1. Introduction
Quality in healthcare services remains a widely-spread challenge for many contexts. Within the global Sustainable Development Goals (SDGs) framework, healthcare quality can no longer be considered a luxury. It is, instead, viewed as a necessity for all countries and a right for their people. This is in accordance with SDG #3 Ensure healthy lives and promote well-being for all at all ages, and especially target 3.8: Achieve universal health coverage, including financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all (United Nations, 2015).
Translating this premise into the complexity of healthcare services networks can take the face of different outcomes. In the case of clinical laboratories, quality may be guaranteed through various national or international schemes: the ISO 15189 and 17025 standards, the Joint Commission International (JCI) Clinical Laboratory standard, the Clinical Pathology Accreditation (CPA) in United Kingdom, the College of American Pathologists (CAP) Laboratory Accreditation program in USA, Clinical and Laboratory Standards Institute (CLSI) GP26A-1 guideline, among others.
The accreditation of clinical laboratories by ISO 15189 continues to be a goal sought by many organizations in Costa Rica. For instance, in the public sector, the implementation of quality management in clinical laboratories of the Costa Rican Social Security Fund (Caja Costarricense de Seguro Social, CCSS) is a major challenge. It has arisen from the need to unify administrative guidelines, internal standards, technical procedures and reference parameters, in all laboratories of the CCSS regardless of level of complexity (from primary care to specialized hospitals) (CCSS, 2012).
Furthermore, interest in accreditation is not only present in the public healthcare institutions. Private organizations may also find several advantages in the process: added value for their customers, improved communications with partners, a structured framework for risk management (especially patient safety-related risks), personnel engagement and standardization/optimization of laboratory procedures (Zima, 2017; Tzankov & Tornillo, 2017).
Health inequality in Costa Rica has been pointed out elsewhere, mainly with a focus on access to basic public services and social determinants of health (Pan American Health Organization, 2004; Morera, 2009). However, to our knowledge, no study has been carried out regarding quality of clinical laboratory services throughout the Costa Rican territory. We hypothesized that, due to the fact of accreditation culture still not being widely spread among the countrys population, the number of accredited laboratories will be low with an uneven distribution given that laboratories do not currently face such demand from key stakeholders such as users and communities. Therefore, the aim of this investigation was to explore the distribution of ISO 15189-accredited and non-accredited laboratories in Costa Rica, in order to identify possible gaps in the fulfillment of the quality healthcare for all SDG.
2. Theoretical framework
2.1 Quality in healthcare
According to the World Health Organization (WHO), the World Bank Group (WB) and the Organization for Economic Cooperation and Development (OECD) (2019), quality of care can be defined as the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge (p. 30). This implies that process improvement in healthcare settings should be aligned with the interests of users and communities as key stakeholders.
Taking this into consideration, many programs and accreditation schemes have been proposed to implement and maintain quality management systems in the field of clinical laboratory services. Out of many accreditation schemes currently available, the ISO 15189 is the most widely used standard to ensure quality in clinical laboratories worldwide (Datema, Oskam & Klatser, 2011; Zima, 2017). Hence, it is employed in the present research article to assess the quality accreditation status of clinical laboratories in Costa Rica.
2.1.1 Clinical laboratory ISO 15189 accreditation
Since the decade of 1970s, the adoption of quality management systems became an increasingly common practice among clinical laboratories. However, laboratory professionals (especially from the European community) considered that existing standards such as the ISO 17025 and the Essential Criteria of the European Communities Confederation of Laboratory Medicine fell short to the necessity of ensuring quality throughout the whole process cycle within a clinical laboratory: from pre-analytical to post-analytical stages (Thelen & Huisman, 2018).
Consequently, a technical committee was installed by the International Standardization Organization (ISO), which drew upon the requirements of existing standards (e.g. ISO 17025, ISO 9001, Essential Criteria of EC4, the British Clinical Pathology Accreditation program, among others) and adjusted them to the context of a clinical laboratory so that equal attention was paid to pre-analytical, analytical and post-analytical processes. This led to the development and publication of the ISO 15189:2003 standard, titled Medical laboratories Requirements for Quality and Competence (Thelen & Huisman, 2018).
