The genus Hemibrycon is a group of freshwater chara cid fish species first recognized by Gunther (1864) as a subgenus of Tetragonopterus. Subsequently the subge nus was defined by Eigenmann (1927) as a new genus of Characidae and species were added and subtracted by several authors (Gery, 1962; Bertaco, Malabarba, Hidalgo & Ortega, 2007; Bertaco & Malabarba, 2010; Roman-Valencia & Arcila-Mesa, 2010; Roman-Valencia, Ruiz-C, Taphorn, Mancera-Rodriguez & Garcfa-Alzate, 2013). Mirande (2010) placed Hemibrycon in the subfam ily Stevardiinae in his hypothesis of phylogenetic rela tionships of the Characidae, but only two species werem included in that analysis. Javonillo, Malabarba, Weitzman & Burns (2010) used DNA sequencing techniques and found evidence for the monophyly of the group.
Fishes of the genus Hemibrycon are typically found in clear freshwater habitats in rivers of the Pacific wa tersheds in Panama, coastal Caribbean drainages, Lake Maracaibo and Orinoco River basins in Venezuela, streams of Trinidad and Tobago, coastal basins of French Guiana and Suriname, coastal Caribbean and Pacific drainages, Catatumbo, Andes and Orinoco drainages in Colombia and upper Amazon River drainages of Bolivia, Peru and Ecuador (Bertaco et al., 2007; Bertaco & Malabarba, 2010; Roman-Valencia et al., 2013). Today there are 36 species reported in the genus (Eschmeyer & Fricke, 2013). Of these, 21 species are distributed in the rivers of Colombia, but only one species, H. santamartae, has been reported previously in drainages of the Sierra Nevada de Santa Marta.
This description of a new species of Hemibrycon from the La Sierra Nevada de Santa Marta in Colombia is a result of an ongoing revision (C.G-A and collabora tors) of the genus, and is further proof of the as yet un documented biodiversity of the genus by Central and South America.
Material and methods
Fishes were captured using seines and were pre served with 10% formalin and later stored in 70% ethanol. Measurements and counts follow Roman-Valencia, Garcfa-Alzate, Ruiz-C & Taphorn (2010). Measurements were made with digital calipers to 0.01mm precision and are expressed as percentages of standard (SL) and head length (HL). In count ranges, values for the holotype are indicated with an asterisk (*). Counts and measurements were taken on the left side of specimens when possible. Osteological observations were made on cleared and stained specimens (C&S) prepared according to Taylor & Van Dyke (1985) and Song & Parenti (1995). Bone nomen clature follows Weitzman (1962), Vari (1995), and Ruiz-C. & Roman-Valencia (2006). Type specimens are deposited in the University of Atlantico Caribbean Region, Dept. Biology, Museum Collection, Barranquilla, Colombia (UARC-IC), Collection of Fishes of Auburn University Natural History Museum, Auburn, Alabama (AUM) and the Ichthyology Laboratory at the Universidad del Quindfo, Armenia, Colombia (IUQ). In the lists of paratypes, the number of individuals is given in parentheses immediately after the catalog number. Institutional acro nyms follow Sabaj-Perez (2010).
We performed a canonical discriminate analysis on the covariance matrix of morphometric characters with the software program R version 2.15.3 (available free at the website http://cran.r-project.org/bin/windows/base/). The Burnaby method to eliminate the influence of over all size, with the Past program, version 3.0 for Windows.
