Introduction
Ascidians are the most diverse class within the subphylum Tunicata, with around 3000 described species (Shenkar & Swalla, 2011). The ascidians are characterized for having an external tunic made up mainly of cellulose (Abbott, Newberry, & Morris, 1997). The current classification for the class divides them into three orders, which are determined by the internal structure of the branchial sac: Aplousobranchia, Stolidobranchia y Phlebobranchia (Lahille, 1886). These organisms exhibit solitary individuals or colonial forms, with zooids with different structural and functional relationships inside a colony (Moreno-Dávila, 2013). Ascidians inhabit many marine environments, from the intertidal zone to abyssal depths (Shenkar & Swalla, 2011). They mostly feed by filtering organic matter and plankton (Petersen, 2007).
This group serves as a substrate and/or refuge for smaller organisms, it is also part of the diet of marine turtles, such as Eretmochelys imbricata. Besides, they are successful competitors for primary substrate (Carrión-Cortez, Canales-Cerro, Arauz, & Riosmena-Rodríguez, 2013; Darwin & Klebba, 2007; Fletcher, Forrest, & Bell, 2013; Marin & Anker, 2008).
Ascidians are among the taxa with the highest number of introduced species reported (Evans, Erwin, Shenkar, & López-Legentil, 2017), which are transported principally as macrofouling attached to the ships hull’s and their larvae through ballast water (Lambert, 2002). Ascidians are successfully facing rough predatory and environmental conditions (Lambert, 2002; Lambert, 2007). It is crucial to set a baseline in order to keep track of invasive species and their effects, hereby the importance of taxonomic knowledge of the group.
Despite their ecological importance and usefulness, and being reported as abundant in the rocky bottom of the North Pacific of Costa Rica; few studies have been carried about their taxonomy and ecology (Cortés, 2009; Nova-Bustos, Hernández-Zanuy, & Viquez-Portuguez, 2010; Tokioka, 1971; Tokioka, 1972). The ascidians identification involves a very detailed, time consuming and specialized process; demanding high taxonomic experience and motor skills for delicate dissections and preservation (Stefaniak, Lambert, Gittenberger, Zhang, & Whitlatch, 2009; Turon, Casso, Pascual, & Viard, 2020); which could have led to the limited knowledge in the region. This study aims to describe the diversity of ascidians in Costa Rica, based on new samplings in the North Pacific, previous reports in the literature, and museum databases available online, in order to improve and update the knowledge on species diversity.
Materials and methods
Sites: Sampling took place on two field trips: from July 1st to August 3rd 2018, and from July 15th to July 19th 2019. The samples were collected at nine sites distributed in the Área de Conservación Guanacaste (ACG, Guanacaste Conservation Area) in Costa Rica’s North Pacific (Fig. 1). This area has a seasonal upwelling that goes along December through April-May, which brings up cooler waters rich in nutrients, helping keep an average superficial seawater temperature around 22°C (Alfaro et al. 2012; Jiménez, 2001).
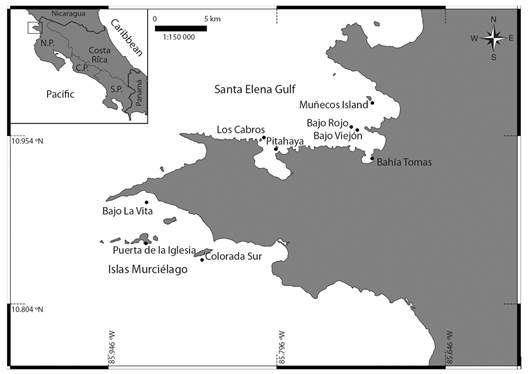
Fig. 1 Collecting sites in the Área de Conservación Guanacaste, North Pacific of Costa Rica. N.P.: North Pacific, C.P.: Central Pacific and, S.P.: South Pacific.
Sampling: The sampling focused mainly on solitary organisms since previous studies in this area have focused on colonial species. Samplings were carried out at depths between 1 and 13 m on rocky bottoms by roving with SCUBA gear, a metal spatula and/or a knife were used to carefully remove the specimens. Sampling in Bahía Tomas was carried out on an artificial substrate (fish breeding cages) at 1 m depth. A chisel and mallet were used to break up the substrate for specimens embedded in the rock to avoid sample damage.
