Introduction
Periodontitis is an infectious disease that induces low-grade systemic inflammation caused by the disbiosis of the subgingival microbiota (1,2,3). Both the presence of pathogenic bacteria and the inflammatory mediators produced in the periodontium have been linked to the onset or progression of a wide range of chronic non-communicable diseases (4). Among the bacteria associated with disbiosis, Porphyromonas gingivalis has been widely associated with the onset and progression of periodontitis (5,6,7). The ability of P. gingivalis to invade the host’s tissues, migrate and establish itself in other tissues or organs, associate it with preterm born and low birth weight, and atherogenesis (8,9,10,11,12,13). Different studies have detected the presence of P. gingivalis in the brain’s tissues of people who died due to Alzheimer's disease (AD) or another cause (14,15). In fact, it has been proposed that periodontitis could be a risk factor of AD and, the invasion of P. gingivalis into the brain could induce unwanted effects in the brain, such as cognitive decline.
AD is a type of dementia characterized by learning and memory decline, and is characterized by the degeneration of hippocampal pyramidal neurons (16). The amyloid cascade begins with neuroinflammation caused by the presence of chronic pro-inflammatory mediators, such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α (17,18). In this context, the onset of AD would be outside the brain, mainly in chronic non-communicable diseases (16). The presence of pro-inflammatory mediators would induce a functional imbalance in brain cells, generating the activation of microglia and astrocytes, which together with the mediators generated in other tissue, would produce the energetic, metabolic and oxidative imbalance that would lead to death of neurons (16). Recently, it has been proposed that neuroinflammation could be initiated by an infectious cause (19,20). In this context, P.gingivalis has been detected in the IV ventricle, and in the cerebro-spinal fluid (CSF) of subjects affected by AD (14,15). However, until today the effect that the bacteria has generated in these patients is unknown, giving rise to speculations and hypothesis that associate P. gingivalis with neuroinflammation. Different experimental models have tried to explain this association, using different animal models and techniques to inoculate P. gingivalis. Thus, after inoculation of different virulence factors of P. gingivalis or the ad integrum bacterium, neuroinflammation, astrogliosis or microglia activation, cognitive or behavior decline, and AD-like histopathological markers has been detected (14,21,22,23,24,25,26,27). The hypothesis that periodontitis could be able to initiate AD through the presence of gram negative bacteria, which would invade the brain, and induce the secretion of Aβ is on the rise.
An important histopathological marker of AD in the last 25 years has been the amyloid β peptide (Aβ) (18). The Aβ is the central molecule that initiates the amyloid cascade, the main hypothesis that explains the origin of the neuroinflammatory events that lead to the onset of AD (16, 28). One of the physiological phenomena that make it possible to assess the progression of AD is the clearance of the Aβ peptide. Indeed, during the life the Aβ is produced constantly, but it is transported towards the CSF via glymphatic flow, avoiding its cerebral accumulation (29). Thus, the detection of Aβ in CSF or in serum, has been used to determine the progression of AD (30). However, given that the presence of Aβ is not necessarily associated with higher or lesser cognitive impairment, new markers that have more precision have been sought. For more than 20 years, apolipoprotein E (ApoE)-ε4 -marker of Aβ clearance- polymorphisms have been considered risk factors for AD and other co-morbidities, and their quantification at serum level has been used to evaluate the progression of the disease (31,32,33). In this way, it is unknown if ApoE-ε4 can be assessed in gingival crevicular fluid (GCF) and if periodontitis affects its levels.Thus, the present study aims to evaluate the periodontal status, and the pro-inflammatory mediators, P. gingivalis load, and ApoE-ε4 levels in GCF samples from patients affected or not with AD.
Materials and methods
Patient selection periodontal screening
In the present study, subjects older than 60 years of age, residents of the Brunet Celarain Asylum, who met all the criteria established during the period between june-december 2019, were recruited. All patients were informed of the objective of the study and the informed consent was signed by their attorneys. The protocol was approved by the Ethics Committee of the Faculty of Dentistry of the Autonomous University of Yucatán (Protocol # FODO-2019-0002).
