Introducción
Fishery Length-Weight Relationship (LWR) models allow useful biomass estimates. This procedure is also essential in fish biology and fisheries, to evaluate the growth type and condition or “wellness” of fish populations and assemblages (Froese, 2006; Neumann et al., 2012). Fish “condition indices” asume that heavier individuals are healthier than lighter individuals of the same length (Miller et al., 2015). Fish condition will vary in response to food availability, habitat condition, sex and developmental stage. Therefore, the models and condition indices are good indicators of the effect of habitat on a specific population (Nash et al., 2006; Famoofo & Abdul, 2020).
The paradigm of mangrove-supported fisheries is being redefined by understanding the degree of dependence of tropical coastal fish on these ecosystems. Within a coastal landscape, some fish species are residents of mangroves or may use them during some stage of their ontogenetic development (zu Ermgassen et al., 2020). Current studies show that the use of mangroves as nurseries by fishes depends on spatiotemporal changes in hydrology, connectivity between different habitats, population dynamic, species ecophysiology, food/predation trade-offs and availability of resources (Hylkema et al., 2015; Sheaves et al., 2015). Therefore, some population parameters of fish assemblages such as individual density or biomass can vary significantly from one type of mangrove to another even within the same area (Faunce & Layman, 2009; Bradley et al., 2019). A recurring hypothesis proposes that mangrove's specialized root systems (i.e. stilt-roots or pneumatophores) offers differential protection and feeding resources mainly for juvenile fish or short-sized adults, although supporting evidence for this hypothesis is still pending (Rönnbäck et al., 1999; Zhang et al., 2019).
For the Mexican Pacific, LWR models of coastal-estuarine fishes have been previously estimated (e.g. González-Acosta et al., 2004; Rojas-Herrera et al., 2009; Velázquez-Velázquez et al., 2009; González-Sansón et al., 2014; Sandoval-Huerta et al., 2015), however, these studies often correspond to organisms caught within coastal lagoons, estuaries or by commercial fishing. Studies on condition and LWR models for mangrove fishes are scarce (Possamai et al., 2020).
Considering that growth parameters can be affected by habitat conditions, this work provides information about length and weight for 10 fish species (Actinopterygii) inhabiting two mangrove root microhabitats: pneumatophores (Avicennia germinans) and stilt-roots (Rhizophora mangle); as well as the variation of the allometry coefficient and relative condition for three shared species. Ultimately, the main goal is to consider the use of LWR models and fish condition as metrics to assess fish population responses to changes in habitat or the hydrology, which could be used ecosystem monitoring.
Materials and methods
Specimens were collected monthly between May and November (wet season) of 2019 from 40 sample points on eight transect lines in mangrove forests of the Boca del Cielo-San José estuary (15°52′-15°45′N, 93°42′-93°31′W; Chiapas, Mexico). Variable-length transects (range 250-770m) were located perpendicular to the waterbody with five equidistant sample points. We classified the sample points in two types of microhabitat by the root system from dominant mangrove: Stilt-roots (R. mangle) and pneumatophores (A. germinans). Fishes were caught in the submerged root systems using telescopic dip-nets (>0,1cm mesh) and seine-nets (0,5cm mesh), with an effort of two collectors for an average effort of four hrs per transect. After anaesthesia, using 1% clove oil solution, specimens were fixed in 10% buffered formalin and immediately transported to the laboratory for measurements.
Species identification and nomenclature were done following Fischer et al. (1995), Castro- Aguirre et al. (1999), Miller et al. (2009) and Fricke et al. (2020). Standard length (SL; measured from tip of the snout to the base of caudal fin) were recorded to the nearest 0,1cm, and the weight (W) was measured with and accuracy of 0,01g. We used the SL to estimate the models, considering that this measure is more consistent than the total length to analyse growth patterns in juvenile or short- sized adult fishes (Moser, 1996). Only species with more than 20 individuals per microhabitat were considered for analyses. Length-Weight relationships by microhabitat were estimated using the expression W = a SL b (Le Cren, 1951). Previously, outliers were identified and removed through a visual inspection for length and weight values (Froese, 2006). The intercept (a-value) and the slope of the regression line (b-value) were calculated by a least-squares method, through the expression log W = log a + b log SL. Confidence limits (95% CL) of a and b were also estimated. The coefficient of determination (r 2 ) provided an estimate of model fit (Froese et al., 2011).
Differences for mean standard length, regression slopes and relative condition among the species found in both microhabitats were tested using Student's t-tests. In cases where the t-test assumptions failed, Mann-Whitney's U-tests were performed. We estimated relative condition factors (K n) as a metric of species fitness using the expression K n = (W / Wʼ) x 100; Wʼ is the expected weight of a fish of the same length predicted by the regression model calculated for each population (Le Cren, 1951). All data were analysed using STATISTICA 8.0 software (StatSoft, 2007).