Sample processing: The collected specimens were kept and transported in seawater, then anaesthetized by adding a mixture of 70 % alcohol and dissolved menthol crystals. This process can take several hours depending on the different morphological structures that characterize each one of the three ascidian orders; being Stolidobranchia the one that takes more time due to the strong musculature, according to our observations. During this period, small amounts of 70 % alcohol were added every hour to start the preservation process, without morphological alterations caused by alcohol fixation.
For the taxonomic identification, it was necessary to carry out a process of external and internal dissection of the organisms. This process began by opening the tunic with a cut from the atrial siphon to the oral one using dissection scissors, cutting carefully to avoid deep cuts that might cause internal structure damage. This was followed by a cut of the body wall in the same direction as in the previous cut. To visualize the internal structures, Harris’s hematoxylin or Bengal Rose were used as a stain. To finish, the pharynx was removed, allowing the structures found inside to be observed. Monniot, Monniot and Laboute (1991) and Rocha, Zanata and Moreno (2012) keys were used for taxonomic identifications, as well as the original descriptions of the species and taxonomic reviews of each species. Specimens were deposited at the Museum of Zoology of the University of Costa Rica.
Taxonomic list: Research on literature and collections databases of the National Museum of Natural History (NMNH), Smithsonian Institution (https://collections. nmnh.si.edu) were performed. Based on this information, and the new material collected for the present study in the North Pacific, an updated list of both solitary and colonial ascidians species present in Costa Rica was developed.
Results
A total of eight species (70 specimens) were obtained from the sampling, corresponding to five genera, four families and three orders within the class Ascidiacea. The species richness was higher in Santa Elena Gulf (8 species) and lower in the Murcielago Islands (4 species). The most frequent species in both areas was Rhopalaea birkelandiTokioka, 1971 (Table 1).
Table 1 Ascidian species (Subphylum Tunicata, Class Ascidiacea) found in different locations of Área de Conservación Guanacaste, North Pacific Costa Rica.
| Species | Murciélago Islands | Santa Elena Gulf | |||||||
| PI | CS | BLV | BR | BV | MÑ | PT | TB | LC | |
| Ascidia sideralis | X | X | X | X | X | ||||
| Ascidia sidneyensis | X | X | |||||||
| Pyura lygnosa | X | X | X | X | |||||
| Pyura carmanae | X | X | X | X | X | ||||
| Pyura bradleyi | X | X | |||||||
| Microsmus cf. exasperatus | X | X | |||||||
| Poliandrocarpa anguinea | X | ||||||||
| Rhopalaea birkelandi | X | X | X | X | X | X | |||
PI: Puerta de la Iglesia, CS: Colorada Sur, BLV: Bajo La Vita, BR: Bajo Rojo, BV: Bajo Viejón, MÑ: Muñecos, PT: Pitahaya, TB: Tomas Bay, LC: Los Cabros.
In our sampling, we found three of the 17 species previously reported, plus five new reports for the North Pacific area, totalling 22 species in Costa Rica (Table 2). From ascidians previously reported, 16 belong to the North Pacific and one to Puntarenas (Central Pacific).
Table 2 Complete list of ascidian species reported for Costa Rica.
