Introduction
Anthropogenic activities such as petroleum exploration, mining, industrial activities, and other human urban activities are the main sources of trace elements in mangroves (Celis-Hernandez et al, 2020). In Brazil, in 2015, the collapse of the Fundão Dam in Mariana occurred, releasing 40 to 60 million m3 of mining tailings into the Doce River, the sediment plume, containing trace metals, reached the ocean and coastal region, potentially affecting mangrove areas (Sá et al., 2021; Tognella et al., 2022). These compounds impose an energy drain on plants; at least in the long-term, persistently high concentrations in mangrove sediments can cause damage to environmental health and affect plant growth, energetic metabolism, and cell structure, thus inducing changes in the ecosystem structure (Wang et al., 2003).
Iron (Fe) and manganese (Mn) are bioessential micronutrients for plant growth and development for being directly involved in metabolic processes (Najafpour et al., 2014; Taiz et al., 2017; Varma & Jangra, 2021). Both elements have a direct relationship with plant photosynthesis. Fe plays an important role as a component of enzymes that participate in electron transfer (redox reactions), such as cytochromes, being reversibly oxidized and reduced through electron transfer between Fe2+ and Fe3+. Besides, chlorophyll (Chl) biosynthesis and maintenance of the structural integrity of photosynthetic reaction centers and light-harvesting compounds (LHC) subunits require Fe. In the photosynthetic electron transport chain (ETC), Fe acts as a cofactor in photosystems II and I (PSII and PSI, respectively) and in the cytochrome (Cyt) b 6 /f complex (Taiz et al., 2017). Mn ions (Mn2+), in turn, activate several plant enzymes of the citric acid cycle (Krebs cycle), such as decarboxylases and dehydrogenases. The best-defined function of Mn2+ is to constitute the oxygen-evolving complex (OEC) associated with PSII through which oxygen (O2) is produced from water. The OEC is a manganese-calcium (Mn4CaO5(H2O)4) cluster housed in a protein complex (Najafpour et al., 2014).
Despite being important for electron transfer in chloroplasts, excessive Fe and Mn accumulation in leaf tissues leads to increased cellular toxicity due to reactive oxygen species (ROS) overproduction (Gill & Tuteja, 2010). The chloroplast, more specifically the electron acceptor side of PSI associated with the thylakoid membrane, is the main site of ROS production in plant cells (Gill & Tuteja, 2010). In conditions of superreduction of the ETC as an effect of physiological disorders, part of the electron flow is diverted from ferredoxin to O2, which is reduced to O2 •- via the Mehler reaction (Taiz et al., 2017). Due to high reactivity and toxicity, ROS cause damage to proteins, lipids, carbohydrates, and DNA, ultimately resulting in cell death (Bailey-Serres & Mittler, 2006). O2 •- ions can donate electrons to Fe+3 to generate the reduced form Fe+2, which reduces the H2O2 formed by dismutation of O2 •- into OH• (Gill & Tuteja, 2010). In this sense, oxidative stress is triggered by disturbances in the photosynthetic electron flow through the ETC. Under normal conditions, electron flow leads to reduction of NADP+ to NADPH, which is used in the Calvin Cycle to reduce CO2 to carbohydrates (Taiz et al., 2017). Oxidative stress occurs when ROS generation exceeds the capacity of the plant to maintain cellular redox homeostasis or to scavenge the toxic O2 molecules (Gill & Tuteja, 2010).
Nevertheless, plants have evolved a sophisticated antioxidant system comprised of specific protective mechanisms to defend themselves against oxidant injury (Gholami et al., 2012). It is well established that the ability to tolerate environmental stress is associated with the expression of an efficient antioxidative system, which provides the first line of defense against the toxic effects of enhanced ROS levels (Gill & Tuteja, 2010). This antioxidative system is composed of antioxidant enzymes as superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), catalase (CAT), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and glutathione reductase (GR) as well as nonenzymatic metabolites of low molecular weight like ascorbic acid (Vitamin C), glutathione (GSH), proline (Pro), α-tocopherol (Vitamin E), carotenoids and flavonoids (Mittler et al., 2004).
The antioxidant potential can also be evaluated in plant tissues by assessing the capacity to scavenge the 1,1-Diphenyl-2-picrylhydrazyl free radical (DPPH•) (Menezes et al., 2021). The DPPH• assay provides rapid results to evaluate the scavenging efficiency of extracts from Rhizophora mangle leaf tissues against ROS, maintaining the flow of electrons along the ETC as well as CO2 assimilation at high rates.
This work aims to evaluate the photosynthetic performance and the integrity of the antioxidant defense mechanisms of the true mangrove species R. mangle in response to high levels of Fe and Mn in leaf tissues. The present findings contribute to better understand the role of Fe and Mn in carbon assimilation and evidence how beneficial these elements are. Thus, carbon assimilation, Chl a fluorescence, photosynthetic pigments, and DPPH• radical scavenging activity were compared in plants from different mangrove areas containing the highest and lowest leaf concentrations of Fe and Mn. Such evaluations allowed to demonstrate how these elements drain energy from plants, and that their persistence in the sediment can induce a long-term loss of environmental health. These approaches are highly relevant in the current century, since coastal eutrophication and the increase in atmospheric carbon have been widely discussed (Gilman et al., 2008; Sanders et al., 2014). Our experiment was conducted in several mangrove systems in the states of Espírito Santo and Bahia, Brazil, following the failure of the Fundão tailings dam in 2015 (Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis [IBAMA], 2019).
