1. Introduction
The chikungunya fever is a viral disease, isolated for the first time in Tanzania in 1952, characterized by intense arthralgia (Ang et al., 2017). The transmission of the virus involves non-human primate hosts and vector mosquitoes of the genus Aedes aegypti and Aedes albopictus (Wilder-Smith et al., 2017).
The chikungunya virus (CHIKV) belongs to the genus Alphavirus, and features four genetically distinct strains: West African (WAf), Eastern, Central, and Southern African (ECSA), Asian and Indian Ocean lineage (IOL) (Volk et al., 2010; Weaver & Forrester, 2015). This virus emerged in East Africa in 2005, through an urban epidemic in popular tourist destinations by infected travelers. In 2006, it was imported to the western hemisphere (including the United States) and Europe, causing an epidemic in Italy (Volk et al., 2010; Wahid, Ali, Rafique, & Idrees, 2017).
In September 2014, the first autochthonous case of CHIKV was confirmed in Brazil, with other subsequent cases in the Northeast region of the country (Ministério da Saúde, 2018; T. M. L. Souza et al., 2019). The following year, in Mato Grosso, of the 141 municipalities, 17 were at risk of epidemic, 44 were in a state of alert and 35 had no risk of epidemic (Secretaria de Estado de Saúde de Mato Grosso [SESMT], 2015).
In Brazil, in 2018, the incidence of chikungunya fever was 42.1 cases per 100 thousand inhabitants (Ministério da Saúde, 2019). From its entry in Mato Grosso until the year 2018, the incidence rates of probable cases gradually increased. At the end of the year 2019, 87.687 probable cases of chikungunya fever were notified in the country. Mato Grosso occupied the first place in the country with an incidence of 387.6 probable cases of chikungunya fever for every 100 thousand inhabitants (Ministério da Saúde, 2018, 2019). The municipality of Tangará da Serra, in this same year, presented incidence rate of confirmed cases of chikungunya fever of 54 for every 100 thousand inhabitants (Secretaria Municipal de Saúde de Tangará da Serra [SMSTS], 2020).
After the confirmation of the first cases of chikungunya fever, researchers discussed the possibility of dissemination and establishment of the disease in Brazil (Honório, Câmara, Calvet, & Brasil, 2015), since it is one of the arbovirus fever in which the vector is widely disseminated in the country and that, in addition to causing prolongation of symptoms for several weeks due to subacute forms developed by the disease, also has an association with precarious socioeconomic and sanitary factors of the country (Balasubramaniam, Krishnakumar, Stephen, Gaur, & Appavoo, 2011; Nunes, Faria, Vasconcelos, Golding, & Kraemer, 2015).
The economic development does not protect countries from diseases transmitted by vectors; and modern lifestyles can contribute to expansion of epidemics, through travel, solid waste production and generation of vector breeding sites, such as Aedes (Honório et al., 2015). Urban population growth in cities, as well as in Brazil, with precarious infrastructure, began to generate inadequate housing conditions, hindering the supply of basic services that may lead to the occurrence of communicable diseases, such as chikungunya fever (Westphal & Oliveira, 2015).
In this way, considering the high burden brought by chikungunya fever to both the society and citizens individually, the objective of this study was to characterize, through epidemiological survey, the profile of chikungunya infection in the population of a mid-sized municipality in Mato Grosso according to sociodemographic and sanitary factors.
This study was funded by the Ministry of Health of Brazil from resources provided for the matrix project “The natural history of the Zika virus epidemic in a Brazilian community: incidence in the general population, pregnant women, congenital anomalies in newborns and consequences for child development ”, of which the present study is part.
2. Theoretical reference
The chikungunya virus belongs to the genus Alphavirus of the Togaviridae family. The alphavirus vírions are contained in icosahedral particles of approximately 70 nanometer (nm) in diameter, the particle nucleus is 40 nm and consists of 240 copies of capsid, C or CP, arranged in a symmetry of T4 around the genomic RNA (Powers, Huang, Roehrig, Strauss, & Weaver, 2011).