Comparative material examined. Hemibrycon beni: UMSS 09585, (18) (35.2-82.6mm SL), Bolivia, Amazonas/Madera/Beni/Bopo, Río Pekhenkhara, Imanblaya, 3 Jan. 1990;UMSS 0890, 50, 47.3-75.4mm SL, Bolivia, Amazonas/ Beni/Madera/Kaka, afluente Taipiplaya-Taipiplaya, 3 Oct. 2008; UMSS 8895, (25) (46.3-77.7mm SL), Amazonas / Madera/Beni/Bopi, Puri bridge, 4 Dec. 2008. H. boquiae (see Roman-Valencia, 2001;). H. colombianus: (see Roman-Valencia & Arcila-Mesa., 2010). H. dariensis: (see Roman-Valencia & Ruiz, 2007). H. guppyi: (see Roman-Valencia & Ruiz-C., 2007). H. helleri: (see Arcila-M., 2008). H. jelskii: (see Roman-Valencia & Arcila-Mesa., 2010) MUSM 36126,(7) (30.2-85.1mm SL), Peru, Cusco state, Amazonas, La Convencion, Echarate, Urubamba, Río Perotoni, 28 May 2009; MUSM 35492, (13) (32.8-56mm SL), Peru, Ucayali state, Amazonas, Atalaya, Sepaliva, que-brada Lazaro tributario Río Mishahua, 28 May 2009. H. jabonero: (see Roman-Valencia & Arcila-Mesa., 2010). H. metae: (see Roman-Valencia & Ruiz-C., 2007) IAvH 3122, (10) (47.6-93.9mm SL), Colombia, Casanare, Aguazul, Orinoco, Chichaca Creek, tributary of Río Cachiza, V. Ortiz-Munoz, 1 Mar. 1994; IAvH 3125, (33) (51.1-71.4mm SL) Colombia, Casanare, Aguazul, Orinoco, Unete, Cravo Sur and Tua River drainage, V. Ortiz-Munoz, 4 May 1996; IAvH 3129, (50) (49.3-78.7mm SL) Colombia, Casanare, Aguazul, Orinoco, Quebrada Cupiagu, Río Unete drain age, V. Ortiz-Muñoz, 4 Mar. 1994. All from Venezuela: MCNG 26774, (2) (38.1-47.1mm SL), Barinas, Río Santa Barbara, 3Km NE, Santa Barbara, Apure drainage; L. Page, 1 Jan. 1992; MCNG 26774, (26) (38.1-47.1mm SL), Barinas, Río Santa Barbara, 3Km NE Santa Barbara, Apure drain age; L. Page, 1 Jul. 1992; MCNG 7916, (1) (59.4mm SL), Barinas, Apure, Dtt. Pedraza, Cano Ticoporo at bridge on road from Acequia River, Apure; D.C. Taphorn, 7 Dec. 1982; MCNG 50011, (1) (54.9mm SL), Río Ventuari, Salto Tencua 58Km. E of San Juan de Manapiare 5°2.86' N, 65°36.95' W, N. Lujan, O. Leon, A. Luna, A.Valmore, 21 Apr. 2004; MCNG 41903, (2) (59.5-65.2mm SL) Barinas, upper La Yuca River; D.C. Taphorn, 3 Nov. 1998; MCNG 32396, (30) (30.5-57.9mm SL), creek NE of San Antonio, Hwy. 5, Río Curito; D.C. Taphorn, L. Page, 3 Feb.1993. H. santamartae: (see Roman-Valencia, R. Ruiz-C, Garcfa-Alzate & Taphorn, 2009b) ICNMNH 10839, (22) (39.8 64.3mm SL), Guajira, Río Rancherfa, Río Marocaso, 26 Oct. 2004; ICNMNH 8878, (4) (64.6-72.0mm SL), Guajira, Río Rancherfa, Chorreras, Paso Ancho, 18 Feb. 2004; ICNMNH 10881, (25) (34.9-57.5mm SL), Guajira, Río Rancherfa Marocaso, Río Marocaso, 26 Oct. 2004; ICNMNH 11598, (20) (44.9-80.6mm SL), Guajira, Río Rancherfa Marocaso, Río Marocaso, 1 Mar. 2005. H. virolinica (see Roman-Valencia & Arcila-Mesa, 2010) ICNMNH 16028, (39) (43.4 77.7mm SL), Santander, El Carmen, Magdalena River system, Quebrada El Carmen, 20 Feb. 2005.
Results
Hemibrycon sierraensis
new species (Figure 1)
Holotype. UARC-IC 134, 67.2mm SL, female, Colombia, Magdalena, Caribbean slope, Río Gaira beneath the bridge at Minca, 11°08'37.8"N - 74°07'08.1''W, 120 m.a.s.l., 25 Aug. 2013, C. Garcfa-Alzate.