Patients with AD were diagnosed by specialists in neurology according to the guidelines of the Alzheimer's Association (34). As inclusion criteria, AD patients were admitted consecutively when they met the following inclusion criteria: over 60 years old, no smokers or drinkers, whose cognitive status allows them follow instructions, and had not received antibiotic, anti-inflammatory treatment, or periodontal therapy 6 months before enrollment. Patients with AD who were unable to cooperate or follow instructions were not included. As a control, subjects older than 60 years who attended the Periodontics Clinic of the Faculty of Dentistry of the Autonomous University of Yucatán and who were not affected by any type of dementia were enrolled. All patients underwent a complete periodontal examination by a single operator (D. S-E), where deep probing (DP), clinical attachment level (CAL), bleeding on probing (BOP), plaque index (PI), complete periodontogram, furcation lesions, mobility and suppuration were recorded. The periodontal diagnosis was performed according to the current classification of periodontal diseases (35, 36).
Montreal cognitive assessment
All patients underwent the Montreal Cognitive Assessment (MoCA). Previously, the operator was trained and certified in the MoCA test via e-learning on the platform http://www.mocatest.org/training- certification/. Briefly, the test consists of 7 items that assess the visuospatial/executive orientation, the identification of elements, memory, attention, language, the capacity for abstraction, delayed recall and orientation. Each question has assigned scores and from a total of 30 a normal value of 30 to 26 is considered. The data of each test was numbered in a correlative way by the Director of the study, who collected daily the tests performed by the study members and then managed the appointment for dental evaluation, if applicable.
Clinical periodontal examination
Each patient underwent a periodontal examination by a single operator (D.S-E) and the probing depth, position of the marginal gingiva, BOP, CAL, and PI in 6 periodontal sites per tooth, excluding third molars were recorded. The periodontogram used was the Periodontal Chart, a program designed by the University of Bern and free to access: http://www.periodontalchart- online.com/es/. For the periodontal diagnosis, the parameters of the 2017 classification of periodontal diseases of the AAP and EFP were taken into account. The diagnosis of "periodontal health" was determined using the presented criteria of clinical periodontal health defined as: a state characterized by an absence or minimal levels of clinical inflammation in a periodontium with normal supporting structures. The diagnosis of gingivitis was established when it was detected a BOP between ≥10% (localized) and ≥30% (generalized); in patients with a reduced periodontium, CAL and BOP were considered on probing with ≥10%, and no BOP and probing site with ≥4mm depth. Periodontitis was diagnosed according to the presented staging criteria. Stage I was diagnosed by observing the presence of CAL loss of 1 to 2mm, a maximum probing score of ≤4mm, and no tooth loss due to periodontitis. Stage II was diagnosed by observing the presence of CAL loss of 3 to 4mm, a maximum probing score of ≤5mm, and no loss of teeth due to periodontitis. Stage III was diagnosed by observing the presence of CAL loss of ≥5mm, stage II complications, and additionally a probing score of ≥6mm with tooth loss due to periodontitis in at least 4 teeth. Stage IV was diagnosed by observing the presence of CAL loss of ≥5mm, stage III complications, and tooth loss due to periodontitis of more than 5 teeth.
Biological sampling
GCF and subgingival microbiota samples were taken from all patients, as was described previously (37,38,39). Briefly, the GCF and microbiota samples were taken from the periodontal pockets with the highest deep on probing. A total of 4 samples for each patient were taken, and those samples contaminated with blood or saliva were discarded. Subsequently, both the GCF and microbiota samples were pooled and treated according the assay to be performed. The GCF was quantified and normalize at similar total protein concentration by spectrophotometer (Synergy, Bio-Tek, USA) readings at 460 and 530nm, and stored in aliquots at -80ºC until Multiplex assay. The microbiota samples were treated in order to purified the total DNA for P. gingivalis detection.