Thanks to the existence of such international standard since the early 2000s, harmonization of quality schemes across countries was facilitated as laboratories could reach out to their countrys respective national accreditation body to include ISO 15189 in their scope. In the case of Costa Rica, the homologue version of the standard was first published some years later, with INTE/ISO 15189:2008 being the first version of the norm of which record is kept in the country. Currently, there is a second version in force, INTE/ISO 15189:2014, which is based on the international standards 2012 version (Instituto de Normas Técnicas de Costa Rica (INTECO], 2014).
Regarding implementation of the standard in Costa Rica, the CCSS authorities have provided guidance for public clinical laboratories to meet a series of requirements in order to facilitate the accreditation process. These requirements are divided into four phases: a first phase covering regulatory aspects such as the registration of the laboratory before the College of Microbiologists and Clinical Chemists of Costa Rica (CMQC) and the operating permit granted by the Ministry of Health of Costa Rica. In addition, this phase contains other aspects such as: managing documentation related to authorized tests, registration of complaints or suggestions, continuous improvement processes, among others (CCSS, 2012).
The second phase is mainly aimed at the development of documentation and compliance with the requirements according to the ISO 15189 standard. The third phase comprises a large part of the requirements of the standard, focused on the matter of production: equipment, reagents and various supplies. Finally, the fourth phase focuses on the full implementation of the quality management system throughout the laboratory (CCSS, 2012).
As for the private sector, implementation of a quality management system in accordance to ISO 15819 relies on the willingness and capabilities of each organization to carry out such a process. Each laboratory needs to outline its own path, which ought to be aligned with the organizations structure, goals, resources and potential benefits to obtain from the process (Antúnez & Murillo, 2014).
Since factors to be taken into consideration for ISO 15189 accreditation may vary from one setting to another, this can influence the ability of certain types laboratories or located within certain geographies, to create, maintain and improve a robust quality management system. This, in turn, might lead to an unequitable landscape where high quality services are only available to small population groups. In such a scenario, we would be talking about health disparities in the access to healthcare of quality.
2.2 Health disparities
Health inequalities or health disparities are differences that exist among specific population groups in the attainment of full health potential. Disparities can exist across many dimensions, such as gender, ethnicity, sexual orientation, age, disability status, socioeconomic status, and geographic location (Weinstein et al., 2017).
Actually, one of the most consistent findings in the health disparities literature is that location matters. Less populated or more geographically-isolated communities may face difficulties with recruiting and sustaining an adequate healthcare workforce because of factors such as resource limitations, provider shortages, among others (Giraldi, García, Kundu & Famitangco, 2018).
This has shown to be true for clinical laboratory services as well. In the United States, for instance, states with many rural areas have less clinical laboratory technologists and technicians than states with fewer rural areas (Giraldi et al., 2018, p. 41). These inequalities have also been evidenced across Latin America, with laboratory services being one of the diagnostic capabilities that are in often shortage relative to demand in rural or lower income regions (Roa et al., 2020). If equal access to services in general remains a challenge, more even so is this a pending challenge with regard to access to healthcare of quality.
For this reason, detecting and assessing these disparities in healthcare is of major relevance given that the only way to properly address the issue of inequality is by understanding the overall landscape as well as its underlying factors. With the methodology we describe below, we look forward to shedding some light on the topic in order to raise awareness on the current state of quality of clinical laboratory services throughout Costa Rica.
3. Methods
3.1 Experimental design
The current research was carried out following a quantitative approach with a descriptive scope. The study design is cross-sectional, given that secondary data on clinical laboratory registration and accreditation status was analyzed for a single time point (January 2021). Since the data consisted of secondary, anonymous, aggregate and public statistics, consent from individual organizations or a confidentiality commitment form was not necessary.
3.2 Population
The study unit for the present investigation was the clinical laboratory. Data was analyzed at a population level, without recurring to any sampling methods, given that the total number of clinical laboratories in Costa Rica was taken into account. Laboratories included were those with an operation permit by the CMQC and categorized as either Clinical Laboratory or Clinical Laboratory and Blood Bank.