| Order/Family | Species | Reported by |
| Aplousobranchia | ||
| Diazonidae | Rhopalaea birkelandi (Tokioka, 1971) | Tokioka, 1971, 1972; Nova et al,. 2010; NMNH; this study |
| Rhopalaea abdominalis (Sluiter, 1898) | NMNH | |
| Didemnidae | Didemnum cf. perlucidum (Monniot, 1983) | Roth et al., 2017 |
| Didemnum moseleyi (Herdman, 1886) | Tokioka, 1972; Nova et al., 2010 | |
| Didemnun candidum (Savigny, 1816) | Tokioka, 1972 | |
| Lissoclinum caulleryi (Ritter & Forsyth, 1917) | Tokioka, 1972; Nova et al., 2010 | |
| Lissoclinum fragile (Van Name, 1902) | Tokioka, 1972 | |
| Diplosoma listerianum (Milne- Edwards, 1841) | NMNH | |
| Polycitoridae | Cystodytes dellechiajei (Della Valle, 1877) | NMNH |
| Polyclinidae | Aplidium constellatum* (Van Name, 1902) | Tokioka, 1972 |
| Polyclinum laxum (Van Name, 1945) | Tokioka, 1972 | |
| Phlebobranchia | ||
| Ascidiidae | Ascidia ceratodes (Huntsman, 1912) | Tokioka, 1972; Nova et al., 2010 |
| Ascidia sydneiensis (Stimpson, 1855) | This study | |
| Ascidia sideralis (Rocha, 2011) | This study | |
| Stolidobranchia | ||
| Pyuridae | Microcosmus cf. exasperatus (Heller, 1878) | This study |
| Pyura bradleyi (Van Name, 1931) | This study | |
| Pyura carmanae (Rocha, 2019) | This study | |
| Pyura lignosa (Michaelsen, 1908) | Tokioka, 1972; this study | |
| Styelidae | Botryllocarpa viridis* (Pizon, 1908) | Tokioka, 1972 |
| Eusynstyela tincta* (Van Name, 1902) | Tokioka, 1972; Nova et al., 2010; NMNH | |
| Polyandrocarpa anguinea (Sluiter, 1898) | This study | |
| Styela canopus* (Stimpson, 1852) | Tokioka, 1972 | |
*Species name has been modified according to WoRMS database.
Systematics
In this section, we made a brief description of the eight species collected through our sampling in Área de Conservación Guanacaste. We only considered the most particular characteristics of the species or characteristics that have slight variations, mainly structure counts. These variations do not produce any changes in the taxonomic identity of the species and these are mentioned in order to broaden the known range in terms of the number of structures. The characteristics not mentioned in the description match with the original description of each species.
Subphylum Tunicata Lamarck, 1816
Class Ascidiacea Blainville, 1824
Order Phlebobranchia Lahille, 1886
Family Ascidiidae Herdman, 1882
Genus Ascidia Linnaeus, 1767
Ascidia sideralisBonnet & Rocha, 2013
Material Examined: 14 specimens (MZUCR-ASC-0066, MZUCR-ASC-0071, MZUCR-ASC-0079, MZUCR-ASC-0081, MZUCR-ASC-0089, MZUCR-ASC-0093).
Description: The specimens present a greyish tunic, whose coloration can vary to reddish and blue tones; characterized by the presence of white dots that become more abundant near the siphons and conspicuous musculature on the right side of the body. The individuals examined match with the original description by Bonnet, Rocha and Carman (2013); however, they present two remarkable differences. Individuals (two) with only four oral siphon lobes were found, thus expanding the range reported in the original description (from seven to eight lobes). The same situation occurs for the oral tentacles. Ranges from 76 to 92 tentacles were reported in the literature, but we found individuals with numbers ranging from 35 to 72. Individuals belonging to this species are quite abundant in the North Pacific area (Fig. 2A).
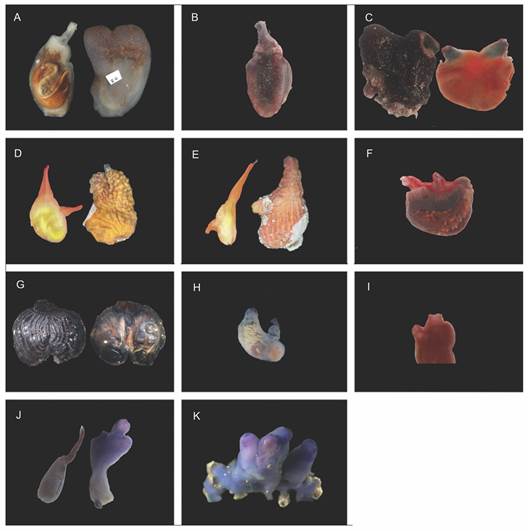
Fig. 2 Specimens collected in Área de Conservación Guanacaste, North Pacific of Costa Rica. A. Ascidia sideralis; B. Ascidia sydneiensis without the tunic; C. Pyura lignosa, D. Pyura carmanae; E. Pyura carmanae, a specimen that represents the group that differs in morphology regarding the tunic, siphons, and does not have developed gonads; F. Pyura bradleyi; G. Microcosmus cf. exasperatus; H. Polyandrocarpa anguinea zooid; I. Rhopalaea birkelandi, pink specimen without tunic; J. Rhopalaea birkelandi blue zooid, on the left side the abdomen, on the right side the thorax; K. Colony of R. birkelandi with four blue zooids. Scale = 1 cm.