CHIKV is maintained in a wild cycle in West and Central Africa involving non-human wild primates and Aedes spp. mosquitoes, as the virus has been isolated in wild mosquito species in several countries, including Senegal, Ivory Coast, Central African Republic, and South Africa (Powers & Logue, 2007).
Chronologically, the Kenyan epidemic was the first to be identified in the succession of chikunugnya fever epidemics that later affected the islands of the Southwest Indian Ocean in 2005-2006 and the Indian subcontinent in 2006. The CHIKV epidemic reached the Comoros Islands in December 2004, and the peak of the epidemic started in January 2005 and ended in March 2005. Of the three islands that comprise the Federal Islamic Republic of the Comoros, the most affected island was Grande Comoro, followed by Moheli and Anjouan (Sergon et al., 2007).
The emergence of CHIKV in the Americas was announced in December 2013, when France's National Reference Center for Arbovirus diagnosed the first local cases of Chikungunya in San Martin, a French-Dutch island located in North America (Cassadou et al., 2014). Since the first reported case in San Martin, native CHIKV transmission has been identified in 50 countries including the Caribbean, North America, South America and Central America (PAHO, 2011).
Brazil confirmed CHIKV autochthonous cases in 2014, simultaneously in two regions of the country, in the North, in Oiapoque, a municipality in the state of Amapá, and in the Northeast, in Feira de Santana and Riachão do Jacuípe in the state of Bahia. The cases found belonged to two different lineages of CHIKV, Asian and ECSA (Cunha et al., 2017; Nunes, Faria, Vasconcelos, Golding, & Kraemer, 2015; Teixeira, Andrade, Costa, Castro, Jesuína, & Oliveira, 2015). Data from epidemiological investigations suggested that the first case patient of chikungunya fever in Brazil was a Brazilian citizen living in Luanda, Angola, who visited his family in the municipality of Feira de Santana, in the state of Bahia, in 2014, but a recent study conducted in Rio de Janeiro with 1,835 individuals suggests that the virus has been circulating in Brazil since 2013, one year before it was detected by surveillance programs (Souza et al., 2019; Teixeira et al., 2015).
Epidemiological Bulletin No. 20 of 2014, which contained chikungunya fever monitoring through Epidemiological Week (SE) 36 of 2014 (the first to report confirmed cases of chikunugnya fever), recorded 36 confirmed cases of the disease, all of which were imported (Ministry of Health, 2014). According to the Monitoring of cases of dengue and chikungunya fever through SE 53 of 2014, 3,195 indigenous suspected cases of chikungunya fever had been reported, where 2,196 were confirmed (Ministry of Health, 2015). From the first confirmed cases in 2014 to the last confirmed cases in 2019 by the Epidemiological Bulletin, there has been an exponential increase of chikungunya fever cases in Brazil, with decreases between 2015 and 2017 and a further increase between 2018 and 2019 (Ministry of Health, 2016, 2017, 2018, 2019, 2020).
In this study, the cluster sampling method was used. The choice of this method was due to the design chosen for the study, where the sampling by clusters analyzes a natural grouping of elements of the population, which are quite heterogeneous internally in relation to the studied characteristic, but with similar behavior between the conglomerates. The cluster sampling method is valuable when a population is very dispersed and it is practically impossible to have a list of all those who are part of the population (Medronho, Block, Luiz & Werneck, 2008).
3. Materials and methods
3.1 Focus
This is an epidemiological study, with cross-sectional, quantitative, descriptive design, of the epidemiological-survey type, conducted in the urban population of a municipality located in mid-north Mato Grosso. Data collection occurred between January and March 2018, being conducted by a previously trained team, with the accomplishment of a pilot study in a census tract that was not included in the final sample.
3.2 Study population
Individuals included in the study were adults, aged 18 years or more, who lived in the urban area of the municipality of Tangará da Serra before 1 April 2016 and remained as residents until the data collection in 2018. Women of childbearing age and pregnant women were also included. Individuals aged under 18 years old, and all those institutionalized, i.e., living in prisons and long-term institutions were excluded. Thus, of the 660 individuals randomly chosen, just 596 constituted the final sample of the study due to losses caused by rejection and absence. The individuals were selected by simple random sampling among the inhabitants of the selected household and data collection started after their acceptance to participate in the study and signing of the Informed Consent Form ICF. The confidentiality of information provided by each participant was assured, emphasizing that all results were delivered to the individuals and those with confirmed presence of the disease were treated by the municipal health team, duly notified and included into a flow of research and clinical management of chikungunya.