Materials and methods
Study area, plant material, and sampling: The sampling locations are shown in Fig. 1 and further described in Tognella et al. (2022). The areas were selected according to the concentrations of Fe and Mn in leaf tissues (hereafter, “Feleaf” and “Mnleaf”, respectively) of R. mangle, which is the dominant species in the studied plots. Thus, four estuaries (Aracruz, Barra Nova, São Mateus and Caravelas, within the geographic coordinates (19°93’97”-17°72’70” S & 40°21’32”-39°28’32” W) showing low and high Feleaf and Mnleaf were selected in fringe and basin forests in the North region of Espírito Santo State and the extreme South of Bahia State (Brazil).
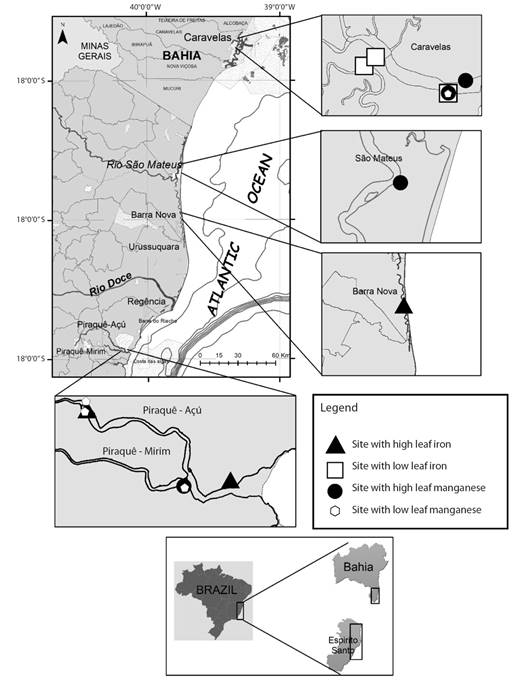
Fig. 1 Location of the sampling sites in Northern Espírito Santo and Southern Bahia. Triangle, square, circle and hexagon identify the sites with high and low leaf Fe concentration and high and low leaf Mn concentration, respectively.
Five plants were randomly selected and marked in each plot. Sampling and measurements were performed in fully expanded but not visibly senescent leaves of the second pair of the branch from the apex. Measurements were made in the morning (between 8:00 and 12:00). Plant height varied from 0.68 to 1.64 m and 0.88 to 1.54 m at the low and high Feleaf sites, respectively. All sites were inserted within predefined areas (fixed plots). The sampling locations showed statistical similarities regarding the minimum and maximum values of salinity (3.7-33.4 psu and 3.7-29.6 psu at the low and high Fe and Mn sites, respectively). Mean annual rainfall ranges between 1 100 and 1 400 mm at the study sites (Alvares et al., 2014; Instituto Nacional de Meteorologia, 2019). In addition, precipitation and relative air humidity in the source areas ranged from 115.76 to 119.17 mm and 75.5 to 87.0 %, respectively between April and August 2019 (Instituto Nacional de Meteorologia, 2019), characterized as dry period. Sampling was conducted at low tide.
Sediment and leaf sampling: Sediment samples were collected in the intertidal area using collectors built with a 50 cm PVC pipe. The material was obtained in two depths of 0 to 5 cm (surface) and 5 to 15 cm. In each plot, simple samples were randomly collected (for each depth) and homogenized to remove roots, shell fragments, leaves and branches and thus form a composite sample for each depth. Next, the sediment samples were placed into previously identified plastic bags, kept in a cool box with ice and taken to the laboratory for freezer storage.
To quantify the concentration of metals in leaf tissues of R. mangle, healthy mature leaves showing no signs of herbivory were sourced from five trees in each location, stored in paper bags and maintained under refrigeration until processing. In the laboratory, leaves were dried at 60 °C until constant weight was attained.
Sediment and leaf chemical analysis: Analysis of metals in sediments was performed according to the United States Environmental Protection Agency (USEPA) Method 3051A (USEPA, 2013). Dry and homogenized sediment (about 0.5 g) was digested with HNO3:HCl (3:1; 12 ml) in Teflon tubes in a microwave oven (CEM, Marx X-Press) using the following program: 1st ramp 25-175 °C in 5:30 min; and 2nd ramp 25-175 °C in 4:30 min (both with a power setting of 1 600 W). Afterwards, the solution was cooled, filtered through a Whatman n° 1 filter, diluted to 100 ml in a volumetric flask and analyzed by ICP-MS (Inductively Coupled Plasma Mass Spectrometry; Agilent, CX7 500).
The plant material was dried, ground and submitted to nitroperchloric digestion. Feleaf and Mnleaf were subsequently quantified by atomic absorption spectrophotometry (Silva, 2009).
Pigment quantification: Leaf samples of 5 g (fresh mass) were frozen at -30 °C and ground in liquid nitrogen with a mortar and pestle to form a fine powder, which was transferred to test tubes and homogenized in 15 ml of 90 % acetone solutions and 0.5 g l-1 of calcium carbonate (CaCO3). Next, the test tubes were immediately stored at 2 °C for 24 h for complete pigment extraction (modified by Arar, 1997). The samples were then filtered, and the supernatant was collected and stored in amber flasks at -30 °C pending analysis by spectrophotometry. The extraction procedure was carried out at room temperature in dark conditions to minimize Chl degradation by enzymatic action. Ice cold extraction solvents were used. The extraction time was kept to a minimum to reduce degradation of the analyzed pigments.