Facilities whose permit category was beyond a clinical scope were excluded from the study. The following are examples of excluded settings: commercial establishments (laboratory reagent and equipment vendors), food and water microbiology laboratories, specialized laboratories, biohazardous waste management facilities, breast milk banks and transfusional units.
Regarding accreditation status, only clinical laboratories accredited as per the INTE/ISO 15189:2014 standard were included. ISO 17025-accredited or ISO 9001-certified institutions or organizations accredited by any other program were excluded from the analysis.
3.3 Data collection techniques
Data regarding clinical laboratory registration and ISO 15189 accreditation in Costa Rica were gathered from two official sources: the CMQC, which holds the database of all registered clinical laboratories allowed to operate in the country, and the list of accredited laboratories by the Costa Rican Accreditation Body (Ente Costarricense de Acreditación, ECA).
3.4 Data analysis
The aforementioned information was arranged by province -Costa Ricas main administrative divisions- and later utilized to calculate normalized indicators on the basis of population and territorial area. Reference data for these two parameters was obtained from the Costa Rican Census and Statistics Institute (Instituto Nacional de Estadística y Censos, (INEC], 2011). Such indicators were calculated by dividing the number of registered (or accredited) laboratories within a province by the respective population or area. To facilitate interpretation, indicators are presented as laboratories/100 000 population and laboratories/100 km2.
Furthermore, national averages were calculated for each indicator alongside with range and standard deviation as measures of dispersion. Descriptive comparisons were made between provinces and with respect to national values.
4. Results and discussion
According to the CMQC database, a total of 480 clinical laboratories and blood banks are properly registered to operate in Costa Rica. The distribution of these laboratories by province and whether they belong to the public or private sector, as well as their ISO 15189 accreditation status, are shown in Figura 1.
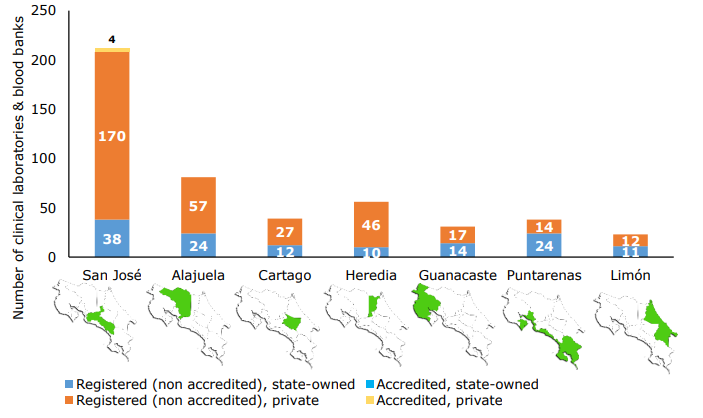
Sources: (CMQC, 2020; ECA, 2020).
Figura 1 Number of clinical laboratories and blood banks in each province of Costa Rica (presented in administrative order), by sector and accreditation status (N=480).
As it can be seen, private facilities outnumber the public clinical laboratories in almost all provinces with the exception of Puntarenas. The provinces with the highest overall count of laboratories are San José, Alajuela, Heredia and Cartago; whereas Guanacaste, Puntarenas and Limón hold the most modest numbers.
It should be noted that clinical laboratories with ISO 15189 accreditation account for less than 1,0% of the overall number of registered facilities. Two of them belong to the private sector, one to a public institution (University of Costa Rica) and the other one operates as a health cooperative (co-op) model. All of them are located in the province of San José. It should be noted, however, that all of them offer their services through laboratories in two or more locations, but only the main or central laboratory is presented in the accreditation certificate, with the exception of the Clinical Laboratory of the University of Costa Rica, which features both of its locations in the certificate (ECA, 2020).
Moreover, two of the accredited entities -ServiSalud and Hospital Clínica Bíblica- provide outsourcing services at the primary care level to the CCSS in several communities of the province of San Jose (Hospital Clínica Bíblica, 2017; ECA, 2013). Additionally, accredited laboratory of the University of Costa Rica operates as a hospital laboratory and blood bank for the National Insurance Institute in a trauma-specialized hospital (ECA, 2012). Therefore, their accredited services might reach a broader population and would not be limited to those who can afford private medical care.