Results
In the stilt-roots, a total of 1322 specimens belonging to seven families and nine species were analyzed: Mugil setosus Gilbert 1862, Poecilia nelsoni (Meek 1904), Poeciliopsis fasciata (Meek 1904), Lutjanus argentiventris (Peters 1869), Centropomus robalito Jordan & Gilbert 1882, Diapterus brevirostris (Sauvage 1879), Amphilophus trimaculatus (Günther 1867), Dormitator latifrons (Richardson 1844) and Gobiomorus maculatus (Günther 1859). While in the pneumatophores, a total of 188 specimens belonging to two families and four species were analyzed: P. nelsoni, P. fasciata, Poeciliopsis pleurospilus (Günther 1866) and D. latifrons. In addition to the specific composition, 11 other fish species were found associated with the mangrove root systems, but due to the limited number of specimens collected (<20), they were not analyzed: Lile gracilis Castro- Aguirre & Vivero 1990, Ariopsis guatemalensis (Günther 1864), Ariopsis seemanni (Günther 1864), Atherinella guatemalensis (Günther 1864), Anableps dowii (Gill 1861), Poeciliopsis turrubarensis (Meek 1912), Caranx caninus (Günther 1867), Lutjanus novemfasciatus Gill 1862, Astatheros macracanthus (Günther 1864), Eleotris picta Kner 1863 and Gobionellus microdon (Gilbert, 1892). The results of the models for stilt-root and pneumatophore microhabitats for species are given in Table 1.
In stilt-roots microhabitat, the coefficient of determination (r 2 ) ranged from 0,87 to 0,99, and b-values ranged from 2,844 to 3,461. For the pneumatophores, the coefficient of determination (r 2 ) ranged from 0,81 to 0,98, and b-values ranged from 3,008 to 3,847. Overall, the specimens of the three species collected in both microhabitats were smaller in pneumatophores (Mean=1,93, standard deviation= 1,1cm SL) than in stilt-roots (Mean= 5,89, standard deviation= 3,3cm SL). These differences in size were significant for all species (t-test or U-test, P<0,05). Therefore, significant differences (t-test or U-test, P<0,05) in slopes (b-values) (Fig. 1) were also reflected due to the variability in length for all species between both microhabitats.
Table 1 Estimated parameters of length-weight relation of fishes from stilt-root and pneumatophore microhabitats in the Boca del Cielo-San José estuary, Chiapas, Mexico
| Microhabitat/ Family/Species | N | SL range (cm) | W range (g) | a | b | 95% CI (a) | 95% CI (b) | r2 |
| Stilt-roots | - | - | - | - | - | - | - | - |
| Mugilidae | - | - | - | - | - | - | - | - |
| Mugil setosus* | 179 | 1,6-21,5 | 1,81-209,34 | 0,013 | 3,229 | 0,004-0,017 | 3,103-3,455 | 0,87 |
| Poeciilidae | - | - | - | - | - | - | - | - |
| Poecilia nelsoni* | 33 | 1,1-5,3 | 0,10-5,96 | 0,017 | 3,439 | 0,009-0,026 | 3,106-3,772 | 0,92 |
| Poeciliopsis fasciata | 295 | 0,8-4,7 | 0,01-2,25 | 0,032 | 2,844 | 0,026-0,038 | 2,691-2,976 | 0,96 |
| Lutjanidae | - | - | - | - | - | - | - | - |
| Lutjanus argentiventris | 32 | 3,9-10,5 | 2,05-26,71 | 0,034 | 2,931 | 0,019-0,045 | 2,731-3,104 | 0,99 |
| Centropomidae | - | - | - | - | - | - | - | - |
| Centropomus robalito | 209 | 5,1-13 | 2,59-45,22 | 0,018 | 3,011 | 0,009-0,023 | 2,868-3,199 | 0,97 |
| Gerreidae | - | - | - | - | - | - | - | - |
| Diapterus brevirostris | 51 | 4,5-13,9 | 2,61-66,24 | 0,015 | 3,461 | 0,009-0,024 | 2,958-3,991 | 0,98 |
| Cichlidae | - | - | - | - | - | - | - | - |
| Amphilophus trimaculatus* | 23 | 3,1-12,3 | 2,32-83,57 | 0,059 | 2,909 | 0,022-0,097 | 2,640-3,178 | 0,95 |
