Introduction
Since the 18th century, sea urchins have been used as models in scientific studies (Crespi-Abril & Rubilar, 2018; Dufossé, 1847). Recently, there has been a growing recognition of the importance of including all invertebrates, including echinoderms, under specific ethical regulations (Crespi-Abril & Rubilar, 2021). Welfare is a broad concept that provides information about the state and conditions of animals in a variety of situations (Broom, 1991; Damián & Ungerfeld, 2013). It can be measured using specific indicators, including behaviour.
Indicators are conceptual tools for quantifying dimensions and producing numerical results that can be applied to any animal phylum (Damián & Ungerfeld, 2013). This tool must meet certain criteria, including representing correlations between variables, being geographically and temporally contextualised, being simple to collect and interpret, and being based on valid scientific research (Tilbury, 2007). In the context of aquaculture, where animal welfare and health are critical to survival, developing control measures and disease prevention is critical. High crop density, poor water quality, incorrect or insufficient nutrition, incorrect temperature, and the presence of pathogens, among other factors, have been a significant source of illness and mass mortality in aquaculture, resulting in significant losses (Food and Agriculture Organization (FAO), 2016).
In echinoderms, the mechanism of righting behaviour involves a 180° turn on the axis to return to the original position, with the oral face facing the substrate and the aboral face facing the medium (Challener & McClintock, 2017) (Fig. 1). This is a simple nervous reflex action studied in sea urchins and sea stars to better understand their behaviour (Lawrence & Cowell, 1996). It appears to be essential for protection against predators and strong surges, and it is important for sea urchin fitness (Brothers & McClintock, 2015; Percy, 1973; Shi et al., 2018).

Fig. 1 Righting behaviour. A. A sea urchin with its aboral face visible in the water column. B. A sea urchin in a half-righting position. C. Sea urchin reaching the complete righting position. Modified from Binyon (1972).
Because sea urchins lack muscular tissue, they rely on their tube feet to propel them forward (Binyon, 1972). As a result, their ability to right themselves is determined by their physical condition and neuromuscular coordination (Himmelman et al., 1984). Furthermore, as mentioned by Lawrence (1975) and Challener & McClintock (2017), sea urchins with larger spines have an advantage in recovering their position, with shorter righting times compared to those with smaller spines. The speed of a sea urchin’s righting action can be used to predict its physiological activity, health, and overall state (Himmelman et al., 1984; Percy, 1973). Various factors, including diameter, salinity, acidification, light intensity, covering material, temperature, and others, can influence this behaviour (Brothers & McClintock, 2015; Dihel et al., 1979; Hamilton, 1922; Kleitman, 1941; Sun et al., 2019; Taylor et al., 2014). Furthermore, the most commonly used method for spawning sea urchins involves an invasive puncture in the peristomal membrane to inject KCl 0.5 M, but the potential stress, mortality, and effect on righting behaviour caused by this method have not been studied (Strathmann, 1987).
Furthermore, there are two methods for measuring righting behaviour in sea urchins: half righting behaviour (HRB), which involves reaching 90° of the surface, and complete righting behaviour (CRB), which involves the animals returning to their natural state, with their oral side on the substrate (Fig. 1). For a long time, these two techniques have been described and used with a variety of species, including Lytechinus variegatus (Lamarck, 1816), Strongylocentrotus purpuratus (Stimpson, 1857), and Strongylocentrotus droebachiensis (O.F. Müller, 1776) (Challener & McClintock, 2017; Sonnenholzner et al., 2010). The reasons for using one parameter over another in various studies are rarely clarified, nor is an analysis of the benefits and drawbacks of using one parameter over another.
There have been no previous studies of the righting behaviour of the sea urchin species Arbacia dufresnii (Blainville, 1825) in nature or in the laboratory. The study of this species’ righting behaviour in response to stressors can provide insights into the health and welfare of sea urchins that can be used in aquaculture systems.
Materials and methods
Animal collection and acclimatization: A total of 300 individuals of A. dufresnii were collected by scuba diving from the Golfo Nuevo, Chubut, Argentina (42.70° S & 65.60° W) during the winter of 2022. They were transferred to the experimental aquarium of the technology-based company EriSea S.A in 10 buckets of 20L with 30 animals each one, without added oxygen, in seawater of the collection site. Transportation lasted approximately 15 minutes by car from the collection site to the experimental aquarium. The sea urchins were acclimated for seven days at 9 ± 1 °C (the same temperature at which they were collected) to ensure that their digestive systems were completely empty and that they were all in the same conditions. They were kept in pond tanks of 570 L with filtered sea water at a density of 2 animals per litre, with oxygen near 8 mg/L, temperature 171, salinity 35 ppm, pH near 8, and ammonium and nitrite levels less than 1. The water was changed 3 times per week or when some parameter was destabilised.