In order to normalize the number of laboratories according to indirect demand indicators, population and territorial density ratios were calculated. These normalized ratios, calculated for all registered clinical laboratories as well as for accredited laboratories, are presented in Table 1.
Table 1 Laboratory accreditation density indicators in Costa Rica, by province.
| Administrative Division | Population | Territory(km2) | Clinical laboratories & blood banks | |||||||
| Registered(3] | Accredited | |||||||||
| per 100 000 population | per 100 km2 | per 100 000 population | per 100 km2 | |||||||
| Public | Private | Total | Public | Private | Total | |||||
| San José | 1 661 547 | 4 965,9 | 2,3 | 10,5 | 12,8 | 0,8 | 3,5 | 4,3 | 0,2 | 0,1 |
| Alajuela | 1 029 568 | 9 757,5 | 2,3 | 5,5 | 7,9 | 0,2 | 0,6 | 0,8 | 0,0 | 0,0 |
| Cartago | 541 259 | 3 124,7 | 2,2 | 5,0 | 7,2 | 0,4 | 0,9 | 1,2 | 0,0 | 0,0 |
| Heredia | 526 092 | 2 657,0 | 1,9 | 8,7 | 10,6 | 0,4 | 1,7 | 2,1 | 0,0 | 0,0 |
| Guanacaste | 393 893 | 10 140,7 | 3,6 | 4,3 | 7,9 | 0,1 | 0,2 | 0,3 | 0,0 | 0,0 |
| Puntarenas | 498 779 | 11 265,7 | 4,8 | 2,8 | 7,6 | 0,2 | 0,1 | 0,3 | 0,0 | 0,0 |
| Limón | 460 083 | 9 188,5 | 2,4 | 2,6 | 5,0 | 0,1 | 0,1 | 0,2 | 0,0 | 0,0 |
| Costa Rica(4] | 5 111 221 | 51 100,0 | 2,6 (1,0) | 6,8 (3,0) | 9,4 (2,5) | 0,3 (0,2) | 0,7 (1,2) | 0,9 (1,4) | 0,1 (0,1) | 0,01 (0,04) |
Source: Own creation based on data from INEC (2011), INEC (2015), CMQC (2020) and ECA (2020)
Population-normalized indicators in Table 1 follow a pattern similar to that of the absolute numbers: the number of private clinical laboratories registered per 100 000 population is higher than that of public ones across all provinces, except for Puntarenas. When comparing between provinces, those with the most clinical laboratories (public and private combined) per 100 000 population are San José and Heredia, while Alajuela, Guanacaste, Puntarenas, Cartago and Limón rank below the national average.
However, if disaggregated by sector, the pattern for the public sector is quite different. In this case, the provinces of Puntarenas and Guanacaste lead the rank with ratios of clinical laboratories per 100 000 population well above the national average, while the rest of the provinces hold very homogeneous below-the-average values.
When assessing territory-normalized ratios, the combined sum of public and private laboratories per 100 km2 follows the same patterns evidenced with absolute numbers in Figure 1: the highest values concentrate in San José, Heredia, Cartago and Alajuela. The pattern remains the same for public and private sector laboratories analyzed individually.
With respect to ISO 15189-accredited laboratories, normalized ratios were only calculated for the private sector since there are no public clinical laboratories accredited to date. The five accredited facilities concentrated in San José yield a ratio of 0,3 laboratories/100 000 population and 0,1 laboratories/100 km2.
4.1 Discussion
As mentioned before, equal access to health services of high quality is a goal to which Costa Rica has committed through the SDGs. However, the scenario pictured in this researchs results might indicate that considerable challenges are still ahead.
For instance, the first set of data to be highlighted is the number of clinical laboratories accredited nationwide. Only four clinical laboratories have been accredited in compliance with INTE/ISO 15189:2014 (the current version of the international standard for Costa Rica). All of these laboratories are located in the province of San José (Figure 1). From a descriptive point of view, this translates into a low proportion of accredited establishments (approximately 0,8%) relative to the total, which, at the same time, are geographically concentrated in a single province.
