includes genes involved in autophagosome formation, in addition to genes needed for selective autophagy (18).
Sequestosome-1 (SQSTM1) is an autophagy gene. It possesses domains that support the transport of targets to the autophagosome after interacting with a membrane receptor (19). In established tumors, autophagy is upregulated to protect malignant cells (20).
Abnormal expression of autophagy proteins is detected in human cancer tissues, such as prostate carcinomas, where the ATG5 protein is upregulated (21,22). Deletion of autophagy genes increases oxidative stress, mitochondrial dysfunction, and susceptibility to proinflammatory stimuli. Such conditions result in DNA damage and subsequent genetic instability (23). Autophagy shows an anticarcinogenic function achieved through its role in controlling homeostatic turnover of mitochondria and by removing protein aggregates. These functions assist cells to handle metabolic stress (22,24).
Conversely, autophagy may promote the survival of tumor cells under some stress conditions. The process may support a high proliferation rate and counter hypoxia caused by impaired vascularization. Inhibition of autophagy reduces pancreatic cancer cell growth. Also, a reduction in autophagy proteins, such as ATG5 and ATG7, increases the resistance of tumor cells to chemotherapy. However, this effect is controversial since some studies show the opposite results. This conflict highlights the need for more studies to understand the complex relationship between autophagy proteins and tumor cell resistance (24).
miRNA controls almost all autophagy stages, which implies importance in both cellular homeostasis and disease conditions (25). miRNA are short nucleotide sequences that participate in various regulatory processes. Mechanisms include direct action, via targeting mRNA either by cleavage or suppression of translation. Deregulation of miRNA expression caused by either genetic or epigenetic alteration induces chromosomal abnormalities observed in human cancers (26).
This study aimed to characterize and compare the effects of grape (resveratrol) and tomato (lycopene) extracts individually and in combination on modulating miRNA interactions with its target ATGs in squamous cell carcinoma cells.
Materials and methods
Cell line and phytochemical extracts
Laryngeal carcinoma cell line (HEp-2) was obtained from the Cell Culture Department- VACSERA-EGYPT in frozen vials. HEp-2 cells were imported from the ''American Type Culture Collec- tion,''reference number CCL-23. A miRNA inhibitor was used with HEp-2 cells to substantiate miRNA presence. Inhibition of miRNA causes cell death, and the presence of dead cells after adding the inhibitor demonstrates active molecules. Synthetic miRNA that inhibits the action of the inhibitor was also added to cell cultures. This addition restores normal miRNA function thus preventing cell death. Highly purified liquid chromatography of grape extract (resveratrol) and tomatoes extract (lycopene) were purchased from Sigma-Aldrich, USA in the form of white and red powder respectively.
Bioinformatic analysis for mirna selection
Bioinformatic analysis was used to identify miRNA species related to laryngeal cancer based on earlier microarray studies. For this cancer, miRNA-20a was identified as a likely effector in the mirWalk database and SQSTM1 was identified as a key target gene for miRNA-20a in an atlas of gene expression.
Purification and quantification of mirna
Clinical validation of the selected miRNA and target gene was done by purification of total RNA and miRNA from HEp-2 cells using a miRNeasy mini kit, (catalog no. 217004, Quiagen,USA). The selected miRNA and its target gene in extracted miRNA were quantified by real-time PCR (qRT- PCR) using a miScript reverse transcription kit (Qiagen, Valencia, CA) for cDNA generation, then the SYBR Green miScript PCR system (Qiagen) on a light- cycler, with software v2.2.2 (StepOne™ Software).