When the animals arrived at the aquarium, they were separated by sex and age (juveniles and adults) based on their size and placed on 2 different tanks. The diameter of each animal (at the ambitus) was measured with a precision calliper and expressed in millimeters. Individuals with diameters ranging from 16 mm to 55 mm were used in the experiments. This allowed us to assess the entire diameter range of A. dufresnii in the Golfo Nuevo area, from juveniles to adults.
Pre-test definitions: The initial position was defined as the point at which the individual’s aboral face is directed toward the substrate and the oral face is directed toward the environment (Fig. 1A). HRB was defined as the point at which the individual made a 90° angle with respect to its oral plane (Pearcy, 1973) (Fig. 1B). The moment when the individual fully rested all of its tube feet on the substrate, returning to its original position with the oral face toward the substrate and the aboral face toward the environment (Fig. 1C), was defined as CRB.
CBR and HBR tests were carried out in tanks with aeration, controlled water quality parameters, and enough water volume to completely cover the animals while avoiding contact with tank walls and other individuals. All animals were handled with extreme caution to avoid damage to the tube feet, which could have an impact on the final results. Each animal was positioned with its aboral face exposed to the water column, and the time it took to perform HRB and CRB was recorded using a stopwatch (Fig. 2).

Fig. 2 Different patterns of righting and half righting behaviour. A. initial position. B. Half righting position at 70°. C. Half righting position at 90° (HRB). D. Complete righting position (CRB).
Experimental design:
Half-righting behaviour (HRB) vs. complete righting behaviour (CRB): these behaviours were tested on 100 adult and juvenile A. dufresnii specimens. To reduce individual variability in the righting starting rate, measurements of both HRB and CRB were initiated as soon as the urchin was inverted in this study.
Influence of sex and diameter: The difference between sexes and the influence of diameter were tested for CRB with different objectives. On the one hand, to see if it was necessary to separate the individuals in the experiments into females and males as separate groups, and on the other hand, to see the effect of the diameter on the CRB.
Experiment 1- Serial turnover repetitions: Three times in a row, the same animal was inverted, and the HRB and CRB times were recorded each time. In this experiment, we were able to determine the effect of fatigue after the effort required for righting behaviour. Animals were kept at 9 °C (± 1 °C) during the experiment.
Experiment 2- Thermal shock: The animals were placed in three separate temperature-controlled recirculation aquaculture systems (RAS). The animals were acclimated at 9 °C (±1 °C). The HRB and CRB were recorded after a thermal shock at 13 °C, 16 °C, and 19 °C (animals were exposed for 24 hours), and the animals were gradually returned to their original temperature after the experiment was completed.
Experiment 3- Spawning stress: A total of 30 animals were induced to spawn by injecting 0.3 mL of KCl 0.5 M through the peristomal membrane to see if this stressor affects CRB. Another group of 20 animals served as controls and received no injections. Both sets of animals’ HRB and CRB righting times were recorded 24 hours after injection.
Data analysis: The experiments and the effects of sex and diameter were analysed using Generalised Linear Models (GLM). Because each stressor effect was studied in different groups of animals, each dataset obtained was analysed using a specific model. Temperature and post-spawning effects on CRB and HRB were tested using diameter as a covariate. To investigate the effects of serial turnover repetition on HRB and CRB, a GLMM analysis was performed (Zuur, 2009). To begin, a graphical data exploration was performed to better understand the relationship between the response variable and each explanatory variable. The amount of variance explained by the model and the deviance (D2 complex that includes the interaction between the factors, indicating their dependence) were used to evaluate model fit to the data. Three criteria were used to select the best model: i) The Akaike Information Criterion (AIC), which assesses the model’s fit to the data as well as its complexity; ii) Residual analysis (observed versus estimated residual plots); and iii) The Principle of Parsimony (the simplest possible model). The AIC values are included in the results. All analyses were carried out with the help of the free software R Studio.