Paratypes. All from Colombia, Magdalena, Sierra Nevada de Santa Marta and collector C. Garcfa-Alzate: AUM 61620, (2) (77.2-87.3mm SL), IUQ 3689, (4) (67.3 88.3mm SL), and IUQ 3629 (2 C&S) (58.9-76.3mm SL), collected with holotype. UARC-IC 135, (20) (50.3-72.1mm SL), Quebrada el Congo, afluente del Río Frfo, vereda el Congo, 10°58'37.9''N - 74°04'16''W, 557 m.a.s.l, 24 Nov. 2012; ICNMNH 5749, (12) (49.7-58.9mm SL), Vereda El Congo, Quebrada El Congo, 500 m.a.s.l, tributary of Río Frfo, 1 Feb. 2002; ICNMNH 6439, (4) (51.9-60.8mm SL),Vereda El Congo, Quebrada El Congo, 500m.a.s.l, tribu tary of Río Frfo, 16 Jan. 2002; ICNMNH 6931, (3) (71.5 83.3mm SL), Río Manzanares, 1 Feb. 2002; ICNMNH 5748, (4) (55.7-85.6mm SL), Río Cordoba, elevation 450 m.a.s.l. 1 Feb. 2002; UARC-IC 136, (2) (48.8-56.5mm SL), Caribe versant, Río Gaira beneath the bridge at Minca, 11°08'37.8''N - 74°07'08.1''W, 120 m.a.s.l, 10 May 2013; UARC-IC 137, (15) (44.5-88.3mm SL), collected with holotype; UARC-IC 138, (2 C&S), Vereda El Congo, Quebrada El Congo, tributary of Río Frfo, 10°58'37.9''N - 74°04'16''W, 557 m.a.s.l, 24 Nov. 2012; UARC-IC 139, (93) (58.8-85.9mm SL), Caribe versant, Río Gaira beneath the bridge at Minca, 11°08'37.8''N - 74°07'08.1''W, 120 m.a.s.l, 10 May 2013.
Diagnosis. Hemibrycon sierraensis n. sp. is distin guished from all other species of the genus by having an iridescent red adipose fin in life (vs. hyaline or trans parent in life, except H. divisorensis that has a reddish adipose fin) and by having a dark brown adipose fin in alcohol (vs. hyaline or transparent in alcohol). It differs from H. divisorensis, H. pautensis and H. santamartae in having the last dorsal-fin ray unbranched. It further dif fers from H. beni, H. boquiae, H. brevispini, H. colombianus, H. mikrostiktos, H. microformaa, H. metae, H. palomae and H. rafaelense in having a vertically elongate humeral spot that extends three to four scale rows below the lateral line series (vs. humeral spot roughly circular not extend ing ventrally three to four scales rows below lateral line series, or with inconspicuous, diffuse vertical extensions). It differs from H. pautensis by the number of scale series between the lateral line and the pelvic-fin insertions (four-five vs. six-seven). It differs from H. divisorensis in the number of unbranched anal-fin rays (iii vs. iv), num ber of dentary teeth (ten vs. 13-16) and the number of maxillary teeth (six-seven vs. ten-13). In addition to the above characters, we found the following differences that distinguish this new species from those that occur in the same basin (and in allopatry with H. santamartae): fewer supraneurals (six vs. eight), greater caudal pedun cle depth (16.5-17.7 vs. 8.7-15.6% SL), longer head length (25.0-29.6 vs .18.4-25.2% SL) and smaller orbital diameter (23.9-34.8 vs. 33.1-45.7% SL).

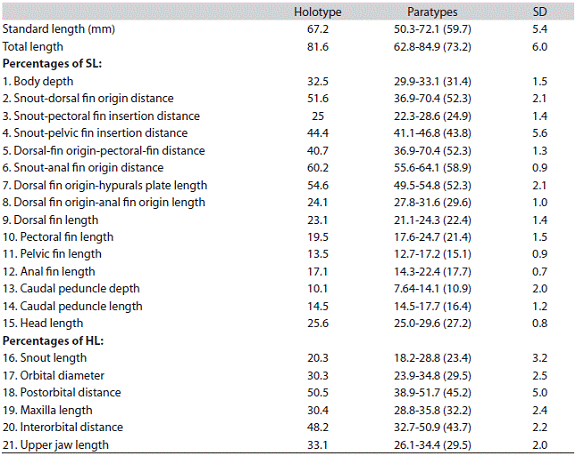
Figure 1: Hemibrycon sierraensis, Holotype just after fixation, UARC-IC 134, 67.2mm SL, male, Río Gaira Sierra Nevada de Santa Marta. Bar = 1cm.