Molecular docking
Molecular docking was used to validate and visualize fitting of resveratrol and lycopene into binding sites of miRNA-20a and SQSTM1 using the Dock Ligands (CDOCKER) protocol from BIOVIA Discovery Studio Visualizer version 4.0 software. Resveratrol and lycopene were prepared for docking, using the Prepare Ligands protocol. The miRNA-20a pdb code 4F3T (27) was chosen from the NCBI database and downloaded from the Protein Data Bank. It was optimized and organi- zed with the ''Prepare Protein protocol,''and the binding site was identified from vacant cavities in the structure associated with the lowest energy with ligands, i.e., sites where ligands were most stable. SQSTM1 pdb code 4UF9 (28) was chosen based on gene sequence; 4UF9 showed 88% similarity using optimization and binding as for miRNA-20a.
Culture treatment, cell viability assay and QRT-PCR
Methyl Thiazol Tetrazolium (MTT) assays were used to assess cell viability after treatment with different doses of resveratrol and lycopene separately and in combination for 24 and 48h.
Doses were higher and lower than the IC50 of both extracts, as calculated in previous studies (4,7) (Table 1).
Total RNA and miRNA were purified from 48 h treated HEp-2 cells and quantified by qRT-PCR as indicated above.
Statistical analysis
Numerical data were assessed for normality using Kolmogorov-Smirnov and Shapiro-Wilk tests. Data showed normal distribution. Data are reported as means and standard deviations (SD). Repeated measures analysis of variance (ANOVA) test was used for comparison of percent viability. One-way ANOVA was used for comparisons of fold change. Bonferroni's post-hoc test was used for pair-wise comparison when ANOVA tests were significant. The significance level was set at p≤0.05. Pearson's correlation was used to assess fold change in miRNA-20a and percent viability, and between miRNA-20a and SQSTM1 fold changes. Statistical analysis was performed using IBM (IBM Corporation, NY, USA) SPSS Statistics Version 20 for Windows (SPSS, Inc.).
Table 1 Doses of resveratrol, lycopene, and their combination used in the study over different time intervals.
| Drug | Dose | 24h | 48h |
|---|---|---|---|
| Resveratrol | 50 μM | GP1 | GP10 |
| - | 125μM | GP2 | GP11 |
| - | 200 μM | GP3 | GP12 |
| Lycopene | 10μM | GP4 | GP13 |
| - | 20 μM | GP5 | GP14 |
| - | 40 μM | GP6 | GP15 |
| Combination of both drugs | 50 μM Res.+10 μM Lyc. | GP7 | GP16 |
| - | 125 μM Res.+20 μM Lyc. | GP8 | GP17 |
| - | 200 μM Res.+40μM Lyc. | GP9 | GP18 |
Res: resveratrol, Lyc: lycopene, GP: group.
Results
Molecular docking
Molecular docking within the binding site of mirna-20A
Resveratrol interacted within the binding site of miRNA-20a with a free binding energy of -22.51 kcal/mol. It displayed hydrogen bonding with amino acid residue, Lys655 (distance of 2.67, 2.71, and 2.78 Å). Moreover, hydrophobic interac- tions were predicted with amino acid residues, Ile651 (distance of 4.85 Å), Tyr654 (distance of 5.16 Å), and Leu694 (distance of 5.02 Å) (Figure 1. A). Lycopene interacted within the binding site of miRNA-20a with a free binding energy of -13.12 kcal/mol, which is less than that for resveratrol. Lycopene showed only hydrophobic interactions with amino acid residues, Ile651 (two interactions, distances of 4.46 and 4.91 Å), Tyr654 (distance 4.41 Å), Lys660 (distance of 3.35 Å), Leu694 (distance of 4.73 Å), and Tyr698 (distance of 3.39 Å) (Figure 1. B).
Molecular docking within the binding site of sqstm1
Both resveratrol and lycopene show less affinity toward SQSTM1 than toward miRNA-20a. Resveratrol interacted with a free binding energy of -20.24 kcal/mol through hydrogen bonds with amino acid residues, Ser28 (distance of 1.87 Å), Pro29 (distance of 2.42 Å), and Met87 (distance of
2.39 Å), and through hydrophobic interactions with Pro43 (distance of 5.31 Å) and Met87 (two interactions, distances of 4.39 and 4.65 Å) (Figure 1. C). Lycopene interacted with a free binding energy of -7.34 kcal/mol, which is less than that for resve- ratrol. No hydrogen bonds were identified with amino acid residues at the active site; however, similar hydrophobic interactions were found with amino acid residues, Pro43 (distance of 4.53 Å) and Met87 (two interactions, distances of 4.74 and 5.39 Å) (Figure 1. D).