Substrate: Rocky bottom.
Distribution: Panama (Bonnet et al., 2013) and Costa Rica (this study, North Pacific).
Ascidia sydneiensisStimpson, 1855
Material Examined: six specimens (MZUCR-ASC-0072, MZUCR-ASC-0076).
Description: Individuals with pale greyish tunic and characterized for having a distinctive musculature, on the margin of the right side of the body, muscle fibers project from the margin to the interior without reaching the center. The characteristics of the examined specimens are consistent with the original description (Stimpson, 1855) and reports from Panama (Bonnet & Rocha, 2011; Bonnet et al., 2013) (Fig. 2B).
Substrate: Artificial substrate.
Distribution: Australia (Stimpson, 1855), Brazil (Granthom-Costa et al., 2016), Cuba and Puerto Rico (Van Name, 1921), Guadeloupe and Martinique (Monniot, 1985), Costa Rica (this study, North Pacific), Panama (Carman et al., 2011), Colombia (Sluiter, 1898), Mozambique (Monniot & Monniot, 1976), Zanzibar (Traustedt & Weltner, 1891), South Africa (Millar, 1962), India (Swami & Chapgar, 2002;Ali et al., 2009), Guam (Lambert, 2002), Virgin Islands, Belize, Mexico, French Polynesia, Hawaii, Japan, Phillipines and Papua New Guinea (https://collections.nmnh.si.edu/search/iz/).
Order Stolidobranchia Lahille, 1886
Family Pyuridae Hartmeyer, 1908
Genus Pyura Molina, 1782
Pyura lignosa Michaelsen, 1908
Material Examined: five specimens (MZUCR-ASC-0070, MZUCR-ASC-0074, MZUCR-ASC-0084).
Observations: A thick and robust tunic characterizes the collected specimens. The siphons are both in the apical position and have a globose shape with a greenish tonality, unlike their body, which is orange. In these individuals, the longitudinal vessels extend at the end of the folds near the esophagus. The observed characteristics of the specimens match the description provided by Michaelsen (1908) and the review by Monniot (1994) (Fig. 2C).
Substrate: Rocky bottom.
Distribution: Costa Rica (this study, North Pacific), Central Pacific (Michaelsen, 1908), Panama-Pacific (Carman et al., 2011), Mexico (Gulf of California) (Van Name, 1931), Majuro, Japan, Philippines (Nishikawa, 1984).
Pyura carmanaeRocha, 2019
Material Examined: 17 specimens (MZUCR-ASC-0083, MZUCR-ASC-0085, MZUCR-ASC-0090, MZUCR-ASC-0091, MZUCR-ASC-0094).
Observations: The characteristics of these individuals match with those given in the original description of the species (Rocha & Counts, 2019). Three specimens differ in the position of the siphons, since they are widely separated one from each other: the oral in apical position and the exhalant in a lateral position. One of these three specimens has gonads only on the left side of the body wall, larger than in other specimens, and completely covering the first intestinal loop. These three individuals could be a different species, but related to P. carmanae. However, two of these specimens did not present gonads, so we could not make a satisfactory comparison between the internal structures of these three with the rest of the examined specimens. We consider the use of genetic tools to test this hypothesis. (Fig. 2D, Fig. 2E).
Although it has already been pointed out by Rocha and Counts (2019), it is important to mention that Tokioka (1972) described organisms belonging to the species P. carmanae as Pyura vittata. In this updated list we discard the presence of P. vittata as part of the Costa Rican marine fauna studied so far.
Substrate: Rocky bottom.
Distribution: Panama (Rocha & Counts, 2019), Costa Rica (this study, North Pacific).
Pyura bradleyiVan Name, 1931
Material Examined: three specimens (MZUCR-ASC-0075, MZUCR-ASC-0078, MZUCR-ASC-0092).
Observations: The individuals examined had an opaque tunic covered by sand, which matches with the reported by Van Name (1931) in the original species description. Furthermore, when observing the internal structures, a strong red coloration can be seen on the siphons and the whole mantle has a reddish color. Under its branched tentacles, red dots can be observed, which are also present under the branchial sac. Similar to that observed by Van Name (1931), its dorsal lamina is divided into barbs and there are six folds on each side of the body. Regarding the original description, these specimens showed a remarkable difference in terms of the number of gonadal sacs. The original description indicates that there are about 50 elongate-oval sacs per gonad; our specimens showed up to 106 on the left side and 99 on the right side, increasing the range for this characteristic (Van Name, 1931). (Fig. 2F).