Description. Morphometric data are in Table 1. Body slender and elongate (mean maximum body depth 31.4% SL). Area between orbits slightly convex. Dorsal profile of head straight to supraoccipital, then slightly convex from supraoccipital to dorsal origin and from last dorsal-fin ray to caudal peduncle, then straight to base of caudal fin. Ventral profile of body convex from snout to base of pelvic fin, oblique along anal-fin base, then straight to base of caudal fin. Head and snout large, mandibles equal; mouth terminal, lips soft and flexible, not covering outer row of premaxillary teeth; ventral border of upper mandible oblique; posterior edge of maxilla reaching anterior edge of orbit; opening of pos terior nostrils vertically ovoid.
Premaxilla with two rows of teeth, outer row with four to five tricuspid teeth; internal row with four, the first three pentacuspid and the last tricuspid, diminish ing gradually in size laterally. Maxilla extends beyond posteroventral edge of the second infraorbital, and has 6-8 tricuspid teeth in a series not reaching anteroventral margin of that bone. Four anterior most dentary teeth larger, with five cusps, followed by six medium-sized tri-cuspid teeth (Figure 2).
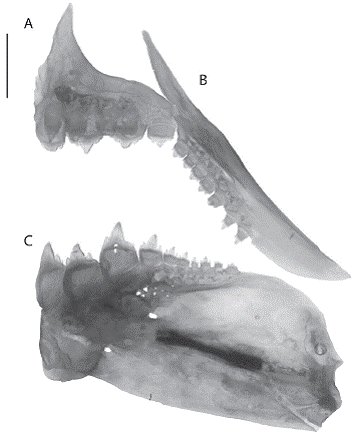
Figure 2: Hemibrycon sierraensis, UARC-IC 138, paratype, 62.2mm SL. Photograph of the premaxilla (A), maxilla (B) and dentary (C), left side. Bar = 1cm.
Lateral line complete, perforated scales 38(3), 39(15), 40(52), 41(4) or 42*(12). Scale rows between dorsal-fin origin and lateral line seven*(40) or eight (46); scale rows between lateral line and anal-fin origin six*(28) or seven (58); scale rows between lateral line and pelvic-fin inser tion four (30) or five*(56). Predorsal scales 14(4), 15*(44), 16(30) or 17(8), arranged in regular series. Anal-fin rays iii, 26*(28) or 27(58). Anal-fin origin posterior to verti cal through base of first dorsal-fin ray. Pectoral-fin rays i,10*,i (46) or 11(40). Dorsal-fin rays ii,7,i*; Pelvic-fin rays i,6,i*; in both fins the last ray is simple; first unbranched ray approximately one-half length of second ray, its tip reaching first bifurcation of first branched ray. Pelvic-fin insertion anterior to vertical through dorsal-fin origin. Caudal fin forked with short pointed lobes not covered with scales. Total number of vertebra 40-41 (n=4).
Six infraorbitals; first infraorbital extending over dor sal surface of maxilla, bearing laterosensorial canal pores, its anterior portion has short process with blunt tip, that does not extend towards antorbital; its posterodorsal margin not modified. Seven supraneurals between head and first proximal pterygiophores of dorsal fin, with car tilage on upper and lower margins. Four proximal radials, third postcleitrum with a small laminar lateral process on medial surface. Pelvic bone short, straight, blunt with cartilage at anterior tip. Pelvic bone ischial process with cartilage. Fifth and sixth hypurals united, posterior mar gin of hypurals without cartilage. Medial and proximal pterygiophores of first four anal-fin rays fused.
Color in alcohol. Body dark brownish-yellow, chro-matophores more densely concentrated on dorsum, most intense on head and extending to anterior part of dentary. Midlateral body with dark stripe from posterior margin of eye, interrupted at humeral spot and then con tinuing posteriorly onto middle caudal-fin rays. Humeral spot vertically elongate, located near posterior margin of opercle, extending from second to fifth scale of lat eral line series and extending three to five scale rows below the lateral line series. Ventral part of body light yellow. Posterior margin of scales on dorsal region of body dark. Dorsal and anal fin with strong concentration of chromatophores along distal margin. Adipose dark brown. Caudal-fin with dark chromatophores on middle rays. Pectoral and pelvic fins as well as caudal-fin lobes hyaline. Dorsal fin with chromatophores concentrated mostly on interradial membranes and distal margins of anterior rays.