| Eleotridae | - | - | - | - | - | - | - | - |
| Dormitator latifrons | 469 | 1,3-14,5 | 0,02-116,7 | 0,025 | 2,994 | 0,016-0,031 | 2,874-3,116 | 0,95 |
| Gobiomorus maculatus | 31 | 1,4-15,4 | 0,06-58,74 | 0,012 | 3,105 | 0,008-0,016 | 2,97-3,239 | 0,99 |
| Pneumatophores | - | - | - | - | - | - | - | - |
| Poeciilidae | - | - | - | - | - | - | - | - |
| Poecilia nelsoni* | 23 | 0,8-3,7 | 0,01-1,23 | 0,021 | 3,053 | 0,015-0,026 | 2,821-3,319 | 0,96 |
| Poeciliopsis fasciata | 112 | 0,7-2,7 | 0,01-0,41 | 0,016 | 3,267 | 0,014-0,018 | 3,092-3,443 | 0,81 |
| Poeciliopsis pleurospilus | 22 | 1,1-3,6 | 0,01-1,37 | 0,008 | 3,847 | 0,003-0,015 | 2,161-3,864 | 0,95 |
| Eleotridae | - | - | - | - | - | - | - | - |
| Dormitator latifrons | 31 | 1,1-6,3 | 0,02-5,89 | 0,029 | 3,008 | 0,013-0,045 | 2,532-3,277 | 0,98 |
N: number of specimens; SL: standard length; W: body weight; a: intercept; b: slope; CI: confidence intervals; r2: coefficient of determination. *: no data about LWR in FishBase (Froese & Pauly, 2021).
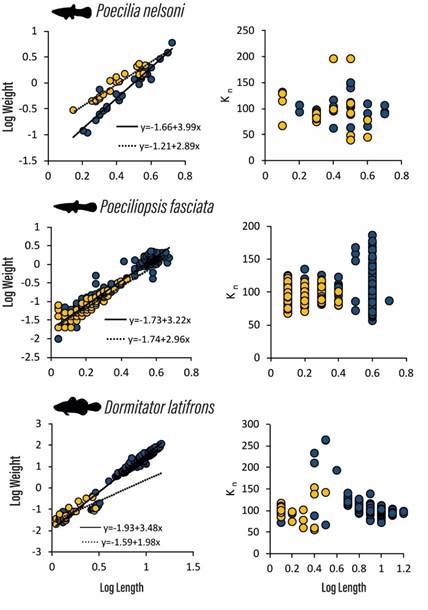
Figura. 1 Length-weight (on left) and relative condition (on right) plots for Poecilia nelsoni, Poeciliopsis fasciata and Dormitator latifrons collected from the Boca del Cielo-San José estuary, Chiapas, Mexico, in stilt-roots (blue dots) and in pneumatophores (yellow dots). Dotted and solid lines represent predicted values from stilt-roots and pneumatophores regression lines respectively.
Poeciliopsis fasciata exhibited different growth behaviour (Positive and negative allometries) between stilt-roots and pneumatophores, while P. nelsoni and D. latifrons maintained a positive allometry and isometry respectively in both cases. The relative condition in P. fasciata appeared to be influenced by length, where the smallest and the largest individuals showed high K n-values. For P. nelsoni and D. latifrons K n-values appeared more evenly distributed with regards to length. Although the mean relative condition for the three species was overall higher in stilt-roots, the differences between microhabitats were only significant (P<0,05) for P. fasciata (Table 2).
Table 2 Comparisons of mean standard length (I), slopes of regression (II) and relative condition (III) for three fish species between two mangrove microhabitats in the Boca del Cielo-San José estuary, Chiapas, Mexico.
| Species | Mangrove microhabitat | N | Mean SL±SD (cm) | I p | b | II p | Mean Kn±SD | III p |
| Poecilia nelsoni | Stilt-roots | 33 | 3,05±1,0 | 0,03* | 3,439(+) | 0,005* | 99,89±22,5 | 0,22 |
| Pneumatophores | 23 | 2,48±0,8 | 3,053(+) | 95,63±42,2 | ||||
| Poeciliopsis fasciata | Stilt-roots | 295 | 2,54±1,1 | <0,0001** | 2,536(-) | <0,0001** | 104,08±21,2 | 0,042* |
| Pneumatophores | 112 | 1,46±0,4 | 3,267(+) | 99,68±11,9 | ||||
| Dormitator latifrons | Stilt-roots | 469 | 8,06±2,2 | <0,0001** | 2,994(i) | <0,0001** | 103,13±15,9 | 0,86 |
| Pneumatophores | 31 | 3,23±1,6 | 3,008(i) | 100,5±22,6 |
N: number of specimens; SL: standard length; SD: standard deviation; b: slope; growth behavior: (+) positive allometry, (-) negative allometry, (i) isometry; Kn: relative condition; p: p-value for Student's t-test (*) or Mann-Whitney's U-test (**) comparing (I) the mean SL of fishes, (II) the slopes of the regressions for stilt roots and pneumatophores. Significant p- values (p<0.05) are in bold.