Protein quantification by multiplex immunoassay
From 100μL of GFC, the secretion levels of the IL-1β, IL-2, IL-4, IL-5, IL-6, IL- 9, IL-10, IL-12, IL-13, IL-17A, IL-21, IL-22, IL-23, IL-27, and GM-CSF molecules were quantified by Multiplex immunoassay (Invitrogen™ Cytokine 25-Plex Human Panel, ThermoFisher Scientific®), following the manufacturer's instructions and evaluated the absorbance using an automated plate reader of the Luminex™ system (Bio-Tek, Winooski, VT, USA). Additionally, from 100 μL of GCF, ApoE-ε4 levels were quantified by ELISA immunoassay, following the manufacturer's instructions (Invitrogen™ Apolipoprotein E4 Human ELISA Kit-Thermo Fisher Scientific®), and evaluated the absorbance using an spectrophotometer at 460nm (Synergy, Bio-Tek, USA).
Porphyromonas gingivalis absolute quantification
From the total volume of the microbiological samples, the total DNA was purified using the FavorPrep™ kit (Tissue Genomic DNA extraction Mini Kit, Favorgen Biotech Corp), and according the protocol published previously (21).
Data analysis
The clinical data obtained from each patient (MoCA, PI, BOP, and CAL) are represented as mean values ± standard deviation. The data of the quantification of the pro-inflammatory mediators are represented in graphs that express the mean value in ng/mL ± standard deviation. The detection of P. gingivalis are represented in graphs that express the mean value of the CFU/mL ± standard deviation. The ApoE-ε4 levels are represented as the mean value in μg/mL ± standard deviation. All these data was analyzed using the Shapiro-Wilk test to determine the homogeneity of the data and the ANOVA-Tukey. Additionally, the association analysis was performed by the Pearson’s correlation, and the sensitivity-specificity association was determined by the area under the curve (AUC) quantification. All the statistically analysis were carried out by using the SPSS v5.0 software
results
Epidemiological data
The recruited patients correspond to 10 patients with AD and 20 patients without AD. Of the 20 patients without AD or another type of dementia,10 were affected with periodontal disease and 10 were healthy subjects. Table 1 summarized the main epidemiological results of the selected patients. Briefly, patients with AD averaged 71±4.8 years, 50% corresponded to women. Patients with periodontitis and non-AD averaged 69.6±3.83 years, 50% corresponded to women and those in the control group average an age of 69.10±4.72 and 60% corresponded to women. In relation to the clinical parameters, patients with AD had higher DPP, more CAL loss, higher BOP and PI, compared to patients without AD affected by periodontitis or healthy subjects. Finally, when evaluating the MoCA test results, the patients affected with AD had an average of 16.56±2.65 corresponding to the 100% to stage 2. Patients non affected with AD had an average of 25.20±1.36, and the 70% was classified at stage 1, and 30% in stage 2. Besides, healthy controls MoCA score averaged 25.80±1.03, with 80% in stage 1 and 20%, stage 2. Also, the patients affected with AD, the 80% had periodontitis stage IV, 10% periodontitis stage III, and 10% periodontitis stage III. Patients without AD had 20% periodontitis stage IV, 50% periodontitis stage III, 30% periodontitis stage I (Table 2).
Additionally, when correlate the association among MoCA score and CAL, BOP or PI values, we detected that the higher CAL loss, higher BOP, or higher PI correlate with lower MoCA values, suggesting that at more cognitive impairment the periodontal state is more severe (Supplementary Figure 1).
Table 1 Clinical profile.
| - | - | Periodontitis | - |
|---|---|---|---|
| - | Health (n=10) | non-AD (n=10) | AD (n=10) |
| Age | 69.10±4.72 | 69.60±3.83 | 71.00±4.8 |
| Gender (female) | 60% | 50% | 50% |
| MoCA score | 25.80±1.03 | 25.20±1.61 | 16.56±2.65 |
| MoCA score 2 | 20% | 30% | 100% |
| MoCA score 1 | 80% | 70% | 0% |
| Periodontal Parameters | - | - | - |
| DPP (mm) | 2.70±0.48 | 6.70±1.25 | 7.2±2.04 |
| CAL (mm) | -0.67±0.89 | -3.35±1.65 | -6.8±1.97 |
| BOP (%) | 5.30±2.90 | 28.80±5.78 | 41.90±10.23 |
| PI (%) | 12.70±2.49 | 31.70±7.273 | 45.50±7.09 |
| P.gingivalis load (CFU/mL) | 5.78±0.74 | 9.01±2.10 | 9.31±2.77 |
MoCA: Montreal Cognitive Assessment, DP: Deep on Probing, CAL: Clinical Attachment Level, BOP: Bleeding on Probing, PI: Plaque Index, P. gingivalis: Porphyromonas gingivalis, CFU: Colony Forming Units.