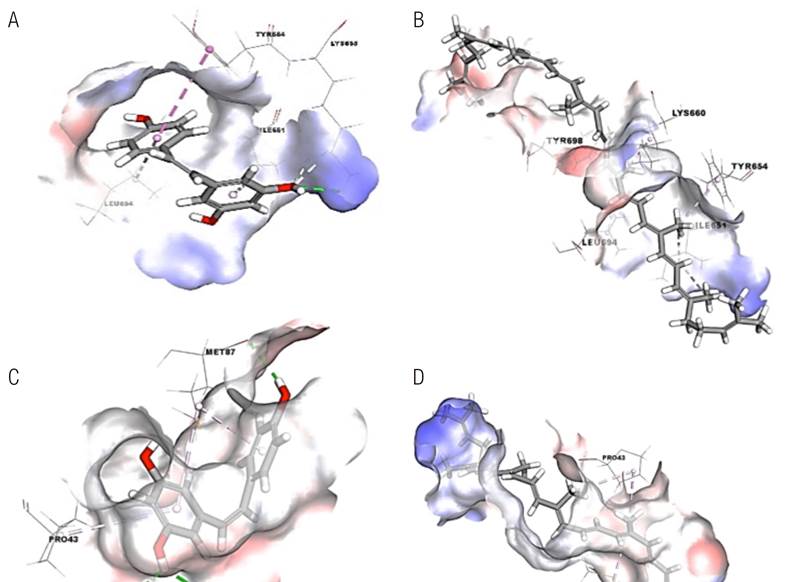
Figure 1 Three-dimensional interactions of (A) Resveratrol and (B) Lycopene in the binding site of miRNA-20a, and (C) Resveratrol and (D) Lycopene in the binding site of SQSTM1. The dotted lines stand for interactions with the amino acid residues within the binding site (photos were generated by BIOVIA Discovery Studio Visualizer version 4.0 software).
MTT assays
MTT assays were performed 24 and 48h after treatment (Table 2). GP4 showed the highest viability among all groups of cells at both times (88% and 85.3%, respectively). Conversely, GP 12 and GP18 showed the highest cytotoxicity with viabilities of 31% and 33%, respectively.
QRT-PCR
MIRNA-20A
GP13 showed the highest mean fold change in miRNA-20a expression (1897.7), followed by GP14 (989.1) at 48h. The smallest mean fold changes were seen with a combination of the high doses of both resveratrol and lycopene, with mean changes of only 15 were seen (Table 3). A non-significant positive correlation between miRNA-20a and percent viability was seen after 48h (Table 4).
SQSTM1
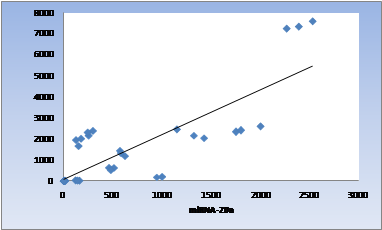
Almost no difference between GP13 and GP16 was seen after 48h; mean fold changes were 2481 and 2400, respectively; this increase in expression was the highest observed for the SQSTM1 gene. The smallest increase was observed for GP18, which is 13.7 (Table 3). miRNA- 20a and SQSTM1 were significantly and positively correlated (R=0.812, p≤0.001) (Table 4, Figure 2).