3.3 Collection techniques:
The process of cluster sampling was performed in two stages (census tracts and households). Initially, there was the recognition of the tracts for identification of households, through beating (tract analysis, through the verification of the presence of households, businesses, vacant lots and others) as shown in Figure 1. From this updated listing, a proportional number of households was selected systematically and in intervals in each census tracts sampled. To estimate the prevalence of CHIKV serology in the population, with a margin of error of 5 percentage points, a sample of 660 individuals was necessary. Thus, the standard error adopted was of 0.025 for a sample of 400 individuals, adding to this number 10% of loss more inflation of 1.5, reaching the total of 660 individuals.
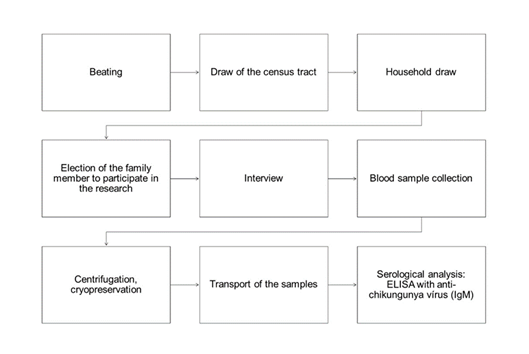
Source: Own elaboration, 2020
Figure 1 Sample selection of the epidemiological survey of chikungunya fever, Tangará da Serra MT, Brazil, 2018
The data collection was performed in the household, with an approximate duration of 40 minutes. The interview consisted of the application of a structured questionnaire composed of questions that addressed sociodemographic, sanitary and health factors.
3.4 Analysis processing:
The dependent variable in this study were confirmed (positive) cases of chikungunya fever by enzyme immunoassay. The independent variables analyzed were sex, age, schooling, race/color, work in the last 12 months, marital status, type of household, paving of the street, main form of water supply, water availability by general network, destination of garbage, use of mosquito nets, travel to another state, visit of agents of endemics, presence of larvae, presence of mosquitoes breeding, application of product in tanks or other containers and fogging truck.
For the serological analysis, the peripheral venipuncture was performed with Vacutainer blood collection tube with a separator gel. The samples were subsequently sent to the laboratory of the department of Epidemiological Surveillance of the Health Municipal Secretariat of Tangará da Serra. They were centrifuged, cryopreserved and transported to the Laboratory of Virology of the Medical School of the Federal University of Mato Grosso (VL/MF/FUMT) in Cuiabá - MT for their maintenance in ultrafreezer (-80°C).
The serological analysis was performed at the Central Laboratory of Public Health of Mato Grosso (CLPH-MT) in Cuiabá - MT, according to the standards of biosafety through immunoassay (Enzyme-Linked Immunosorbent Assay - ELISA) with the anti-chikungunya virus ELISA kit (IgM) - EUROIMMUN®, making: 171108BC.
The answers of the interviews and the laboratory results were doubly entered using a form built on the EpiInfo 7 software. The inconsistencies were subsequently verified using the Excel 365® software, constituting, at the end of these steps, the final database for analysis. For data processing, the IBM software Statistical Package for the Social Sciences - SPSS 20.0 was used. The analyses used descriptive and inferential statistical techniques. The descriptive analyses used proportions and tables; the inferential analyses used chi-square tests, Fisher's exact test and crude prevalence ratios, with their respective 95% confidence intervals. For all the inferences, the significance level was of 5%.
This study integrates a matrix project and was approved by the Research Ethics Committee of the Clinical Hospital of Porto Alegre, under opinion n. 2.068.222.