Results
Half righting behaviour vs. Complete righting behaviour: The righting behaviours followed a distinct pattern. Some people reached 70° and paused for a long time before continuing to 90°, from which they fell back down and completed the CRB. During the righting behaviour, three different situations were observed: 1) individuals reached 90° and quickly fell down without braking, 2) individuals reached 90°, briefly paused, and then slowly completed the righting behaviour, taking approximately the same amount of time for both phases, and 3) individuals began the movement, nearly reached 90°, and then fell back down to the initial position (aboral face to the substrate) (Fig. 2).
There was no difference in the times for righting and half-righting behaviour. Fig. 3 shows the paired data of each individual’s righting and half-righting times. The data points formed a single cluster rather than a discernible pattern, as shown in the figure. Furthermore, residual analysis revealed that the HRB model provided the poorest fit for explaining the data (Fig. 4). Table 1 displays the GLM analysis results along with the corresponding Akaike values and degrees of freedom.
Table 1
Generalised linear models and generalised linear mixed models analysis for sex and diameter and stressors variables in Arbacia dufresnii.
ANALYSIS
d.f.
Akaike
Half righting vs. complete righting behaviour
Righting
976.143
Half righting
961.737
Sex and diameter (GLM)
Null model
1077.347
Righting ~ diameter + sex
1050.731
Serial repetitions (GLMM)
Righting ~ serial repetitions
1901.6
Temperature (GLM)
Null model
257.599
Righting ~ diameter + stress
239.729
Post spawning (GLM)
Null model
417.649
Righting ~ diameter + stress
392.390
For the GLMs, in bold there are the best adjustments according to the Akaike criterion.

Fig. 3 Half righting behaviour and Complete Righting behaviour (in seconds) of each individual with different diameters (in mm) in adults of Arbacia dufresnii.

Fig. 4 Analysis of residuals of the best models for Half Righting Behaviour (A) and Complete Righting Behaviour (B) in adults of Arbacia dufresnii.
Influence of sex and diameter: The findings reflect the wide range of sizes examined in this study. Larger individuals took longer to complete CRB, with values ranging from 150 to 250 seconds in animals larger than 30mm compared to values less than 150 seconds in animals smaller than 30mm (Fig. 3). According to the GLM analysis, sex had no effect on CRB time (Fig. 5), but diameter did (Fig. 6).

Fig. 5 Arbacia dufresnii. Sex influences complete righting behaviour (CRB). Female and male righting behaviour times in seconds without stress (N = 108). There were no statistically significant differences between sexes.

Fig. 6 Arbacia dufresnii. The effect of diameter on complete righting behaviour (CRB). Juveniles and adults (N = 247) righting behaviour times in seconds.
Experiment 1: Serial turn over repetitions revealed no evidence of extenuation, with CRB times less than 100 seconds in three repetitions. The GLMM analysis revealed no statistically significant differences in time between serial repetition experiments (Fig. 7).

Fig. 7 Arbacia dufresnii The effects of serial repetitions on complete righting behaviour (CRB). Righting behaviour times in seconds.
Experiment 2: Temperature: The model that took into account both diameter and temperature provided the best fit to the data, with a lower AIC value. This suggests that diameter and temperature had separate effects on the righting response. According to the GLM analysis, the temperature shock only influenced CRB time at the higher temperature of 19 °C, increasing the time required to complete CRB to 200 seconds. The righting time remained constant at temperatures of 16 °C and 13 °C, with CRB times of less than 100 seconds (Fig. 8).

Fig. 8 Arbacia dufresnii. The effect of temperature on complete righting behaviour (CRB). Righting behaviour is measured in seconds. The 19 °C treatment was significantly different from the 13 °C and 16 °C treatments (*denotes significant differences with a P-value of 0.00955).
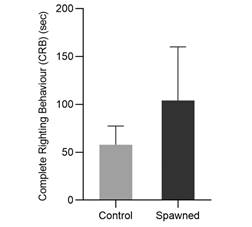
Experiment 3-Post-spawning: According to the GLM analysis, the stress caused by spawning had no significant effect on CRB. However, post-spawned sea urchins exhibited greater variability in righting time with higher CRB times, reaching 150 seconds compared to non-spawning sea urchins who reached values less than 75 seconds (Fig. 9). Furthermore, spawned individuals died at a 10 % rate, whereas non-spawned sea urchins (control group) died at 0 %.