Table 2 Periodontal diagnosis distribution.
| - | - | Periodontitis | - |
|---|---|---|---|
| - | Health (n=10) | non-AD (n=10) | AD (n=10) |
| Periodontal health | 100% | - | 0% |
| Gingivitis | 0% | 0% | 0% |
| Periodontitis stage I | 0% | 30% | 0% |
| Periodontitis stage II | 0% | 0% | 10% |
| Periodontitis stage III | 0% | 50% | 10% |
| Periodontitis stage IV | 0% | 20% | 80% |
Frequency of periodontal disease stages among groups. AD: Alzheimer’s Disease. Periodontal Diagnosis based on the 2017 classification.
PRO-INFLAMMATORY CYTOKINES ARE INCREASED IN THE GCF OF PATIENTS AFFECTED WITH PERIODONTITIS AND AD
From the GCF samples of all patients, the secretion levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL- 9, IL-10, IL-12, IL-13, IL-17A, IL-21, IL-22, IL-23, IL-27, and GM-CSF were quantified by Multiplex (Figure 1). For the pro-inflammatory cytokines IL-1β, IL-2, IL-6, IL-9, IL-12, IL-21, IL-23, and GM-CSF an increase was detected in patients with periodontitis affected or not with AD, compared to healthy subjects. Additionally, for IL-21 a significant difference was detected in patients with periodontitis and AD compared with patients with periodontitis alone. For the pro-bone resorptive cytokines IL-17A, and IL-22, an increase in the secretion levels was detected in patients with periodontitis with or without AD, compared with healthy controls. Except for IL-21, no difference was detected between the periodontitis groups in the pro-inflammatory or pro-bone resorptive cytokines.
When analyzing the immune-modulatory or regulatory cytokines, a significant decrease was detected for IL-4, IL-13, and IL-27 in both conditions of periodontitis with or without AD compared with controls. Besides, for IL-10 a decrease was detected in patients with periodontitis and AD, compared with controls. No differences were detected in the IL-5 levels. Finally, no differences between periodontitis groups of patients with or without AD was detected in the aforementioned modulatory or regulatory cytokines.
Also, we analyzed the secretion levels of IL-1β, IL-4, IL-9, IL-10, IL-17A, and IL-22 stratified by periodontitis stage and the presence or absence of AD (Figure 2). An increased was detected for IL-1β, IL-9, and IL-22 in patients with AD and periodontitis stage III-IV compared with healthy controls. Additionally, in patients with AD and periodontitis stage III-IV an increase in the secretion levels of IL-1β and IL-9 were detected when compared with AD patients affected with periodontitis stage I-II.
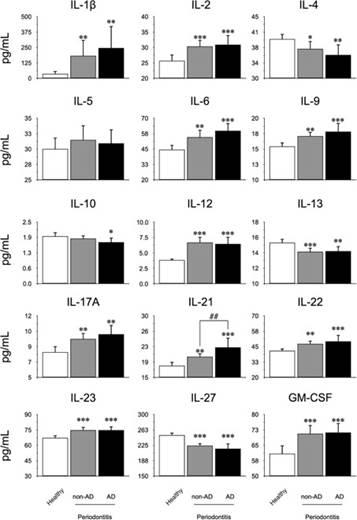
Figure 1 Quantification levels of mediators in GCF. From the GCF of 10 healthy subjects, 10 patients with periodontitis, and 10 patients with periodontitis and AD, the levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL- 9, IL-10, IL-12, IL-13, IL-17A, IL-21, IL-22, IL-23, IL-27, and GM-CSF were quantified by Multiplex. The graphs represent the mean value in pg/mL and standard deviation. The ANOVA-Tukey test was performed for inter-group assessment. *P<0.05, **P<0.01, ***P<0.001, ##P<0.01. IL: Interleukin, GM-CSF: Granulocyte Monocyte- Colony Stimulating Factor, pg: picograms, mL: mililiters, AD: Alzheimer’s Disease, GCF: Gingival Crevicular Fluid.