4. Results
The sociodemographic profile of the study participants was mainly composed mainly of women (67.8%), aged 18 - 39 years (45.5%), with eight years or more of study (58.4%), non-white reported race/color (67.2%) and not living with companion (51.1%). The distribution of positive cases of CHIKV in the study population, according to Table 1, remains with a predominance of females (9.6%). The age changes, with the majority of participants between 18 and 39 years (28.2%), with more than eight years of study (10.2%), non-white reported ethnic/color (8.6%), not living with a companion (11.3%) and employed in the last 12 months (8.4%). The schooling, color and employment showed no statistically significant relation of CHIKV infection, being the highest prevalence found among individuals with schooling > 8 years (10.2%), non-white color (8.6%) and individuals employed in the last 12 months prior to the survey (8.4%).
Table 1 Prevalence and 95% confidence interval of positive and negative CHIKV (IgM) cases, according to demographic variables of the epidemiological survey, Tangará da Serra - MT. Brazil. 2018.
| Variable | Positive | Negative | cPR | 95% CI | P-value |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Sex | |||||
| Male | 11 (6.3%) | 164 (93.7%) | 0.65 | (0.34 ; 1.27) | 0.204 |
| Female | 31 (9.6%) | 292 (90.4%) | 1.00 | - | - |
| Age range (years) | |||||
| 18 - 39 | 53 (28.2%) | 135 (71.8%) | 0.92 | (0.50 ; 1.67) | 0.78 |
| 40 - 59 | 66 (25.0%) | 198 (75.0%) | 1.13 | (0.83 ; 1.54) | 0.451 |
| ≥ 60 | 14 (12.0%) | 103 (88.0%) | 1.00 | - | - |
| Education (years of study) | |||||
| Illiterate/≤ 8 | 13 (5.9%) | 209 (94.1%) | 0.57 | (0.30 ; 1.08) | 0.079 |
| > 8 | 28 (10.2%) | 246 (89.8%) | 1.00 | - | - |
| Ethnicity/color | |||||
| Non White | 28(8.6%) | 297 (91.4%) | 1.05 | (0.57 ; 1.94) | 0.871 |
| White | 14 (8.2%) | 157 (91.8%) | 1.00 | - | - |
| Residing with companion | |||||
| No | 28 (11.3%) | 219 (88.7%) | 1.97 | (1.06 ; 3.64) | 0.028 |
| Yes | 14 (5.8%) | 229 (94.2%) | 1.00 | - | - |
| Employment in the past 12 months | |||||
| No | 15 (8.2%) | 169 (91.8%) | 0.97 | (0.53 ; 1.78) | 0.927 |
| Yes | 26 (8.4%) | 284 (91.6%) | 1.00 | - | - |
n - sample size by variable, cPR - Crude prevalence ratio, 95% CI - 95% confidence interval, p - Chi-square test.
Source: Own elaboration, 2020
Not residing with affective companion was statistically significant with the CHIKV infection (cPR: 1.97; 95% CI: 1.06-3.64; p<0.028). Studies (Richmond & Roehner, 2017; Verbrugge, 1979) indicate that the marital status has a strong impact on health, since the influence on the abilities of the human being until changes in mortality rates, which are worst among singles than married. Living with affective partners reduces the vulnerability to chronic diseases, in addition to being more conducive to developing fewer incapacitating injuries than singles. A possible explanation for the best health status of married people would be the stability, greater access to health and social networks arising from the union.
The profile of seroprevalence of infection by CHIKV according to social-sanitary characteristics (Table 2) was constituted by individuals who lived in apartments (10.0%), paved streets (8.8%), with no other form of water supply besides the general distribution network (8.6%), with frequent water availability by general network (9.1%) and other garbage destination than the direct collection (9.1%). Of the participants positive for CHIKV, 10.3% reported not using mosquito net, 8.5% had travelled to another state, 10.0% received the visit of an agent of endemic only once, 10.8% found the mosquito larvae at home, in the house of 13.3%, there were breeding areas for the mosquito, 26.2% had not received application of product in tanks or other containers, 9.5% had no fogging trucks.