Discussion
The results of our study indicate that LWR models and relative condition may provide potentially useful metrics for understand some factors that affect the development of mangrove- dependent fish. Estimation of growth parameters is especially important for comparisons, due to the limited information available about the biology of mangrove fish. However, it is necessary to be cautious in the use of biomass estimation factors, which can be affected by the life stage, sex and ecogeographic differences.
Length-Weight Relationship parameters for four species reported here (M. setosus, P. nelsoni, A. trimaculatus and G. maculatus) are not available in FishBase (Froese & Pauly, 2021). Regarding P. nelsoni, previous LWRs data are included with Poecilia butleri Jordan 1889, which is considered a species now confined to the north of the trans-Mexican Volcanic belt according to Palacios et al. (2016). In another case, M. setosus is now recognized as a valid name for Mugil curema Valenciennes 1836 in the eastern Pacific, based on molecular and morphological data (Britzke et al., 2019).
Allometric coefficients for nine species from this study fall within the expected range of 2,5- 3,5 proposed by Froese (2006), from which the type of normal growth observed in most fish is represented (isometric when b = 3 or allometric, negative when b<3 and positive when b> 3). An out-of-range b-value (>3,5) was estimated for P. pleurospilus in pneumatophores. According to the models reported for this species in Southern Mexico (Velázquez-Velázquez et al., 2015), the allometric coefficient was 2,809 and a wide range in the length of the measured specimens (1,0- 5,1cm standard length). The out-of-range b-value obtained here may be attributed by a limited variability in the length frequency of the specimens collected from mangroves (Abu Hena et al., 2017). However, the spatial variation for the species may reveal population, nutritional or ontogenetic stage differences of the measured individuals (Froese, 2006; Froese et al., 2011), indicating a differential use of microhabitats.
The coefficient of determination (r 2 ) values in the stilt-root microhabitat were found to be greater than 0,9 in eight out of nine species, which reflect the proper fitness for the growth model and good health status of the fish (Kodeeswaran et al., 2020). Otherwise, the r 2 values in the pneumatophores microhabitat were <0,9 in three out of four species.
Most of the species analysed in the present study had positive allometric growth (b>3), which means that the fish grows faster in weight than in length. Despite the availability of food and protection for juvenile fish in mangroves, it has been shown that stress caused by fluctuating hydrological conditions (e.g. hypoxia, hypersalinity, and high water temperature), such as those that occur in this estuarine system (Contreras & Zabalegui, 1991), can define the growth pattern in some coastal species (Kimmerer et al., 2005).
The environmental effect on the relative fish condition has been studied trough spatial or temporal comparisons (eg. Blackwell et al., 2000; Miller et al., 2015; López-Pérez et al., 2020). Fish condition generally decreases due to scarce food availability. Blaber (2000), suggests that food items in mangroves are more concentrated among pneumatophores than in less dense stilt-roots which facilitates feeding. In our study, we found no evidence of significant changes in the condition for P. nelsoni and D. latifrons between the two microhabitats. However, the differences in condition and growth type for P. fasciata could reflect a strategy of some species, in which the juveniles develop positive allometric growth to compensate for the size they need to reach sexual maturity. To better understand this pattern, it is necessary to consider ontogenetic shift in the diet and early maturity sizes in these species. Likewise, a lower relative condition could indicate the energy cost of migration between microhabitats. According to the observations of our study, some species may first remain in the pneumatophores to grow and then move towards the stilt roots, an aspect that also should be investigated.
The results of this study demonstrated that difference in the average size and allometric coefficient of the fish between two mangrove root microhabitats exist. Substrate, primary production, root density and the availability of a volume of root-free water are attributes that could drive to a differential provision of food and shelter from predators for fishes (Rönnbäck et al., 1999). Likewise, it is necessary to consider the temporal factor, since the low depth and prevailing anoxic conditions during the dry season offer a barrier for the entry of larger predatory fish into the mangrove swamp.
Ethical, conflict of interest and financial statements
The authors declares that they have fully complied with all pertinent ethical and legal requirements, both during the study and in the production of the manuscript; that there are no conflicts of interest of any kind; that all financial sources are fully and clearly stated in the acknowledgements section; and that they fully agree with the final edited version of the article. A signed document has been filed in the journal archives.
S. S. D., C. T. H. and E. I. R. B.: Study design, data collection and analysis. W. A. M.: Data analysis. All co-authors: Preparation and final approval of the manuscript.












 uBio
uBio 