Table 2 Mean, standard deviation (SD) and ANOVA test followed by post-hoc tests for % viability of different groups of cells at different time intervals.
| Drug | 24h | - | 48h | - | p-value |
|---|---|---|---|---|---|
| - | Mean | SD | Mean | SD | - |
| Res. 50µM | 84.7 | 4 | 74.7 | 3.5 | 0.001 |
| Res. 125 µM | 70.7 | 4 | 46.7 | 4.2 | <0.001 |
| Res. 200 µM | 57 | 3.5 | 31.7 | 4 | <0.001 |
| Lyc. 10 µM | 88 | 7.2 | 85.3 | 7.4 | 0.320 |
| Lyc. 20 µM | 79.7 | 9.6 | 76 | 11.1 | 0.177 |
| Lyc. 40 µM | 73.7 | 4.5 | 48.3 | 5.5 | <0.001 |
| Res.50 µM + Lyc. 10 µM | 78.7 | 5.7 | 75.7 | 6.7 | 0.265 |
| Res. 125 µM + Lyc. 20 µM | 67.3 | 5.7 | 53.3 | 2.9 | <0.001 |
| Res.200 µM +Lyc. 40 µM | 44.3 | 5.5 | 33 | 4.4 | <0.001 |
Res: resveratrol, Lyc: lycopene.
Table 3 Mean, standard deviation (SD), and ANOVA followed by post-hoc test for miRNA-20a and SQSTM1 fold changes after 48 h of treatment of HEp2 cells.
| Drug | Mean | SD | Mean | SD | p-value |
|---|---|---|---|---|---|
| - | miRNA-20a | - | SQSTM1 | - | - |
| Res. 50 µM | 584.5 ᴱ | 85.2 | 1302.5 ᴱ | 154.7 | - |
| Res. 125 µM | 480.6 F | 74.5 | 505.7 F | 84 | - |
| Res. 200 µM | 140 ᴴ | 26.7 | 27.8 ᴴ | 6.6 | - |
| Lyc. 10 µM | 1897.4 ᴮ | 164.8 | 2481 ᴮ | 199.4 | - |
| Lyc. 20 µM | 1315 C | 188.1 | 2301 C | 204.5 | - |
| Lyc. 40 µM | 989.6 ᴰ | 120 | 171 ᴳ | 54.1 | <0.001 |
| Res.50 µM + Ly. 10 µM | 256 ᴳ | 56.7 | 2400 ᴮ | 174.5 | - |
| Res. 125 µM + Lyc. 20 µM | 140 ᴴ | 20.3 | 1830.1ᴰ | 204.6 | - |
| Res. 200 µM + Lyc. 40 µM | 15.1 ᴵ | 3.6 | 13.7 ᴵ | 1.6 | - |
| miRNA inhibitor 0.5 (48h) | 3.9 ᴶ | 0.5 | 0.7 ᴶ | 0.1 | - |
| miRNA inhibitor 1.5 (48h) | 0.8 ᴶ | 0.1 | 1.2 ᴶ | 0.2 | - |
| miRNA inhibitor control | 2402 ᴬ | 191.2 | 7480.5ᴬ | 263.7 | - |
Res: resveratrol, Lyc: lycopene.
Table 4 Pearson's correlation coefficient for miRNA-20a and % viability and miRNA-20a and SQSTM1.
| Time | Comparison groups | Correlation coefficient | p-value |
|---|---|---|---|
| 48 h | miRNA-20 and % viability | 0.264 | 0.104 |
| - | miRNA-20a and SQSTM1 | 0.812 | <0.001 |
Discussion
Cancer is a common cause of death throughout the world. In Egypt, head and neck cancer accounts for about 17% of all malignant tumors (29).
Cancer cells can withstand stress, such as nutrient starvation and lack of oxygen. Hypoxia is countered by induction of autophagy via inducible hypoxia factor-1 alpha. Moreover, it provides an alternate source of energy in the form of amino acids released during protein degradation in the case of starvation (30).
miRNAs act as tumor suppressors or oncogenes depending on cellular conditions (31). miRNA may target ATGs, demonstrating a significant role of these molecules in the regulation of the process (25). miRNA-20a can act as a tumor promoter (32). Conversely, it inhibits cutaneous squamous cell carcinoma (33). The process of autophagy is controlled through a group of ATGs. SQSTM1 is one such gene (20).