Table 2 Prevalence and 95% confidence interval of positive and negative CHIKV (IgM) cases, according to demographic and sanitary variables of the epidemiological survey. Tangará da Serra - MT. Brazil. 2018.
| Variable | Positive | Negative | cPR | 95% CI | P-value |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Type of housing | |||||
| House | 41 (8.5%) | 442 (91.5%) | 0.85 | (0.13 ; 5.58) | 0.593* |
| Apartment | 1 (10.0%) | 9 (90.0%) | 1 | - | - |
| Paved street | |||||
| No | 2 (6.3%) | 30 (93.8%) | 0.71 | (0.18 ; 2.79) | 0.616* |
| Yes | 40 (8.8%) | 413 (91.2%) | 1 | - | - |
| Main type of water supply | |||||
| Other | 3 (7.0%) | 40 (93.0%) | 0.81 | (0.26 ; 2.50) | 1.000* |
| General network | 39 (8.6%) | 412 (91.4%) | 1 | - | - |
| Water availability by general network | |||||
| Alternate | 3 (4.5%) | 63 (95.5%) | 0.5 | (0.16 ; 1.57) | 0.217 |
| Frequent | 39 (9.1%) | 390 (90.9%) | 1 | - | - |
| Garbage destination | |||||
| Other | 3 (9.1%) | 30 (90.9%) | 1.08 | (0.35 ; 3.32) | 0.751 |
| Direct collection | 39 (8.4%) | 426 (91.6%) | 1 | - | - |
| Use of mosquito net | |||||
| No | 38 (8.3%) | 420 (91.7%) | 0.81 | (0.30 ; 2.15) | 0.56 |
| Yes | 4 (10.3%) | 35 (89.7%) | 1 | - | - |
| Travel to another state | |||||
| Yes | 25 (8.5%) | 268 (91.5%) | 1.04 | (0.57 ; 1.91) | 0.885 |
| No | 16 (8.2%) | 180 (91.8%) | 1 | - | - |
| Visit of agent of endemics | |||||
| 2 - 4 months | 12 (8.3%) | 132 (91.7%) | 1.09 | (0.50 ; 2.41) | 0.814 |
| Once | 6 (10.0%) | 54 (90.0%) | 1.32 | (0.51 ; 3.40) | 0.569 |
| Cannot remember/unknown | 12 (8.3%) | 133 (91.7%) | 1.09 | (0.49 ; 2.39) | 0.828 |
| Monthly | 11 (7.6%) | 134 (92.4%) | 1 | - | - |
| Presence of larvae | |||||
| Yes | 7 (10.8%) | 58 (89.2%) | 1.59 | (0.69 ; 3.67) | 0.278* |
| No | 17 (6.8%) | 234 (93.2%) | 1 | - | - |
| Cannot remember/not applicable | 14(9.2%) | 138 (90.8%) | 1.36 | (0.69 ; 2.68) | 0.373 |
| Presence of mosquito breedings | |||||
| Yes | 6 (13.3%) | 39 (86.7%) | 1.81 | (0.77 ; 4.22) | 0.235* |
| No | 22 (7.4%) | 277 (92.6%) | 1 | - | - |
| Unknown/not applicable | 14 (9.2%) | 139 (90.8%) | 1.24 | (0.65 ; 2.36) | 0.505 |
| Application of product in tanks or other containers | |||||
| Yes | 4 (6.3%) | 59 (93.7%) | 1 | - | - |
| No | 83 (26.2%) | 234 (73.8%) | 1.45 | (0.52 ; 4.01) | 0.47 |
| Unknown/not applicable | 13 (8.7%) | 137 (91.3%) | 1.36 | (0.46 ; 4.02) | 0.569 |
| Fogging truck | |||||
| No | 25 (9.5%) | 239 (90.5%) | 1.86 | (0.89 ; 3.89) | 0.091 |
| Yes | 9 (5.1%) | 168 (94.9%) | 1 | - | - |
n - sample size by variable, cPR - Crude prevalence ratio, 95% CI - 95% confidence interval, p - Chi-square test, *: Fisher's exact test.