Color in life. Counter-shaded and with silvery lateral stripe highlighted in iridescent yellowish-green, more conspicuous along dorsal margin of stripe. Dorsal margin of opercle anterior to lateral stripe intense violet. Dorsal margin of eye yellow. Infraorbital along posterior margin of orbit violet, this color extending along dorsal half of opercle. Head beneath orbit intense blue. Scales on sides of body without melanophores, giving it a whitish or silvery appearance. Dorsal region dark violet. Wide dark humeral spot, conspicuous beneath silvery lateral stripe, extending through it but with less intensity. Posterior part of caudal peduncle with dark midlateral stripe that extends on to middle caudal-fin rays; ventral margin of lateral stripe reddish on caudal peduncle. Ventral poste rior portion of caudal peduncle red. Pectoral and pelvic fins hyaline, anal and dorsal fins with reddish bar crossing middle sections of rays, more notable in males, distal tips of dorsal and caudal fins dark. Adipose fin iridescent red.
Sexual dimorphism. Males have hooks on anal, pel vic and pectoral-fin rays. There are five to nine pairs of hooks located on the middle and distal portions of last unbranched and first to seventh branched anal-fin rays. All branched pelvic-fin rays have ten to 20 small hooks all along most of ray's length, no hooks on unbranched rays. There are small, poorly developed hooks on the extreme distal portions of the pectoral-fin rays.
Etymology. Named for the collection locality, in the Sierra Nevada of Santa Marta drainage, north from Colombia.
Distribution and ecological notes: This species is so far known from the upper Gaira and Río Frfo drainages, in the Sierra Nevada of Santa Marta, Caribbean coastal drainages of northern Colombia (Figure 3). Hemibrycon sierraensis was captured in streams characterized by rela tively rapid water current, running over rocky and sandy bottoms with high transparency (Figure 4). The pH was near neutral, dissolved oxygen and percent saturation of oxy gen values were high (Table 2) typical of oligotrophic en vironments. The new taxon is allopatric with Hemibrycon santamartae, a species found in the more eutrophic wa ters of the Rancherfa River and its tributaries. Analysis of stomach contents of four specimens revealed pres ence of filamentous algae, fragments of vascular plants, adults and larvae of two different species of Trichoptera (Hydropsychidae), adult Formicidae and fragments of unidentified arthropods. The presence of allochthonous and autochthonous items suggests that this species is omnivorous.
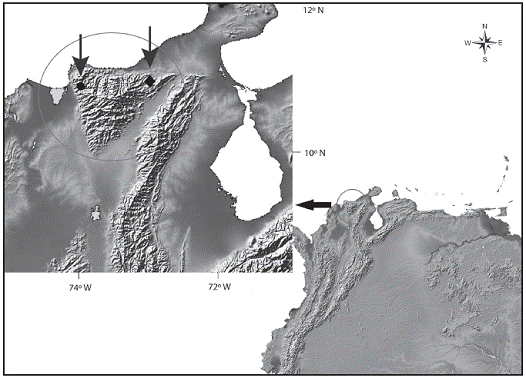
Figure 3: Distribution of Hemibrycon sierraensis. (●) and H. santamartae (♦) in Northeastern South America, Colombia.

Figure 4 Type locality of Hemibrycon sierraensis. Río Gaira in the village of Minca, Sierra Nevada de Santa Marta, Colombia.
Table 2: Physical and chemical variables recorded from the habitat of Hemibrycon sierraensis. Data taken in November 2012 and March of 2013
Comments: A discriminant analysis that included species from adjacent watersheds did not distinguish among most species based on morphology, but did separate Hemibrycon sierraensis. H. sierraensis is distin guished from H. boquiae, H. colombianus, H. santamartae, H. dariensis, H. cardalensis, H. metae, H. palomae, H. poly-odon, and H. jabonero along canonical axis one by differ ences in interorbital distance, postorbital distance, head length and caudal peduncle depth (Figure 5). The first ca nonical axis explained 46,8% of the total variability and the second 19,8%, between the first and third canonical axis explained the 81% of the variation (Figure 6).