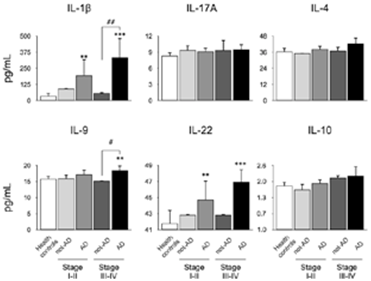
Figure 2 Quantification levels of mediators in GCF according periodontal stage. From the GCF of 10 healthy subjects, 10 patients with periodontitis, and 10 patients with periodontitis and AD, the levels of IL-1β, IL-4, IL-9, IL-10, IL-17A, and IL-22 were quantified by Multiplex. The chosen cytokines are representative of Th1, Th2, Th9, Th17, Th22, and Treg subsets respectively. The graphs represent the mean value in pg/mL and standard deviation grouped according the periodontal diagnosis. The ANOVA-Tukey test was performed for inter- group assessment. **P<0.01, ***P<0.001, #P<0.05, ##P<0.01. IL: Interleukin, pg: picograms, mL: mililiters, AD: Alzheimer’s Disease, GCF: Gingival Crevicular Fluid, Th: T helper lymphocytes, Treg: T regulatory lymphocytes.
Cytokines correlates with moca scores
To determine whether the levels of cytokines associated with inflammation, pro-bone resorption, regulation or modulation of the immune response with the different scores of the MoCA test, we evaluated the association using the Pearson's correlation (Figure 3). When we analyzed the pro-inflammatory IL-1β, and IL-9, and the pro-resorptive IL-17A, and IL-22 cytokines, a negative correlation was detected with the MoCA score. For the IL-4 modulatory cytokine, a positive correlation was observed with the MoCA score, and for the regulatory IL-10, no correlation was detected.
P. GINGIVALIS detection inhigher in patients with periodontitis and ad
The P. gingivalis detection in subgingival microbiota samples from all the selected patients were quantified by qPCR (Table 1, Figure 3.B). A high P. gingivalis load was detected in patients with periodontitis and AD compared to healthy subjects. Curiously, no differences in P. gingivalis load was detected among healthy subjects and patients with periodontitis. Also, when correlates the P. gingivalis load with the MoCA score we observed a negative significant correlation (Figure 3.C).
In addition, we evaluate the association between the P. gingivalis load and the IL-1β, IL-4, IL-9, IL-10, IL-17A, and IL-22 cytokines levels by the Pearson’s correlation. No correlation was detected among the different conditions.
APOE-Ε4 is increased in the GCF of patients with AD
In order to determine if ApoE -a protein associated with the increased risk of AD- is possible to be detected in the GCF and if the periodontal status influences the levels or not, we evaluated through ELISA the secreted levels of the ε4 form in all groups (Figure 4. A).
Firstly, we were able to quantify ApoE-ε4 in the GCF and detect differences. In both periodontitis patients with or without AD presented an increase in the secretion levels compared with healthy controls. Also, patients with periodontitis and AD produced more ApoE-ε4 than patients with periodontitis and not-AD. Secondly, we determine the association of the ApoE-ε4 levels with the MoCA scores by the Pearson’s correlation, detecting a negative correlation (Figure 4.B). Due there is no evidence in the literature of the detection of this molecule in GCF we decided to analyze the ROC curve (Figure 4.C). The area under the curve was higher than 0.8 suggesting that ApoE-ε4 detection in GFC of patients with different cognitive status could have a positive assessment.