Source: Own elaboration, 2020
In relation to health factors observed in the investigation, there was no statistically significant association between the conditions in which the residents are exposed and the acquisition of chikungunya fever. The results indicated that the protective factors against chikungunya fever are residing in first-floor house (cPR: 0.85; 95% CI: 0.13-5.58), non-paved street (cPR: 0.71; 95% CI: 0.18-2.79), using water from another source than the general distribution network (cPR: 0.81; 95% CI: 0.26-2.50), water availability in the household on alternate days by the distribution network (cPR: 0.50; 95% CI: 0.16-1.57) and using mosquito net (cPR: 0.81; 95% CI: 0.30-2.15), but not statistically significant.
The results found may have relation with the level of awareness of the population about the practices of control and protection against A. aegypti and A. albopictus mosquitos, urban vectors responsible for the dissemination and infection of CHIKV. The analysis shows that receiving the visit of agents of endemics once (cPR: 1.32; 95% CI: 0.51-3.40), having larvae (cPR: 1.59; 95% CI: 0.69-3.67) and breeding of mosquitos at home (cPR: 1.81; 95% CI: 0.77-4.22), in addition to not receiving application of products in tanks and containers (cPR: 1.45; 95% CI: 0.52-4.01) are risk factors that culminated in the highest prevalence of acquisition of chikungunya fever in the results above, but without statistical significance.
5. Discussion
The study found a prevalence of chikungunya fever of 8.4%, lower than those found in previous studies with similar populations. In Anagaputhur and Kerala, in India, the prevalence of chikungunya fever was found to be 22.3% and 68%, respectively (Balasubramaniam et al., 2011; Kumar et al., 2011). The study conducted in Managua, Nicaragua, presented a result close to that found in Tangará da Serra, with 13.1% (Kuan et al., 2016).
The different prevalence found in the studies may result from the variability of factors to which a community is exposed, the complex set of sociocultural and urban structural factors can promote and hinder the spread of a virus (Fritzell et al., 2018; L. S. Souza & Barata, 2012). Another possibility is that, in spite of studies being conducted in communities, with adults and seeking to verify positivity for the CHIKV, the collection of samples occurred in different periods of the year. In Brazil, in a rural community, there was a prevalence of 20% of chikungunya fever, but comparisons with the study of Tangará da Serra would not be applicable, since the latter had been carried out in the urban area (Cunha et al., 2017).
In the opposite direction of the results presented in other seroepidemiologic studies where prevalence is higher in male subjects (Ang et al., 2017; Kumar et al., 2011; Moro et al., 2010), the female population presented higher prevalence of the disease (9.6%), a result similar to that found in studies performed in Coimbatore in India and in the Coast Province in Kenya (Labeaud et al., 2015; Mudurangaplar & Peerapur, 2015). Even though the male sex is pointed out as the most affected by chikungunya fever (Fritzell et al., 2018), the female sex composes the epidemiological profile of individuals who suffer with the post-disease chronicity post. In studies conducted to verify the manifestations of the disease and post-infection evolution, women showed more reports of arthralgia, chronic edema and headache (Kohler, Azevedo, Lima, Marinho, & Souza, 2018; Zingman, Paulino, & Payano, 2017). In Tangará da Serra, male sex was a protective factor against illness by chikungunya fever (cPR 0.65; 95% CI 0.34;1.27).
There were no large variations of positive cases when analyzed by age, individuals aged from 18 to 39 years showed the highest number of positive cases (28.2%). Even though the lowest prevalence has occurred among individuals aged ≥ 60 years (12%), studies have called attention to a higher risk of post-disease chronicity, incidence of atypical cases, severe cases and mortality proportional to aging. There is also a greater mortality by chikungunya among people who already showed some comorbidity preceding the CHIKV infection (Dramé et al., 2018; Kohler et al., 2018; Zingman et al., 2017).
Health is defined as a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity, and may be determined by the simultaneous action of factors, whether individual factors, such physical and mental conditions, or, among other factors, the social one, such as precarious urban environment or the lack of basic services (Aith & Scalco, 2015). Frequently, poverty, inadequate housing, unhealthy environments, environmental pollution, unemployment, increasing traffic of vehicles, high demographic density and occurrence of vector-borne diseases (VBDs) are associated with the fast and usually unplanned growth of urban centers, which affects the response of services and generates insufficient provision of sanitation, education and health suitable for the population (Neiderud, 2015).