Subsequently, the optical density readings were determined on a spectrophotometer (Genesys 10S UV-Vis, Thermo Fisher Scientific, Waltham, USA) at 470, 645 and 663 nm. Determination of photosynthetic pigment concentrations was performed according to the equations proposed by Wellburn (1994): chlorophyll a (Chla) a = (12.25 x A663 − 2.79 x A645), chlorophyll b (Chlb) = (21.5 x A645 − 5.1 x A663), and carotenoids (Car) = (1 000 x A470 − 1.82 x Chla − 85.02 Chlb/198); values were expressed in mg ml-1 of fresh mass, where A470, A645 and A663 represent the absorbance at 470, 645 and 663 nm, respectively.
Chlorophyll a fluorescence and leaf gas exchange: Chl a fluorescence was measured at room temperature using a plant efficiency analyzer (Handy-PEA, Hanstech Instruments Ltd., King’s Lynn, Norkfolk, UK), as in Strasser & Govindjee (1992). Previously, leaves were adapted to the dark during 30 min using leaf clips (Hansatech Instruments Ltd.). Fluorescence rise OJIP trace was induced by 1 s pulses of red light (650 nm, 3 000 μmol (photon) m-2 s-1). O and P refer to the initial and maximum fluorescence intensity considered here at 50 μs (F0) and 300 ms, respectively. J (≅ 2 to 3 ms) and I (≅ 30 ms) are inflection points between O and P levels. The fluorescence transients OJIP curves were analyzed according to the JIP-test (Strasser et al., 2004). L and K-bands were calculated as VOK = (F100μs − F0)/(F300μs − F0) and VOJ [(F300μs − F0)/(F2ms − F0)] (Srivastava & Strasser 1997; Strasser & Stirbet, 2001). A detailed description of parameters and their meaning can be found elsewhere (Strasser et al., 2004) and briefly addressed in Table 1.
Table 1 Abbreviations of the JIP-test parameters, formulas, and description of the data derived from the transient of Chl a fluorescence.
| Fluorescence Parameters | Description |
| Ft | Fluorescence at time t after onset of actinic illumination |
| Fo ≅ F20ms | Minimal fluorescence, 11cep all PSII RCs are open |
| FK ≅ F0.3ms | Fluorescence intensity at the K-step (0.3 ms) of OJIP |
| FJ ≅ F2ms | Fluorescence intensity at the J-step (2 ms) of OJIP |
| FI ≅ F30ms | Fluorescence intensity at the I-step (30 ms) of OJIP |
| FP (=Fm) | Maximal fluorescence at the peak P, 11cep all PSII RCs are closed |
| Fv ≅ Fm − F0 | Maximal 11ceptor11 fluorescence |
| Area | Total complementary 11cep between the fluorescence induction curve and F0 and Fm |
| VJ = (FJ − F0)/(Fm − F0) | Relative 11ceptor11 fluorescence at the J-step |
| L-band = VOK = (Ft − F0)/(FK − F0) | Variable fluorescence between steps O (50 µs) and K (300 µs), indicative of the energetic connectivity between the subunits associated with PSII |
| K-band = VOJ = (Ft − F0)/(FJ − F0) | Variable fluorescence between steps O (50 µs) and J (2 ms), which is an indicative of stability of oxygen 11ceptor11n complex (OEC) |
| ABS/RC = M0.(1/VJ).(1/ φP0) | Absorption flux per active reaction center (RC) at t = 0. |
| TR0/RC = M0.(1/VJ) | Trapped energy flux per RC (at t = 0) |
| ET0/RC = M0.(1/VJ).ψЕ0 | Electron transport flux per RC (at t = 0) |
| DI0/RC = [(ABS/RC) − (TR0/RC)] | Dissipated energy flux per RC at t = 0. |
| RC/CS0 = φP0·(VJ/M0)·(ABS/CS) | Total number of active reaction center per cross section |
| φP0 = TR0/ABS = [1 − (F0/Fm)] = Fv/Fm | Maximum quantum yield of primary photochemistry at t = 0). |
| φD0 = 1 − φP0 = (F0/Fm) | Quantum yield of energy dissipation (at t = 0). |
| φE0 = (1 − F0/Fm) (1 − VJ) | Quantum yield for PSII electron transport (ET) |
| δR0 = (1 − VI)/(1 − VJ) | Quantum yield for reduction of the end electron acceptors at the PSI 11ceptor side |
| PIABS = RC/ABS.φP0/(1 − φP0).ψE0/(1 − ψE0) | Performance index based on absorption |
| PITotal = PI(ABS) × [δR0/(1 − δR0)] | Performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of PSI end acceptors |
Leaf CO2 assimilation (A [μmol m-2 s-1]), stomatal conductance (gs [mol m-2 s-1]), intercellular CO2 concentration (Ci [μmol m-2 s-1]) and leaf transpiration rate (E [mmol m-2 s-1]) were estimated in the same leaves used to measure Chl a fluorescence; for that, portable infrared gas analyzers (models Lci, Lci T and Lcpro T, ADC, BioScientific Ltd., Hoddesdon, England) were used. The gas chamber was maintained at ambient conditions, the average photon flux density in the chamber was 200 ± 28.7 and 316 ± 46.9 μmol m-2 s-1, with the average leaf temperature reaching 29.5 ± 0.4 and 31.0 ± 0.5 °C for the Fe and Mn treatments, respectively. Estimation of water-use efficiency was calculated and determined as intrinsic water-use efficiency (WUEint = A/gs [μmol (CO2) mol-1(H2O)]) and instantaneous water-use efficiency (WUEins = A/E [μmol (CO2) mmol-1(H2O)]) (Krauss, et al, 2006). A and Ci were used to estimate the carboxylation efficiency of ribulose-1,5-bisphosphate-carboxylase/oxygenase (Rubisco) (A/Ci) (Zhang et al., 2001).