Substrate: Rocky bottom and artificial substrate.
Distribution: Peru (Van Name, 1931), Ecuador (Van Name, 1931) and Costa Rica (this study, North Pacific).
Genus Microcosmus Heller, 1877
Microcosmus cf. exasperatus Heller, 1878
Material examined: seven specimens (MZUCR-ASC-0077, MZUCR-ASC-0082).
Observations: Large individuals, up to 4.5 cm in length, with a dark reddish tunic, the mantle has a mixture of light orange and yellow. In general, the characteristics of the examined specimens match with the original description of the species (Van Name, 1945). Furthermore, they match with the diagnosis reported by Rocha, Bonnet, Baptista and Beltramin (2012). However, we have not been able to properly find the velum of these individuals in order to check the presence and shape of the spines, and therefore, to confirm the specimens identification (Fig. 2G). It is the first time that M. cf. exasperatus has been reported in Costa Rica.
Substrate: Rocky bottom and artificial substrate.
Distribution: Bermuda, U.S. (Florida), Cuba, Jamaica, Haiti, Puerto Rico, Saint Thomas, St. Lucia, Guadalupe, Martinica, Granada, Belize, Panama, Colombia, Aruba, Curaçao, Venezuela, Brazil (Ceará to Santa Catarina), Senegal, Sierra Leone, Liberia, Ghana, South Africa (Rocha, Zanata and Moreno, 2012), Israel, India (Ratnasingham & Hebert, 2007), Mediterranean Sea, Atlantic Ocean, Indo-Pacific (Rocha, Bonnet, et al., 2012) and Costa Rica (North Pacific).
Family Styelidae Sluiter, 1895
Genus Polyandrocarpa Michaelsen, 1904
Polyandrocarpa anguineaSluiter, 1898
Material examined: one specimen (MZUCR-ASC-0086).
Observations: Grayish and translucent colonies with a cartilaginous tunic; zooids more than 1 cm long. The zooids had approximately 25 smooth tentacles of two interspersed sizes and four folds on both sides of the branchial sac, which coincides with that described by Rodríguez (1977) and Rocha and Faria (2005) (Fig. 2H).
Substrate: Rocky bottom.
Distribution: South Africa, Brazil, Mozambique in the Eastern Atlantic, Mauritius in the Indian Ocean, and in the Tropical Pacific Ocean (Monniot, 1987), Martinica (Monniot, 2018), Panama (Carman et al., 2011), United States (Florida) (Ratnasingham & Hebert, 2007) and Costa Rica (this study, North Pacific).
Order Aplousobranchia Lahille, 1886
Family Diazonidae Seeliger, 1906
Genus Rhopalaea Philippi, 1843
Rhopalaea birkelandiTokioka, 1971
Material examined: 17 specimens (MZUCR-ASC-0067, MZUCR-ASC-0068, MZUCR-ASC-0069, MZUCR-ASC-0073, MZUCR-ASC-0080, MZUCR-ASC-0087, MZUCR-ASC-0088).
Observations: In the examined specimens we noticed two different colors for the tunic: pink and blue. Tokioka (1971) describes this specie as solitary, however, we observed that the blue individuals were linked under the substrate. As for the pink individuals, we observed that they are much more abundant. All the specimens were found mainly on porous rocks, where their tunic extends through the crevices, up to about 10 cm.
Rhopalaea birkelandi has two body parts separated by a thin and fragile peduncle. This, added to the insertion of the tunic in the rock, can lead to the separation of both sections during the extraction and dissection process. Comparing the original description of the species with our specimens, we noted that the peduncle is not mentioned. On the other hand, the original illustrations coincide with the morphology that we found in the specimens whose peduncle was damaged and consequently, the abdomen and thorax separated. Because of this, we believe that Tokioka may not have had access to specimens of R. birkelandi in good condition and thus, the description may be incomplete. (Fig. 2I, Fig. 2J, Fig. 2K).
Substrate: Rocky bottom.