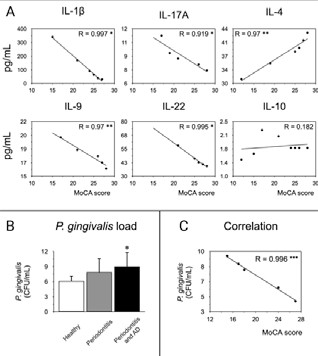
Figure 3 Correlation of mediators with MoCA score. A) From the GCF of 10 healthy subjects, 10 patients with periodontitis, and 10 patients with periodontitis and AD, the levels of IL-1β, IL-4, IL-9, IL-10, IL-17A, and IL-22 were quantified by Multiplex. The chosen cytokines are representative of Th1, Th2, Th9, Th17, Th22, and Treg subsets respectively. The graphs represent the Spearman’s correlation of the cytokines concentrations and the MoCA scores. B) From the subgingival microbiota of 10 healthy subjects, 10 patients with periodontitis, and 10 patients with periodontitis and AD, the levels of the ribosomal 16S subunit of P. gingivalis were quantified by qPCR. C) The Spearman’s correlation of the bacterial load and the MoCA scores was performed. *P<0.05, ***P<0.001. CFU: Colony Forming Units, mL: mililiters, MoCA: Montreal Cognitive Assessment, IL: Interleukin, pg: picograms.
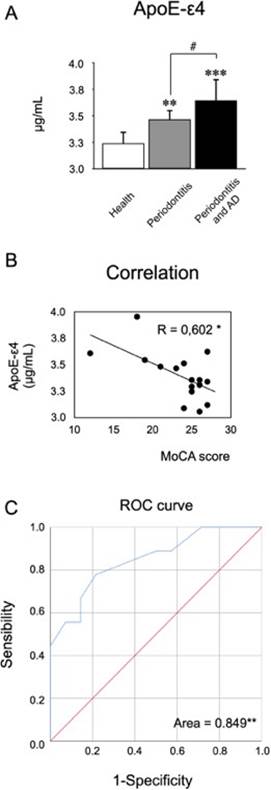
Figure 4 Quantification of ApoE-ε4 levels in GCF. From the GCF of 10 healthy subjects, 10 patients with periodontitis, and 10 patients with periodontitis and AD, the levels of ApoE-ε4 were quantified by ELISA. A) The graphs represent the mean valua in μg/mL. B) The graphs represent the Spearman’s correlation of the ApoE-ε4 concentrations and theMoCA scores. C) The area under the curve was determined by matching ApoE-ε4 concentrations with MoCA scores, in order to determine sensibility over specificity. *P<0.05, **P<0.01,***P<0.001,#P<0.05. ApoE-ε4: Apolipoprotein E-ε4, μg: micrograms, mL: mililiters, MoCA: Montreal Cognitive Assessment, AUC: Area Under the Curve.
discussion
In this work, we evaluate the periodontal status of a population affected by AD and compared the clinical, microbiological and molecular parameters with people affected by periodontitis without dementia and healthy subjects. As a first result, we were able to detect that at the level of GCF, the pro-inflammatory and pro-bone-resorptive mediators are increased both in patients with periodontitis affected or not with AD. Besides, AD patients have more severe periodontitis compared to non-AD patients. To evaluate whether the severe stages of periodontitis present higher levels of pro-inflammatory mediators, we selected 2 pro-inflammatory, 2 pro-bone resorptive and 2 modulatory cytokines. Surprisingly, only IL-1β, IL-9, and IL-22 had a significant increase in patients with periodontitis stage III or IV compared to healthy subjects. Furthermore, for the same selected cytokines, a correlation analysis was performed, where the molecules associated with periodontitis show a negative correlation with the cognitive state. Conversely, IL-4 presented a positive correlation, suggesting that a better cognitive state is related to a higher presence of IL-4. Secondly, we quantified the load of P. gingivalis in the deeper pockets or sulcus of all patients. We detect a higher bacterial load in patients with AD, and those levels correlated negatively with the cognitive status. This data suggests that the periodontal inflammation and severity can be associated by an increase in P. gingivalis. In fact, we performed an association between the mentioned cytokines an P. gingivalis levels, detecting no correlation. Finally and as the most novel aspect of the present work, we measured the levels of ApoE-ε4 in the GCF. We were able to detect the presence of ApoE-ε4 both in healthy patients and in periodontitis patients with or without AD. Indeed, patients with AD and periodontitis presented higher levels of ApoE-ε4, compared to patients without AD and periodontitis and healthy subjects. When evaluating the association of ApoE-ε4 levels with the MoCA scores, we could observe a negative correlation and an area under the curve higher than 0.8.