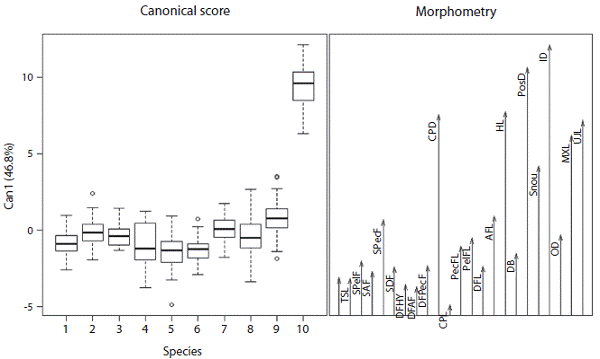
Figure 5: Canonical scores of the morphometric data for Hemibrycon boquiae (1), H. colombianus (2), H. santamartae (3), H. dariensis (4), H. cardalensis (5), H. metae (6), H. palomae (7), H. polyodon (8), H. jabonero (9) and Hemibrycon sierraensis n. sp. (10). The abbreviations correspond to the 23 morphometric measurements given in Table 1.
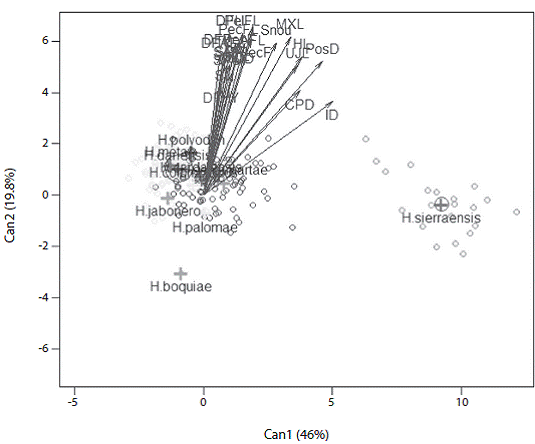
Figure 6: Representation of the first two canonical axes, from morphometric data of Hemibrycon boquiae (n=71), H. colombianus (n=37), H. santamartae (n=122), H. dariensis (n=16), H. cardalensis (n= 29), H. metae (n=81), H. palomae (n=32), H. polyodon (n=33), H. jabonero (n=72) and Hemibrycon sierraensis n. sp. (n=23). The abbreviations correspond to the 23 morphometric measurements given in Table 1.
Discussion
Hemibrycon sierraensis has all of the synapomorphies observed in other Hemibrycon (Mirande, 2010; Arcila-M, 2008). Although most species of Hemibrycon are similar in color pattern throughout their geographic distribu tion, subtle differences in the concentration and distribu tion of black pigment in the humeral and caudal regions, have been used to identify new diversity (Bertaco & Malabarba, 2010; Roman-Valencia, Vanegas-Rfos & Garcfa, 2009a; Roman-Valencia et al., 2009b; Roman-Valencia & Arcila-Mesa, 2010). In addition to a red adipose fin, Hemibrycon sierraensis has a red spot on the ventral por tion of the caudal peduncle, a character that also been observed in several other species of Hemibrycon (Bertaco et al., 2007; Roman-Valencia et al., 2010), leading us to infer that it represents a synapomorphy for the genus as suggested by Arcila-M (2008). The bright red adipose fin is a possible autapomorphy for this new species and has not been observed in other species of the genus.
The character traditionally used to define Hemibrycon is having the toothed portion of anterior margin of the maxilla longer than the untoothed portion (Eigenmann, 1927). However, this character is probably homoplastic and is present in other genera of Characidae such as Brycon, Roeboides and Pseudochalceus.
One species, H. santamartae, has been reported pre viously from the Sierra Nevada de Santa Marta (Roman-Valencia et al., 2009b). It is similar to and probably related phylogenetically to H. sierraensis, but is distinguished from it by: the number of supraneurals, caudal peduncle depth, head length and orbital diameter, and also by the pigmentation pattern observed in alcohol preserved specimens (see Roman-Valencia et al., 2009b).
Contrary to Bertaco & Malabarba (2010), we consider Hemibrycon pautensis Roman-Valencia, Ruiz C. & Barriga (2006), one of the species we compared to the new spe cies, to be a valid species, not a synonym of H. polyodon (Gunther 1864). Roman-Valencia et al., (2013) noted the following differences between H. pautensis and H. polyodon: H. pautensis has a longer caudal peduncle (8,07-11,07% vs. 14,4-16,6% SL) and longer upper jaw (23,4-30,8 vs. 43,7-45,6% SL); and a smaller orbital diam eter (29,5-34,1 vs. 39,51-44,06% SL).












 uBio
uBio