DPPH• radical scavenging assay: The free radical scavenging ability of the extracts was determined using the DPPH• method (Dal Prá et al., 2013; Huang et al., 2005). To obtain the extracts, which were referred to as working solutions (WS), plant samples were dried in an oven and then diluted in methanol (two, five, ten or twenty times). The tests were carried out by adding aliquots of 15, 25 and 35 µL of each WS into cuvettes containing 3.0 mL of DPPH methanolic solution (0.2 mmol l−1), which were kept protected from light for 60 min. Tests were made in triplicate. Measurements were performed on a Perkin Elmer Lambda 16 spectrophotometer, monitoring the absorbance of the samples at 517 nm. The blank consisted of 3.0 ml of methanol containing 15, 25 or 35 µl of the respective WS. A solution containing DPPH served as negative control. The percentage of DPPH• scavenging was calculated as [1 − (As − Ab)/ (Ac − Ab)] x 100, where As, Ab and Ac are the absorbance of samples, blank and negative control, respectively.
The IC50 values (concentration necessary to inhibit 50 % of the DPPH• radical) were determined by means of linear regression. As analyses were performed in triplicate, the mean and the standard deviation were used to represent the IC50 of each sample.
Statistical analysis: Data were submitted to normality (Shapiro-Wilk) and homogeneity (Barttlet) tests. To determine differences between the physiological parameters in relation to the concentration of Fe and Mn (low x high), the non-normal data were analyzed via the non-parametric Mann-Whitney U test, while normally distributed data were assessed through the t-student test. In the basic statistical analyses, Excel or the statistical treatment package Statistica (STATSOFT®) were used. The significant threshold was set at 0.05 for all tests.
Results
Fe and Mn concentration: In R. mangle, the low and high Feleaf were 74 and 195 mg kg-1, while the low and high Mnleaf were 65 and 414 mg kg-1, respectively. The concentration of Mn in the sediment was 101 and 239 mg kg-1 at the sites with the lowest and highest concentrations, respectively, while no variation in Fe concentration was observed among the sites (≅ 22 252 mg kg-1) (Fig. 2).
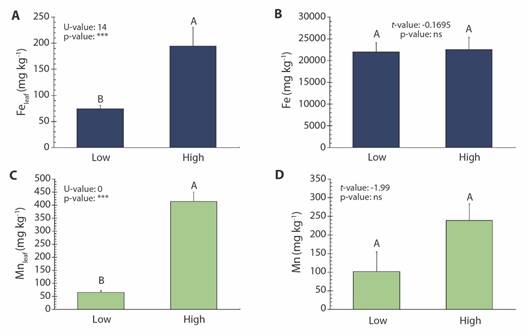
Fig. 2 Concentration (mean ± SE) of Fe and leaves (A) and sediments (B); Mn in leaves (C) and in sediments (D) of the Rhizophora mangle, referring to sampling sites (Low and High concentration). Different letters indicate significant difference between sites (P < 0.05; n.s.: non-significative; U or t : Mann-Whitney U test or t-student, respectively).
Pigment quantification: There was no significant difference in the concentrations of Chla, Chlb and Car in the leaves of R. mangle growing in both high and low Fe and Mn sites (Table 2).
Table 2 Concentration of pigments (mean ± SE) in Rhizophora mangle, referring to sampling sites (low and high concentration of Fe and Mn in the leaf) in Northern Espírito Santo and Southern Bahia.
| Feleaf | Mnleaf | |||||||
| Low | High | U or t value | P-value | Low | High | U or t value | P-value | |
| Chla (μg mL-1) | 297.65 ± 23.21 A | 238.94 ± 139. 00 A | 12.00 U | n.s. | 249.20 ± 31.77 A | 331.09 ± 62.58 A | 50.00 U | n.s. |
| Chlb (μg mL-1) | 212.75 ± 16.70 A | 147.21 ± 76.90A | -1.36 t | n.s. | 165.74 ± 19.22 A | 231.59 ± 43.25 A | 46.00 U | n.s. |
| Car (μg mL-1) | 589.64 ± 58.78 A | 576.89 ± 189.00 A | -0.08 t | n.s. | 597.92 ± 72.67 A | 628.86 ± 68.39 A | -0.31 t | n.s. |
Chla (chlorophyll a- μg ml-1), Chlb (chlorophyll b- μg ml-1), Car (carotenoids- μg ml-1). Different letters indicate significant differences between sites (P < 0.05; n.s.: non-significative; U or t : Mann-Whitney U test or t-student, respectively).