Several studies have evaluated the oral status in patients affected with any type of dementia, such as Parkinson’s disease, AD, vascular dementia, or another (40,41,42,43,44,45,46,47,48,49,50,51,52,53,54). Of these, only one article quantifies the levels of pro-inflammatory mediators at the serum level and correlates them with the presence of CAL and BOP (44). Indeed, in patients with severe periodontitis and mild cognitive impairment, an increase in Epidermic Growth Factor (EGF), Granulocytes-Colony Stimulating Factor (G-CSF), Granulocytes and Monocytes-Colony Stimulating Factor (GM-CSF), Growth Regulated Oncogene (GRO), IL-6, IL-7, IL-8, CXCL-10, Monocyte Chemoattractant Protein-1 (MCP-1/CCL2), Macrophage Inhibitory Protein-1α (MIP-1α/CCL3), MIP-1β (CCL4), and TNF-α levels were observed compared to healthy subjects. Although patients with mild cognitive impairment presented a higher BOP and CAL loss than controls, the authors could not identify whether the presence of mediators at the serum level can associate this condition with periodontitis (44). This is the only study to date that has evaluated pro-inflammatory mediators at the systemic level. Unlike us, we characterize the presence of periodontitis with cytokines representative of pro-inflammatory, pro-bone- resorption or modulatory responses. This is a high strength of our work, being the first to determine this broad pattern of cytokines. It is noteworthy that the presence of mediators such as IL-1β, IL-6, IL-17, and TNF-α is important to determine in patients with AD as they represent a risk for their further progress (16,55). In this sense, low-grade inflammatory diseases are considered risk factors for the onset of AD (56,57,58,59,60). In fact, these mediators participate in the breakdown of the blood-brain barrier (BBB), which is compromised in patients with AD (56,59,61,62). Nevertheless, no study have been determined until today how periodontitis can affect the BBB stability.
When evaluating the presence of periodontitis, AD patients have CAL loss, more BOP, and a higher PI (41,42,44, 46, 47). In these context, the increase in periodontal inflammatory indexes do not necessarily correlates with periodontitis due no study diagnoses periodontitis except for Gil Montoya (44). The studies focus their analysis on poor oral hygiene and how, this is due to the absence of teeth and the presence of removable prostheses (41, 42, 45, 53). Although periodontitis is the main cause of tooth loss, the importance of the presence of teeth is related with the risk of dementia (63,64,65). Indeed, the tooth loss has been proposed recently as a risk factor for developing dementia, and dementia increase the risk of tooth loss, which can be an unexploited two-way relationship (63). Due to this, the evidence tries to demonstrate the need to avoid the population's toothlessness. It is important to mention that poor oral hygiene in patients with AD or another type of dementia may be due to poor fine motor skills, because caregivers do not care about oral hygiene or because patients forget it (66,67,68). Thus, it is important to improve oral hygiene to reduce the bacterial load that can cause oral or intestinal dysbiosis (69). In this sense, our work shows a higher load of P. gingivalis, a keystone bacterium that in recent years has been proposed as a microbiological risk factor to trigger AD due to its high pathogenicity (14,21). In this sense, there are studies that have identified it in the hippocampus, the brain cortex, the IV ventricle and the cerebro- spinal fluid (14,15). Furthermore, experimental studies have clearly shown how this bacterium can initiate neuroinflammation and increased Aβ production (14,21,23,25,27,70). Also, it has been proposed that the higher serum levels of IgG against P. gingivalis can be a hallmark for the onset of AD later in life (48, 49, 71,72,73,74). Besides, maintaining oral hygiene would have three main objectives: decrease the incidence of periodontitis, decrease tooth loss, and decrease the load of anaerobic bacteria.