Different natural extracts were investigated for possible anticancer effects, including resveratrol (grape extract) and lycopene (tomato extract). Both chemicals show anticancer properties against breast, prostate, liver, skin, and ovarian cancer as well as oral squamous cell carcinoma (34,35,36,37,38,39,40). In this study, we evaluated the effect of resveratrol and lycopene and their combination on a newly recognized miRNA and a target autophagic gene in HEp-2 cells.
Viability of HEp-2 cells after 24h of exposure to both resveratrol and lycopene was reduced in a dose-dependent manner. Resveratrol results are consistent with that of Mohammed et al. (4) at the lowest dose; however, these authors did not find any inhibitory effect at the higher dose. As an explanation, the authors stated, ''in the design of cell culture experiment it was important to be aware of the growth state of the culture, as well as the quantitative characteristics of cell strain or cell line. Cultures will vary significantly in many of their properties between exponential growth and stationary phase.''
HEp-2 cells treated with different lycopene doses for 24h showed decreased viability at the higher dose. Other studies, however, reported that lycopene had no cytotoxic effect on HEp-2 cells (37,41). Park et al. (42) did find strong cytotoxicity at low doses of lycopene in Hep3B hepatoma cells.
Cytotoxicity was similar for cells after 48h of exposure. The lowest mean viability was not significantly different between GP12, and GP18. Thus, the addition of lycopene (GP18) did not increase the toxicity of resveratrol alone (GP12).
Almost all doses displayed higher cytotoxicity after 48h of exposure. Exceptions were cells in GP4, GP5, and GP7. Cytotoxicity after 24h of exposure for these cells were not different compared with equivalent groups, GP13, GP14, and GP16, respectively, after 48h of exposure.
Previous findings demonstrate anticancer activity of both phytochemicals. Resveratrol induces apoptosis by inhibiting the mTOR pathway that promotes cell growth and via alterating the expression of anti-apoptotic factors such as Bcl-xL (43). In addition, resveratrol causes depolarization of the mitochondrial membrane, resulting in cell death (44). The cytotoxicity of lycopene is explained partly by strong antioxidant properties and its ability to quench free radicals that might predispose cell transformation in different tissues (45). Also, lycopene reduces cellular proliferation of various cancer cell lines induced by insulin-like growth factor-I (46,47).
Molecular docking in the current study predicted favorable negative binding energies and binding interactions for both resveratrol and lycopene with binding sites of miRNA-20a and SQSTM1. Resveratrol showed more affinity toward both miRNA-20a and SQSTM1, consistent with the biological evaluation.
miRNA-20a and its target SQSTM1 gene expression were examined in this study using qRT-PCR for cells exposed to chemicals separa- tely or in combination for 48h. Lycopene induced higher expression of miRNA-20a than resveratrol did in HEp-2 cells. However, combined resveratrol and lycopene treatment induced lower expression of miRNA-20 than resveratrol alone. The smallest effect on expression was found for GP 13 cells, which also showed the highest absolute levels of miRNA-20a. The greatest impact of treatment was seen in cells in GP18, which displayed the lowest fold change and produced results most similar to cells exposed to the miRNA inhibitor.
miRNA-20a expression was consistent with percent viability after 48h of exposure. Thus, the highest viability (lowest cytotoxicity) and the highest levels of miRNA-20a were found in GP13. Further, the highest cytotoxicity and the lowest levels of miRNA-20a were seen for GP 18. This direct relationship between levels of miRNA-20a and cell viability is consistent with Hui et al., who found that miRNA-20a is upregulated in well-established head and neck cancers, and that a decrease in the levels of the miRNA-20a is associated with a decrease in viability of tumor cells (48).