Chl a fluorescence: The OJIP transients of samples exposed to high and low Feleaf and Mnleaf showed a typical polyphasic rise with the fluorescence signal rising from the initial fluorescence level (F0) to the maximal fluorescence level (Fm), with well-defined intermediate J and I steps (Fig. 3). High Feleaf increased the fluorescence yield (F0 and Fm) and the area beneath the fluorescence curve between F0 and Fm (Table 3). In contrast, high Fe did not affect quantum yield for primary photochemistry, for electron transport and for energy dissipation, φP0, φE0, and φD0, respectively. The specific energy fluxes per reaction center (RC) for absorption (ABS/RC), trapping (TR0/RC) and electron transport (ET0/RC) were lower at high Fe without variation in the specific energy flux for dissipation (DI0/RC). Furthermore, the total number of active reaction centers per cross section (RC/CS0) and the quantum yield for reduction of the end electron acceptors at the PSI acceptor side (δR0) increased about 15.3 % and 4.51 % at high Fe, and the performance indexes (PIABS and PITotal) were statistically similar among sites, with average values of 20.92 and 13.19, respectively. Moreover, the increase in Feleaf increased the energetic connectivity between the subunits associated with PSII and the stability of OEC, which can be visualized by lower values of L and K-bands, respectively, while no changes were registered in the oxidoreduction capacity of QA by electrons originated from P680 (VJ or J-step values) among sites (Table 3).
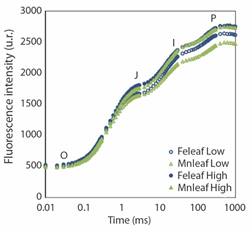
Fig. 3 The OJIP chlorophyll a fluorescence transient curve in Rhizophora mangle, referring to sampling sites (low and high concentration of Fe and Mn in the leaf).
Table 3 Mean values (± SE) of the parameters calculated from the JIP-test obtained from Rhizophora mangle plants in the sampling of Fe and Mn (low and high concentration of Fe and Mn in the leaf) sites in Northern Espírito Santo and Southern Bahia.
| Parameters | Feleaf | Mnleaf | ||||||||
| Low | High | Percentage of change in reduction | U or t value | P-value | Low | High | Percentage of change in reduction | U or t value | P-value | |
| Area | 65 073.51 ± 1 305.51 B | 71 012.28 ± 1573.50 A | -- | 2.92 t | ** | 66 382.32 ± 1136.52 A | 67 127.65 ± 1257.73 A | n. s. | 1 759.00 U | n. s. |
| F0 | 434.37 ± 4.41 B | 474.93 ± 3.65 A | -- | 444.00 U | *** | 438.10 ± 4.15 A | 447.01 ± 4.22 A | n. s. | 1.50 t | n. s. |
| Fm | 2 644.97 ± 26.51 B | 2 768.17 ± 31.95 A | -- | 2.98 t | ** | 2 497.76 ± 29.73 B | 2 751.91 ± 40.58 A | --- | 4.64 U | *** |
| ϕP0 | 0.802 ± 0.001 A | 0.798 ± 0.006 A | n. s. | -1.56 t | n. s. | 0.792 ± 0.002 B | 0.806 ± 0.001 A | --- | 830.00 U | *** |
| ϕE0 | 0.377 ± 0.004 A | 0.361 ± 0.006 A | n. s. | 1 123.00 U | n. s. | 0.357 ± 0.007 B | 0.374 ± 0.005 A | --- | 2.04 t | * |
| ϕD0 | 0.197 ± 0.002 A | 0.202 ± 0.002 A | n. s. | 1.50 t | n. s. | 0.207 ± 0.002 A | 0.193 ± 0.001 B | 6.76 % | 832.00 U | *** |
| J-Step | 0.459 ± 0.005 A | 0.441 ± 0.008 A | n. s. | 1 257.5 U | n. s. | 0.435 ± 0.008 A | 0.454 ± 0.006 A | n. s. | 1.79 t | n. s. |
| ABS/RC | 1.89 ± 0.037 A | 1.75 ± 0.034 B | 7.40 % | -2.68 t | ** | 1.85 ± 0.037 A | 1.74 ± 0.038 B | 5.94 % | -2.11 t | * |
| TR0/RC | 1.51 ± 0.026 A | 1.39 ± 0.025 B | 7.94 % | -3.06 t | ** | 1.46 ± 0.026 A | 1.40 ± 0.030 A | n. s. | 1 559.00 U | n. s. |
| ET0/RC | 0.704 ± 0.011 A | 0.629 ± 0.014 B | 10.65 % | 688.00 U | *** | 0.654 ± 0.016 A | 0.642 ± 0.012 A | n. s. | 1 728.00 U | n. s. |
| DI0/RC | 0.382 ± 0.011 A | 0.356 ± 0.009 A | n. s. | 1 138.00 U | n. s. | 0.390 ± 0.011 A | 0.340 ± 0.009 B | 12.82 % | 1 150.00 U | *** |
| RC/CS0 | 279.12 ± 3.81 B | 321.85 ± 5.26 A | --- | 6.74 t | *** | 284.89 ± 5.69 B | 310.68 ± 5.69 A | --- | 1 156.00 U | *** |
| PIABS | 21.43 ± 0.871 A | 20.41 ± 0.989 A | n. s. | 0.77 t | n. s. | 19.37 ± 1.00 B | 23.33 ± 1.04 A | --- | 1 255.00 U | ** |
| PITotal | 13.02 ± 0.628 A | 13.36 ± 0.652 A | n. s. | 1 224.00 U | n. s. | 12.42 ± 0.621 A | 13.86 ± 0.661 A | n. s. | 1 476.00 U | n. s. |
| δR0 | 0.377 ± 0.004 B | 0.394 ± 0.005 A | --- | 2.55 t | * | 0.396 ± 0.005 A | 0.372 ± 0.004 B | 6.06 % | 1 137.00 U | *** |
| L-Band | 0.167 ± 0.001 A | 0.164 ± 0.001 B | 1.79 % | -2.37 t | * | 0.171 ± 0.001 A | 0.165 ± 0.0008 B | 3.50 % | 1 354.00 U | * |
| K-Band | 0.365 ± 0.006 A | 0.340 ± 0.006 B | 6.84 % | -3.20 t | ** | 0.367 ± 0.001 A | 0.348 ± 0.007 A | n. s. | -1.82 t | n. s. |
Different letters indicate significant differences between sites (P < 0.05; n.s.: non-significative; U or t : Mann-Whitney U test or t-student, respectively). Parameters expressed in relative units.