Discussion
It is critical to have an indicator that can be determined with a small margin of error in order to accurately determine the stress levels of sea urchins. Percy (1973) proposed that sea urchins spend approximately 80 % of their time raising themselves to a 90° angle and can gradually or rapidly descend by releasing their supporting tube feet to achieve the CRB. In the current study, it was discovered that some individuals reached approximately 70° and then paused for a long period of time before continuing up to 90° and eventually reaching the Complete Righting Behaviour (CRB). Three distinct patterns of behaviour were described: 1) reaching 90° and rapidly falling without braking, 2) reaching 90°, pausing briefly, and then gradually descending while exerting the same force used to reach 90°, and 3) beginning the movement, almost reaching 90°, and then falling back to the initial position (aboral face to the substrate). These behaviours observed in A. dufresnii to reach the CRB were similar to those observed in Strongylocentrotus droebachiensis and have previously been reported in other equinoderms, particularly sea urchins (Percy, 1973; Romanes & Ewart, 1881).
HRB has been used as an indicator of stress in species as Lytechinus variegatus and Strongylocentrotus droebachiensis (Böttger et al., 2001; Brothers & McClintock, 2015; Challener & McClintock, 2017; Hagen, 2020; Percy, 1973) as well CRB in species as Strongylocentrotus fragilis (Jackson, 1912), Strongylocentrotus purpuratus, Diadema antillarum (Philippi, 1845), Echinometra lucunter (Linnaeus, 1758) and Strongylocentrotus intermedius (A. Agassiz, 1864) (Shi et al., 2018; Sherman, 2015; Sun et al., 2019; Taylor et al., 2014; Giese & Farmanfarmaian, 1963). Determining the precise position of the Half Righting Behaviour (HRB) can be difficult, especially when the animals complete the behaviour in less than a minute. Establishing a clear indicator is difficult due to the variability observed during the initial stage of this behaviour. Furthermore, determining the precise moment when an animal reaches a 90° angle is difficult and subjective because it can be influenced by factors such as observer position and water reflection. Furthermore, as observed in this study, animals may use a 70° angle rather than a 90° angle. Given that they did not move their spines or ambulacral feet, this appears to be a resting position. It is critical that the observer maintains the same position throughout the trial in order to accurately determine when the animal reaches the 90° angle. The CRB, on the other hand, provides a clear and unequivocal indicator. To calculate the CRB time, the sea urchin must restore its natural oral position by placing all of its tube feet on the ground. In the CRB, the angle or observed position is irrelevant, and there is no doubt when sea urchins have accomplished it. It would be more reliable to concentrate efforts on determining the CRB position rather than the HRB. Furthermore, there was no significant variability between measures in the current study’s treatments, indicating that this would be a reliable indicator. However, no significant differences between HRB and CRB were found in this study of A. dufresnii. Although HRB appeared to be an ambiguous and subjective indicator that was not tested on as many species as CRB, it is important to note that it would be more useful in aquaculture trials. It is known that in stressful situations, some species can take up to 10 minutes to reach the CRB (Taylor et al., 2014), making it difficult to use the CRB to measure many animals in a practical or rutinary manner. As a result, it is critical to consider the type of test that will be performed when deciding whether to use HRB or CRB as a parameter.
The aim of this study was to define an indicator based on righting behaviour, and determining whether sex and diameter had any effect on it was critical. On the one hand, the sex had no effect on the CRB, which was expected based on previous research in this species. However, it is well known that diameter influences motor behaviour (movement) on both horizontal and vertical surfaces (Domenici et al., 2003). In species such as L. variegatus, S. purpuratus, and S. droebachiensis, CRB is also influenced by diameter, with smaller individuals completing the behaviour faster than larger ones (Challener & McClintock, 2017; Sonnenholzner et al., 2010; Percy, 1973). Larger individual have more variance in CRB than the smaller ones (Challener & McClintock, 2017). However, in a study with S. intermedius, the diameter had no effect on the CRB (Zhang et al., 2017). In the current study, there was a difference in CRB between smaller and larger individuals. Larger ones (up to 30 mm) achieved times between 150 and 250 seconds, while smaller ones achieved times less than 150 seconds. This may be due to the force exerted by the animals using their tube feet (Lawrence, 1975), which appears to be proportional to the individual’s size. The size of the spines may also explain the difference in time to reach the CRB (Sherman, 2015). Smaller individuals in A. dufresnii had larger spines, which could justify the effort and time required for larger individuals to perform the CRB, as reported by other authors such as Challener & McClintock (2017). As a result, when using CRB as an indicator, it is critical to consider the individual’s size. To refine the indicator, it may be necessary to examine the canopy (the diameter including the spines) rather than just the diameter to determine the CRB (Nishizaki & Ackerman, 2007). More trials would be conducted to measure the size of the spines in juveniles and adults of A. dufresnii in order to verify the information. Furthermore, CRB may differ between species due to factors such as seawater temperature and predator presence. S. purpuratus, for example, took an average of 12 seconds to perform CRB in individuals ranging in size from 125 to 54 mm in diameter, whereas A. dufresnii took an average of 45 seconds in individuals ranging in size from 16 to 50 mm in diameter. Despite these interspecies differences, the CRB can be used in experiments and aquaculture to evaluate the well-being of individuals within a species under various conditions.