Distribution: Costa Rica (North Pacific) (Tokioka, 1971; Tokioka, 1972; Nova et al., 2010; this study), Panama (Carman et al., 2011) and Mexico (Baja California Sur) (Ratnasingham & Hebert, 2007).
Discussion
Considering that ascidians have been barely studied in Costa Rica, this research shows the necessity of new sampling efforts to increase the known diversity. The present study reports for the first time the presence of five species in the Costa Rican Pacific and delivers an updated list of the 22 species of ascidians reported for Costa Rica.
The species richness and abundance in Santa Elena Gulf was higher than in Murcielago Islands. Both regions are under the effects of the seasonal upwelling phenomenon, which affects the entire North Pacific region (Alfaro et al., 2012; Rodríguez, & Morales, 2012). On the other hand, Santa Elena Gulf is an important artisanal fishing point, in contrast to the Murcielago Islands, which are within the protected Marine Sector of the Área de Conservación Guanacaste. Other studies have reported a greater abundance of ascidians in areas around human activity, which generates movement of organic particles that constitute food for ascidians and facilitate their growth (Naranjo, Carballo, & García-Gómez, 1996). The anthropic influence in Santa Elena Gulf could explain a higher ascidian diversity.
Rhopalaeae birkelandi is the most common and abundant species in our study, similarly, it has been reported as abundant and conspicuous in the rocky reefs of the Pacific of Central America (Tokioka, 1972). Nova and colleagues (2010) also found this species as the most abundant, reporting densities of up to 32 individuals per m2 in Cuajiniquil Bay. The presence of vanadocytes and the high acidity (pH < 2) of their tunic, mainly near the siphons, can reduce epibionts and predation, being abundant in sites with a high predation rate (Stoecker, 1980).
Before this study, there were 17 ascidian species reported for Costa Rica; from these, 13 are colonial. We found only three of the previous ascidians reported (R. birkelandi, Pyura lignosa and Poliandrocarpa anguinea) but our study was focused on the diversity of solitary ones. This approach allowed us to report five species of solitary ascidians for the first time. It is noteworthy that we did not find Ascidia ceratodes, despite being solitary and having been reported as common in the area (Nova et al., 2010).
The marine regions of Costa Rica are considered rich in terms of species diversity (Wehrtmann, Cortés, & Echeverría, 2009). In the case of ascidians, there are reports for only one region of the country, the North Pacific (Cortés, 2009). Most of the studies took place in that region of Costa Rica or referred to individuals collected in that area. Differences in sampling effort, and not a low ascidian diversity in other regions, can explain this lack of data. Costa Rica’s ascidian species inventory is undoubtedly underestimated. In a close by area of the Caribbean, Northwestern Panama, Rocha and colleagues (2005) reported a total of 58 ascidian species, from which 14 were new to science. In fact, we have observed the presence of colonial ascidians in regions such as Cahuita and Punta Uva, on the South Caribbean of Costa Rica, without having the opportunity to study them until now.
Among the ascidians reported in Costa Rica, we find P. anguinea, a species that has been introduced to many countries around the world (Evans et al., 2017). This species, native of Mozambique (Sluiter, 1989), has been found in Brazil (Rodríguez, 1977), Panama (Carman et al., 2011), Mauritius (Monniot, 1987), Martinique (Monniot, 2018) and the Atlantic Ocean in the United States (Villalobos, Lambert, Shenkar, & López-Legentil, 2017). Some introduced species could become invasive (Blackburn et al., 2011), changing the ecosystems by modifying ecological relations and even harming the survival of native species (Evans et al., 2017; Molnar, Gamboa, Revenga, & Spalding, 2008). Taking into account the ability of P. anguinea to become invasive, we recommend monitoring the population of this species in the country.
In conclusion, new samplings in the North Pacific and the compilation of previous reports from the literature allowed us to extend the known diversity of ascidians in Costa Rica, updating the knowledge about this group. We report five species for the first time and provide a list that includes until now, 22 species in total, the majority found in the North Pacific region. Different sampling efforts, but not presumably low ascidian diversity in other regions, suggest the necessity of samplings along with other places in the Pacific and the Caribbean, which for the latter there are no reports. Future investigations should include colonial ascidians, the use of molecular tools, and ecological studies that can clarify the importance of ascidians, and also monitor the impact of exotic species in the area.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