The increase in Mnleaf did not affect the basal fluorescence yield (F0) in R. mangle, but increased Fm and Area. Contrastingly, R. mangle high in Mnleaf showed enhanced values of quantum yield for photochemistry and electron transport (φP0 and φE0, respectively), and decreases the quantum yield of energy dissipation (φD0) at high Mnleaf. A greater number of active reaction centers was recorded at high Mnleaf, the absorption (ABS/RC) and dissipation (DI0/RC) energy flux were lower, although the capture (TR0/RC) and electron transport flux (further than QA -) or ET0/RC did not vary. As for the performance indexes (PIABS and PITotal), only PIABS showed a statistically significant difference among sites, with higher values (23.33) being detected at the sites where Mnleaf was higher. The opposite occurred with the the quantum yield for reduction of the end electron acceptors at the PSI acceptor side (δR0), and L-band at high Mnleaf. No changes were seen in the K-band or VJ (Table 3).
Leaf gas exchange: Leaf CO2 assimilation and gas-exchange variables were analyzed to better characterize the effects of Feleaf and Mnleaf on R. mangle photosynthetic performance (Fig. 4). The present results indicate that photosynthesis was differently influenced by Fe and Mn in leaf tissue. E, gs, A, and CE (carboxylation efficiency = A/Ci) values increased in leaf tissue of R. mangle containing high Fe, while Ci and WUEint (intrinsic water use efficiency = A/gs) and WUEins (instantaneous water use efficiency = A/E) remained unchanged. Conversely, despite exhibiting better photochemical performance, the rise in Mnleaf increased Ci and decreased E, A, WUEint, WUEins and CE (Fig. 4).
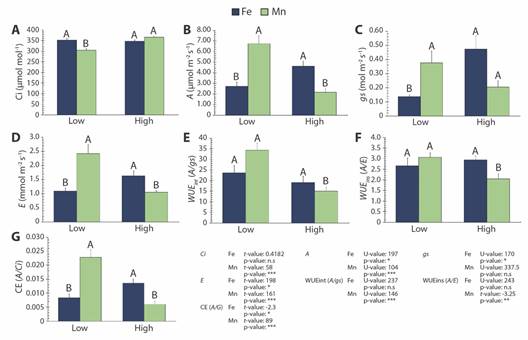
Fig. 4 Gas exchange parameters (mean ± SE) in Rhizophora mangle, referring to sampling sites (Low and High concentration of Fe and Mn in the leaf). A. Ci (intercellular CO2 concentration), B. A (net carbon assimilation rate), C. gs (stomatal conductance), D. E (transpiration rate), E. WUE int (intrinsic water use efficiency - A/gs), F. WUE ins (instantaneous water use efficiency) and G. CE (carboxylation efficiency). Different letters indicate significant differences between sites (P < 0.05; n.s.: non-significative; U or t : Mann-Whitney U test or t-student, respectively).
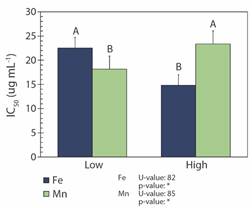
DPPH• radical scavenging assay: Higher Feleaf in R. mangle reduced the concentration of the extract needed to scavenge DPPH• by 50 % (IC50). For Mnleaf, however, the opposite effect was observed (Fig. 5).
Discussion
The effects triggered by Fe and Mn exposure were seen in all evaluated parameters and resulted in physiological changes in R. mangle. We understand that, although iron and manganese concentrations in leaf tissues have a great influence on photosynthetic processes, other factors also act as regulators. Nevertheless, we observed alterations occurred among locations where Feleaf and Mnleaf were significantly different, and involved analyses of photosynthetic pigments, transient Chl a fluorescence, gas exchange, CO2 assimilation, oxidative stress, and the detoxification capacity of plants, evaluated here as DPPH• radical scavenging activity. The achieved results will certainly allow to understand the successful strategies of the R. mangle tree to cope with the altered environment as well as its capacity to cleanse sediment and water.