Similarly, Yang et al. (49) found high levels of miRNA-20a in both gastric and nasopharyngeal cancer, which further supports an oncogenic role for miRNA-20a. Finally, Venkatadri et al. (50) found that resveratrol modulates miRNA-20a, leading to downregulation of anti-apoptotic protein Bcl-2, thus increasing apoptotic cell death. This property of resveratrol suggests a possible target for future cancer treatment options. Resveratrol is likely responsible for decreased levels of miRNA-20a since cells treated with lycopene alone showed the least effect on miRNA-20a expression compared with the miRNA inhibitor.
An inverse relationship was reported between the expression of the miRNA 17-20 cluster family and major histocompatibility complex class I chain-related proteins, A and B (MICA and MICB). MICA and MICB are vital ligands for the recognition of tumor cells by immune effector cells. Resveratrol suppresses c-Myc expression, which inhibits transcription of the miR-17-92 cluster. This suppression upregulates MICA and MICB, promoting death of breast cancer cells by natural killer cells (51).
Tang et al. (52) found that lycopene also inhibits c-Myc involved in cell proliferation, thus, suppressing the progression of colon cancer. This finding explains how resveratrol and lycopene might act via a similar mechanism for their anticancer effects.
In the current study, statistically significant positive correlation was found between viability and mean fold change in miRNA-20a expression. Thus, upregulation of miRNA-20a might promote cell viability.
Cells in GP13 and GP16 showed the highest levels of SQSTM1 expression, along with the least cytotoxicity. Conversely, GP18 showed the lowest expression level, paralleling percent viability from cytotoxicity tests. Han et al. (53) found similar results, reporting that levels of SQSTM1 expression correlated with the viability of established cancer cells. Additionally, SQSTM1 was elevated in small-cell lung cancer (20).
SQSTM1 is a positive regulator of the mammalian target of rapamycin (mTOR) pathway through interaction with the mTOR complex in an amino acid dependent manner (54). Activation of the mTOR pathway stimulates cell growth and activates anti-apoptotic factors. Thus, a decrease in expression of SQSTM1 causes a decline in mTOR pathway activity and an increase in the activity of apoptotic factors. SQSTM1 participates in autophagy by transporting targets to autophagosomes for subsequent degradation. As a result, apoptosis becomes more prominent in cancer cells (55,56). This role explains why resveratrol and lycopene reduce the expression of SQSTM1 and reduce autophagy. Finally, the significant correlation between miRNA-20a and SQSTM1observed in this study demonstrates their direct relationship. Eleva- ted levels of miRNA-20a and SQSTM1 in cancer cells are consistent with elevated levels of autophagy in well-established tumors and decreased autophagy after administration of phytochemicals.
Conclusion
Both resveratrol and lycopene are cytotoxic to HEp-2 cells, in a dose- and time-dependent fashion. Cytotoxicity of resveratrol is more pronounced than lycopene at different doses and time intervals. A combination of a resveratrol/lycopene mixture shows greater cytotoxicity when compa- red with lycopene alone. Resveratrol and lycopene reduce expression of miRNA-20a and levels of its target, SQSTM1, in a dose-dependent manner. Combined resveratrol-lycopene exposure showed no statistically significant difference in miRNA-20a and SQSTM1 expression compared with resveratrol alone. Finally, miRNA-20a and SQSTM1 levels were directly related, indicating a direct linkage. Decreasing expression of miRNA-20a and SQSTM1 induced a decrease in levels of autophagy and an increase in apoptosis in HEp-2 cells.
Author contribution statement
Conceptualization and design: M.M.E., I.M.H. and M.M.S.
Literature review: I.M.S.
Methodology and validation: M.M.S. and D.B.F.
Formal analysis: M.M.S.
Investigation and data collection: S.E.G. and N.S.A.
Resources: M.M.S.
Analysis and interpretation: M.M.E., I.M.H., M.M.S. and D.B.F.
Writing-original draft preparation: I.M.S.
Writing-review & editing: S.E.G. and N.S.A.
Supervision: M.M.E. and I.M.H.
Project administration: I.M.S.
Funding acquisition: I.M.S.