This does not necessarily mean that the rest of (non-accredited) laboratories are unable to provide health care of quality, specifically services related to clinical laboratory analyses. However, it does imply that the large majority of clinical laboratories in Costa Rica have not yet demonstrated their competence for performing clinical examinations or assays, in accordance to international standards.
In fact, the scope of the 15189 standard is defined as follows: This International Standard can be used by medical laboratories in developing their quality management systems and assessing their own competence. It can also be used for confirming or recognizing the competence of medical laboratories by laboratory customers, regulating authorities and accreditation bodies (ISO, 2012). Hence, this reaffirms its importance as an objective and internationally recognized parameter for assessing quality in a specific healthcare setting, either by patients or health authorities.
Being aware of the standards aforementioned relevance, Carboni-Huerta and Sáenz-Flor (2019) carried out a survey on laboratory accreditation throughout Latin America some time ago. Our results for Costa Rica are in line with their findings for the whole region: the number of ISO 15189-accredited laboratories remains low, and it is even lower among public (state-owned) laboratories. When inquired about the reasons for their non-accredited status, most of the surveyed laboratories in the region agreed on two major factors: that laboratories current conditions do not comply with the standards requirements and that potential benefits of accreditation do not outweigh the costs (Carboni-Huerta & Sáenz-Flor, 2019).
In the face of the current situation of only a small number of laboratories being accredited in Costa Rica, we conducted an additional analysis including all settings registered before the CMQC as clinical laboratories and blood banks (entities registered exclusively as blood banking facilities were excluded). Although not as drastically evident as the accreditation analysis, Table 1 reflects that density of laboratories -both by population and by territory extension- can vary between provinces.
With some exceptions (such as Puntarenas high ratio of public/state-owned laboratories per 100 000 population), an overall trend can be observed among most registered clinical laboratories: the density of these facilities is higher in central provinces of the country. This can be seen specially San José and Heredia, where there are between 10,6 and 12,8 laboratories per 100 000 population and the ratio per 100 km2 of territory oscillates around 2,1 and 4,3. On the contrary, coastal provinces such as Limón and Puntarenas exhibit lower laboratory density ratios.
Even though inferring disparity or inequity in access to health care goes beyond the scope of the methodology employed in this research, it is worth providing some context from previous literature. For example, a 2004 study by Rosero-Bixby (the only one available on the topic) demonstrated that, even after the implementation of the health sector reform (which is started in the late 20th century), Costa Rican provinces of Guanacaste, Puntarenas and Limón had the highest proportion of population with poor access to health services in general (Rosero-Bixby, 2004).
Adding to this, the importance of quality clinical laboratory services for public health has been acknowledged for years. A multilateral regional precedent was set by African countries with the Maputo Declaration which, among other statements, included the governments recognition of the need to expand and further develop quality-assured laboratory services as part of a greater framework of health system strengthening within resource-limited settings (WHO – Regional Office for Africa, 2008, p. 1).
According to such statement, low-, middle- and mid-high-income countries should invest in quality assurance for clinical laboratories in order to help driving the whole health systems advancement in terms of quality of care. To this, we would add that the demonstration of competence in delivering high quality services (i.e. accreditation) should be set as a priority as well. Along this line of thought, the remaining ~99% of laboratories registered in Costa Rica face an important challenge ahead.
As a final point in this discussion, it should be highlighted that this study faced some limitations regarding the level of granularity of publicly available information. Future research should inquire about differences in laboratory density at the administrative sub-division level (canton or district). Moreover, analyzing the degree of progress of laboratories currently seeking accreditation could also be a valuable contribution that would allow for a better understanding of the landscape of quality in clinical laboratory services in Costa Rica.
Lastly, findings presented here are descriptive in nature. Subsequent studies should consider the possibility of addressing the topic of disparities and/or inequity in access to laboratory health services with appropriate methodological designs that allow for a more in-depth analysis.
5. Conclusions
In conclusion, by exploring the distribution of ISO 15189-accredited as well as registered/non-accredited laboratories in Costa Rica, we were able to identify geographic disparities in the access to clinical laboratory services. This should raise awareness among public health authorities and private healthcare providers regarding the countrys progress in attaining its quality healthcare for all commitment derived from the SDGs.