The difference in Fe uptake and accumulation allowed us to select the study areas. Such differences in absorption, given the invariability of the element in the sediment, may be associated with the presence of other trace elements at higher concentrations, e.g. Mn, (data not shown), as observed in the sediment of the site showing lower Feleaf (data not shown). Conversely, low Mn sediment concentration was found under high Feleaf (data not shown). The content of clay and soil organic matter also influences the availability of Fe to plants, since there is a tendency to retain Fe in muddy soils. Adequate levels of organic matter improve Fe uptake due to its acidifying and reducing properties; moreover, certain humic substances could form chelates under adverse pH conditions (Dechen & Nachtigall, 2006). Besides, Fe absorption decreases with increased concentrations of Ca, Mg, Cu, Zn and especially Mn (Jones-Junior, 2012). High concentrations of Mn in acidic soils can competitively inhibit Fe absorption (Malavolta, 1980). Such Mn values are independent of the results observed in this study, considering the different sampling sites. Like Fe, Mn in the sediment tends to form stable and insoluble compounds, suitable for the adsorption of other metals (Förstner & Witmann, 1981). However, upon contact with mangrove sediments, imported particulate manganese is reduced, due to the physical-chemical characteristics of this compartment. This process makes the element soluble, facilitating its export to adjacent environments in the form of Mn (II) (Vidal & Becker, 2006). Therefore, it is possible that the variation in Mn concentration in the sediment observed in this study is associated with areas of export (low concentrations) and import (high concentrations).
Chl a fluorescence transient in R. mangle under low and high Feleaf and Mnleaf revealed the three typical OJIP phases (O-J, J-I and I-P), indicating that all samples remained photosynthetically active (Strasser & Stirbet, 2001) independently of metal concentration. The increase in F0 and Fm at the sites with higher Feleaf resulted in higher area above the fluorescence curve (Area) between F0 and Fm. As stated by Joliot and Joliot (2002), the area represents the electron acceptor pool sizes of PSII, including QA and QB. In this investigation, the area over the fluorescence curve was slightly but significantly (P ≤ 0.05) increased by 9.12 % under high Feleaf compared to low Feleaf, showing that the elevation in Feleaf improves the electron transfer rates at the donor side of PSII and increases QA pool size (Gao et al., 2022; Mehta et al., 2010). Furthermore, despite the increase in Fm at high Mnleaf, there were no changes in F0 or Area. It has been suggested that high Fm occurs when thylakoid membranes are preserved, thus leading to the clustering of light-harvesting complexes (LHCII) associated with PSII (Schreiber & Neubauer, 1987).
The levels of F0 were only increased by about 2.03 % (P ≥ 0.05) at high Mnleaf, while at high Feleaf they were 9.3 % (P ≤ 0.05) higher than at low Feleaf. Nonetheless, although F0 values showed a significant rise under high Feleaf, no alterations were registered in parameters related to efficiency, as similarly observed at high Mnleaf. F0 is associated with the donor side of PSII, with the adjustment capacity of antenna pigment level or with the excitation trapping efficiency at the active center of PSII. In this regard, the current findings indicate that the increased F0 values observed at high Feleaf are demonstrated at antenna pigment level, as verified by decreased levels of Chlb, since the values of L and K-bands were reduced and the parameters related to the excitation trapping efficiency at the active center of PSII (φP0, φE0, φR0, TR0/RC, PIABS and PITotal) remained unchanged. K-band and L-band are associated with the donation of electrons from the OEC to PSII and with the connection/disconnection (or energetic connectivity) of the PSII core antenna (LHC), respectively (Strasser et al., 2004). The observed decreases/invariability of K-band and L-band values evidence that concentrations of Fe and Mn, as those registered in leaf tissues of R. mangle grown under in situ conditions, exert some protective effect on electron donor and acceptor sides of PSII, thus maintaining the integrity of the OEC and LHC. It is worth noting that the OEC uses Mn as an essential cofactor in the oxidation of water (Strasser et al., 2004); moreover, as well as the ferredoxin protein, the end electron acceptor of PSI contains Fe in its molecular structure.
Analysis of the efficiency of the energy flow per reaction center (RC) revealed reduction in absorption (ABS/RC), trapping (TR0/RC) and transport (ET0/RC) and no change in dissipation (DI0/RC) in plants growing under high Feleaf. Similar results were obtained for R. mangle from sites with high Mnleaf; there were significant decreases in DI0/RC and ABS/RC and no alterations in TR0/RC or ET0/RC. The energy flow parameters per RC are calculated as the total number of photons absorbed, captured, transported, and dissipated from all RCs divided by the total number of active RCs (Mehta et al., 2010). Thus, the ratio of active/inactive RCs influences the values of energy fluxes. Consequently, as disclosed herein, reductions in all energy fluxes occur when the number of RCs is improved; increases in RC/CS0 of 15.3 % (P ≤ 0.05) and 9.05 % (P < 0.05) were found in R. mangle grown under high Feleaf and Mnleaf, respectively. Increased RC/CS0 in R. mangle can reduce the effects of photoinhibition and thus result in lower energy dissipation. These statements are reinforced by the decreases seen in ABS/RC values, which indicate that both metals increased the antenna size of active RCs; such observations were more important in R. mangle growing under high Feleaf and Mnleaf conditions.
Collectively, these results indicate the efficiency of the photosynthetic apparatus, since the excitation energy was absorbed and captured by the Chl molecules and directed towards electron transport to reduce pheophytin, QA and the other electron acceptors in the ETC (Taiz et al., 2017). The increase in Fm and the reduction and/or invariability of parameters connected to energy dissipation mechanisms, as DI0/RC and φD0, are thus explained. In general, although it is widely accepted that PSII is extremely susceptible to several types of environmental stresses, the observed increases in R. mangle Feleaf and Mnleaf have a positive regulatory effect, at least in terms of preservation of structure and functionality of the plant photosynthetic apparatus. Mechanisms of photosynthetic regulation related to the ability to dissipate excitation energy leading to photoprotection have been suggested and discussed (Wang et al., 2016). Carotenoids participate in the photoprotection system, and the invariability of this pigment is consistent with the results found for the increase in Feleaf and Mnleaf (Szabó et al., 2005).