When evaluating cognitive status, the main question is which test is better to choose. All studies evaluating oral health or disease in patients with cognitive impairment, AD, vascular dementia or other, characterize them with Mini-Mental state examination (MMSE) (45,46,47,51,54,75). Indeed, when patients are affected of both periodontitis and dementia, they presented a decrease in the MMSE score. Nevertheless, for the MMSE the overall accuracy at a cut-off of 25 was a sensitivity of 0.87 and specificity of 0.82 (76). Also, when adjusted estimates of accuracy for educational level, the MMSE was found to have a sensitivity of 0.97 and a specificity of 0.70. The overall accuracy at a cut-off point of 24 was sensitivity 0.85 and specificity 0.90. Based on the results of this meta-analysis, it would be expected that 85% of patients with dementia would be correctly identified with the MMSE, while 15% would be wrongly classified as without dementia (76). This is noteworthy, since a score of 25 points indicates that there could be some degree of cognitive impairment, and 24 points indicates the presence of impairment (77). Conversely, the selection of the MoCA test in our study was due to the fact that its psychometric characteristics describe a high level of reliability and validity with a sensitivity of 0.87 and a specificity in a range of 0.90 for mild cognitive impairment with a cut-off point <26, and a sensitivity of 0.87 for AD-type dementia with a specificity of 1.0, having a cut-off point <18 (78). Thus, the MoCA is highly reliable compared to other screening tests such as the MMSE, which in turn presents a lower sensitivity and specificity (77, 79,80,81,82,83).
Finally, and as a novel antecedent, in this work we detected the presence of ApoE-ε4 in the FCG. This is the first study to carry out this quantification in GCF since there is no scientific evidence of the determination of this important marker of AD in periodontal tissues that can serve as an adjunct for the management of AD. Currently ApoE-ε4 and the gene that encodes it represent the main risk factor for the development of AD and previous studies show that the presence of ApoE-ε4 at an earlier age generates a risk dependent on the concentration detected (84). Similarly, its relationship with the presence of periodontal diseases has been reported, speculating that the over-expression of this protein is associated with the presence of the most severe forms of periodontitis (85). Besides, it has been detected that the presence of ApoE polymorphisms is related to a higher risk of periodontitis, a higher burden of periodontal pathogenic bacteria, and higher serum lipid levels (86). Indeed, Aβ deposition is greater in patients who overexpress ApoE-ε4 and this protein is increased in patients with AD (87,88). This is why this study suggests that the early detection of ApoE-ε4 in GCF through routine periodontal evaluation may be of vital importance due to the role that these proteins have in the development of other diseases (33). Finally, the increase in ApoE-ε4 makes it possible to link AD, at least in part, with co-morbidities that are considered risk factors (33). The present study was able to detect that periodontitis significantly increases the levels of ApoE-ε4 in the GCF compared to healthy subjects and that it increases even more in patients affected by periodontitis and AD. When evaluating ROC, we observed a percentage of 84.9, which indicates that it may be a good marker to link the possible effect of periodontitis on cognitive status (33).
The main limitation of this study was the sample size. Therefore, we could consider this work as a pilot study which determined whether the pro-inflammatory mediators and the load of P. gingivalis are affected in patients with AD. The data allow us to suggest that in patients with AD there is a higher burden of P. gingivalis, CAL loss, higher BOP and PI, and higher levels of pro-inflammatory and pro-bone resorptive mediators in the GCF. Thus, in patients with AD, periodontitis could be more severe and urgently requires the intervention of dental surgeons in order to reduce systemic inflammation and restore periodontal stability.
Funding
This work was supported by a the Regional Development Program of the International Association for Dental Research 2017-2019, and 2021-2023, and Exbecario PRODEP UADY-EXB-241.
Author contribution statement
Conceptualization and design: V.M.A. and J.D.Z.
Literature review: L.D.S.E., F.M., V.M.A. and J.D.Z. Methodology and validation: L.D.S.E., F.A.P.,
S.M.R. and J.A.
Formal analysis: A.P.L., V.M.A. and J.D.Z.
Investigation and data collection: L.D.S.E., F.A.P.,
V.M.A. and J.D.Z.
Resources: V.M.A. and J.D.Z.
Data analysis and interpretation: L.D.S.E., F.M., S.M.R., V.M.A. and J.D.Z.
Writing-original draft preparation: L.D.S.E., V.M.A. and J.D.Z.
Writing-review & editing: All the authors. Supervision: V.M.A. and J.D.Z.
Project administration: V.M.A. and J.D.Z. Funding acquisition: V.M.A. and J.D.Z.