For the urban health, characterized by the impact on health caused by the interventions of the public sector in cities, observing and analyzing the numbers of basic services offered to the population is of extreme importance, since infectious diseases, such as chikungunya, are strongly associated with the provision of sanitation services (Instituto Brasileiro de Geografia e Estatística [IBGE], 2018).
The inadequate distribution of water leads people to store it in containers, the accumulation of garbage in the homes and streets by inefficient sanitation policy or even the accumulation of rainwater due to irregular trash collecting system increases the risk for the occurrence of endemics and epidemics by infectious diseases such as dengue, zika and chikungunya. The provision of adequate sanitation, for example, could contribute significantly to public health, but due to the cost with the management and improvement of infrastructure, the public management choses to deal with the diseases rather than eliminating them (IBGE, 2018; Neiderud, 2015).
As well as the Centers for Disease Control and Prevention (CDC) of the United States of America and the European Center for Disease Prevention and Control (ECDC) are seeking to raise awareness in the population of ways to prevent and control the virus and the disease, Brazil has also sought ways to keep the population aware of the chikungunya fever (Centers for Disease Control and Prevention [CDC], 2017; European Center for Disease Prevention and Control [EDCD], 2014). In Brazil, as soon as the virus was detected in circulation, the Ministry of Health launched strategies for prevention and control of vectors as tools that contribute to rapid notification of cases of diseases, helping the contingency of the fever in the country. The Ministry of Health released the National Contingency Plan for the chikungunya fever and booklets of clinical management of the disease, the chikungunya fever was inserted in the Disease Notification Information System (DNIS) in 2016, as well as control and prevention campaigns against the vectors Aedes aegypti and A. albopictus were strengthened with emphasis to the transmission of CHIKV (Ministério da Saúde, 2014, 2016). Even though the health surveillance, from all the tools employed in the control and awareness of the disease, has responded well in the middle of the zika-dengue simultaneous movement in this country, ongoing assessments of the strategies employed are important for the long-term planning of the health system, aiming to avoid the high burden that the disease can generate (Silva et al., 2018).
6. Cconclusion
The profile of infection by chikungunya fever consists of female individuals, aged between 18 and 39 years, with at least complete basic education, non-white reported color, not residing with a companion and employed in the last 12 months. The overall prevalence of chikungunya fever found in the community was 8.4%.
The study found that women are the most affected by the infection, as well as being single represented a risk factor, and risk behaviors, such as presence of larvae and breeding of mosquitos at home, may reflect a low level of awareness of the disease. In this way, the study was important for the knowledge of the profile of a midsized population exposed to a virus newly present in the country, but with a high representation in numbers in a state with such a territorial extension as Mato Grosso. From the findings in this study, other neighboring municipalities or with similar characteristics can enhance their strategies of health surveillance with a view to curbing the disease directed to the weak points of the population.
Despite the implementation of measures for prevention and control of the disease-transmitting vectors in Mato Grosso, it is valid to emphasize that the chikungunya fever is a disease that can produce large-sized epidemics and generate large impact on populations due to its high limiting morbidity by post-infection chronic arthralgia. Surveillance systems must be effective to maintain the tools used to curb the disease, which generally include strengthening campaigns to control the dissemination of vectors, health promotion from the population’s awareness of the disease, rapid and effective diagnosis of the disease, as well as its notification on DNIS.
The accomplishment of the study in a population with non-institutionalized individuals aged over 18 years was one of the main limitations. The non-inclusion of children and adolescents in the epidemiological profile proposed by this study prevents from knowing how the population of the city of Tangará da Serra may be immuno susceptible to CHIKV, which could hinder the construction of possible management strategies against the occurrence of the disease. The results made it possible to identify situations of risk to the health of the participants and from the knowledge acquired allows focal health surveillance actions to be implemented in order to reduce health risks, treat early and prevent further health commitments.