For a deeper understanding of the role of Fe and Mn as elements in carbon assimilation in R. mangle, a detailed gas-exchange analysis was carried out associated with oxidative stress and antiradical activity. It is generally agreed that environmental alterations directly affect carbon assimilation in plants, considering that photosynthesis is highly vulnerable to metal toxicity (Ahmad et al., 2008; Barcelos et al., 2022); the effects are multi-dimensional and influence photosynthetic CO2 fixation under controlled conditions as well as in situ. The current results evidenced distinct interference patterns of Fe and Mn with the functional processes of photosynthesis, including the antioxidant capacity of R. mangle, despite the improved performance of the ETC of chloroplast.
Unlike the results obtained at high Mnleaf, the antioxidant system of R. mangle, evaluated here through the free radical scavenging activity, undoubtedly functioned as a protection system under high Feleaf. As a result, high CO2 assimilation capacity and carboxylation efficiency (CE) of ribulose-1.5-bisphosphate carboxylase oxygenase (Rubisco) were maintained. Thus, increased Ci as well as decreased CO2 assimilation, WUEint, WUEins and CE under excessive Mn are linked to oxidative damage buildup in R. mangle leaf tissue, despite its plasticity in light energy utilization. These are alarming results, since such estuarine systems undergo great climatic variability (Lopes et al., 2019; Pascoalini et al., 2022). Additionally, the high nutrient content intensifies plant vulnerability to drought, especially under low atmospheric humidity and/or limited freshwater input, thus compromising the use of water by the vegetation (Lovelock et al., 2009; Oliveira et al., 2022).
The increases in A and gs under high Feleaf conditions, which were associated with the invariability of Ci, suggest that the enhanced CO2 flux into R. mangle leaves caused by stomatal opening was a relevant factor for the increase in A. In contrast, the lower A, gs and E values at high Mnleaf, which were associated with higher Ci levels, imply that R. mangle photosynthesis was limited by non-stomatal factors, besides indicating that Mn interfered with stomatal regulation. Reductions in CO2 assimilation is likely to be associated not only with lower CO2 entry into leaves, but also some biochemical limitation in CO2 fixation within the chloroplasts, which is related to lower Rubisco activity and changes in the capacity for ribulose-1,5-bisphosphate regeneration (Alves et al., 2011; Wang et al., 2022). Investigations about the role of Mn on photosynthesis have evidenced repression of specific genes, particularly of those nuclear-encoded small subunits of Rubisco (Sheen, 1994). Consequently, Rubisco content and CO2 assimilation are reduced. According to Li et al. (2010), the regulatory role of Mn-excess appears to be linked with the accumulation of soluble sugars, as sucrose, glucose, and fructose, but it remains to be further clarified. The authors also report that the reduced CO2 assimilation in Mn-excess leaves was not accompanied by Chl, since there were no differences in the contents of Chla or Chlb between the tested Mn treatments. Such finding is consistent with the unchanged concentrations of photosynthetic pigments described here for R. mangle under high Mnleaf conditions.
Globally, mangroves play a particularly important role in maintaining biodiversity hotspots. However, despite the environmental and economic values attributed to mangroves, these ecosystems are constantly prone to anthropogenic or natural actions that result in increased heavy metal concentrations in sediments, which often act as sinks for these toxic elements. Our results showed that Fe and Mn affected the physiological performance of R. mangle in a different manner. Nevertheless, when taking all physiological parameters into consideration, we verified that the effects of high Feleaf at antenna pigment level did not impair the carbon gain of R. mangle trees at the evaluated sites, since K-band values were reduced and there were no changes in parameters related to the excitation trapping efficiency at the active center of PSII. Rises in Feleaf and Mnleaf in R. mangle at the levels recorded in this evaluation increased RC/CS0, thus suggesting a positive regulatory effect at least in terms of preservation of structure and functionality of the plant photosynthetic apparatus. This study also identified distinct interference patterns of Fe and Mn with the functional processes of photosynthesis, especially with CO2 assimilation and ROS metabolism. The most pronounced effects were observed in CO2 assimilation and carboxylation efficiency of Rubisco at high Mnleaf. Thus, interference of high Mnleaf in R. mangle occurs at non-stomatal and biochemical levels. Further research should be conducted under controlled conditions and including higher doses of the metals to expand our understanding of the regulatory role of excessive Fe and Mn on the metabolism of R. mangle at the whole-tree level. The current findings may aid in predicting alterations resulting from the effects of environmental changes, which make the mangrove forest even more vulnerable.
This assessment described the antagonism between Fe and Mn regarding the physiology of R. mangle, which is a dominant species in Brazilian mangroves. Moreover, attention is drawn to the coastal eutrophication processes currently taking place in the global mangrove area. R. mangle appears to be participating in bioremediation, but the physiological responses disclosed herein raise concern about the interference of long-term eutrophication in ecosystem productivity.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives. Funding: This research was financially supported by Renova Foundation via its Technical-Scientific Cooperation Agreement nº 30/2018 with Espírito Santo Foundation of Technology (FEST). Declaration of Competing Interest: The authors declare no conflict of interest.












 uBio
uBio