Fatigue during serial repetitions of the CRB has not been observed in sea urchins (Kleitman, 1941; Lawrence & Cowell, 1996). In this study, A. dufresnii did not show fatigue when the CRB was repeated three times in a row. The absence of fatigue makes routine welfare assessments more practical, and it supports the hypothesis that the CRB is a good indicator of the health and condition of animals in captivity. Because the variation in a single count, or three counts, is not that great, a single test could be performed to evaluate the state of the animals, reducing time and improving data collection. This would also be beneficial to include the CRB in the data collected during routine sampling.
The temperature of the seawater can affect the CRB by affecting metabolism and the mechanical function of the tube feet. At low temperatures, the tube feet stretch and movement and adhesion to the substrate slow (Percy, 1973). Rosales-Schultz (2016) found that high temperatures influenced Tetrapygus niger (Molina, 1782) behaviour, reducing mobility until total cessation near 32.7 °C. High temperatures can cause delays or failures in righting behaviour, possibly due to heat narcosis, which causes weakness, limpness, and contraction of the tube feet (Percy, 1973). A. dufresnii is a temperate species with a seawater temperature range of 0 °C to 20 °C, with 19 °C being near the upper limit. As expected, longer CRB times were observed at the highest seawater temperature (19 °C) in this study. Brothers and McClintock (2015) discovered a 50 % decrease in the number of L. variegatus individuals capable of performing the CRB between the first and tenth day of temperature exposure (28 °C and 32 °C). The current study discovered two distinct failure modes when performing the CRB at high temperatures (19 °C), including the inability to exceed the 30-40 ° angle at low temperatures and remaining in a dorsal position with continuous translational or rotational movements at high temperatures. Furthermore, the times of CRB at 19° were significantly different when compared to 13 °C and 16 °C, with values near 150 seconds and less than 100 seconds, respectively; the lowest temperatures did not show significant differences. To avoid measurement errors, it is critical to consider the influence of temperature on the CRB during experimentation or when using this behaviour as an indicator. Furthermore, the CRB can provide information on a species’ optimal culture temperature because the time to perform the CRB may be the shortest recorded when the animal is in optimal conditions. According to Ancin et al. (2021), 15 °C appeared to be a suitable temperature for culturing A. dufresnii, and these findings would support this hypothesis while also confirming the CRB as a reliable indicator of animal health.
Spawning induction is commonly used in scientific experiments with various applications in aquaculture. Sea urchin gametes are extremely valuable due to their numerous applications in human consumption, antioxidant capacity, and biotechnology (Crespi-Abril & Rubilar, 2021). However, there have been few studies on how spawning stress affects sea urchin behaviour and welfare, and there is no evidence of this effect on A. dufresnii. In this study, sea urchins that were spawned had higher variance but longer CRB times (greater than 150 seconds) than those that were not spawned (less than 60 seconds). The high variability and lack of significant differences observed in the statistical analysis would be explained by the small sample size used for this analysis (30 animals). However, there is a significant difference between the treatments, which is supported by a high mortality rate, indicating the stress that these individuals have experienced. However, because the control of this experiment was only the absence of injection, it would be useful to conduct additional trials with a control that only simulated the injection (with no solution). The effect of the injection’s stress would be studied in this manner. As a result, the sample size combined with the type of control would not accurately represent the true response to this stress, and the response may be overestimated or underestimated.
The current study is the first to examine behaviour in Arbacia dufresnii in aquaculture systems, and it supports the use of righting behaviour as an indicator of stress in this species.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio