In 2012, 915 000 new cases, and 723 000 deaths of gastric cancer (GC) were registered, making it one of the malignant tumors with the highest incidences and mortalities worldwide (Ferlay et al., 2015). To this date, few clinical parameters have proven its accurate early diagnosis and prognosis. This poses an important challenge, namely the identification of factors that can predict gastric carcinogenesis, and that can thus be used in clinical practice for prevention, early diagnosis, prognosis and treatment.
Infection with Helicobacter pyloriis the most recognized risk factor for developing GC (El-Omar et al., 2000b; Uemura et al., 2001; Correa & Houghton, 2007). However, it remains an enigma that only a small percentage of people infected with H. pyloridevelop cancer. The final clinical outcome of the infection depends on complex interactions between characteristics of the infecting strain of the bacterium, the host immune response and environmental factors (Kodaman et al., 2014). The composite and multifactorial etiology of GC presents a second challenge in the battle against the disease; the elucidation of the biological mechanisms underlying how and under which circumstances H. pylori alter normal physiological processes in such a way that sequential events culminate in the development of GC.
Remodeling of the extracellular matrix is a key event during malignant cell invasion and metastasis. It is principally mediated by two families of proteases: matrix metalloproteases (MMPs) and the plasminogen activation (PA) system (Egeblad & Werb, 2002; Danø et al., 2005). The expression of some members of these families has been correlated to certain clinical and pathological parameters of GC. The understanding of the mechanisms that regulate invasion and metastasis of gastric tumors is of great importance for the possibility to predict the clinical course of the disease, optimize treatment and improve the life quality of the patients. This review provides a summary of epidemiological, clinical and biological aspects of GC, as well as an update of studies relating to the PA system in GC and its potential implications in clinical practice. Furthermore, recent findings that suggest the participation of H. pyloriin the induction of some of the components of the PA system are discussed.
Epidemiological aspects of gastric cancer
Gastric cancer is the fifth most common malignant tumor worldwide, following cancer of the lung, breast, colorectum and prostate (Ferlay et al., 2015). Incidence rates vary considerably depending primarily on age, sex and geographic location (Catalano et al., 2005; Forman & Burley, 2006). The majority of GC patients are over 50 years old when diagnosed and it affects two men for each woman (Catalano et al., 2005; Forman & Burley, 2006; Brenner, Rothenbacher & Arndt, 2009). The most substantial variation in incidence is geographic, with high incidence in the Eastern parts of Asia, Eastern Europe and the Pacific coast of Latin America. In contrast, South Asia, Western Europe, North America, Australia and Africa, generally show low incidence (Bertuccio et al., 2009; Sierra, Cueva, Bravo &Forman, 2016).
In the last decades, the incidence of GC has fallen substantially in high- as well as low-risk areas. The reasons for this are not completely understood, although a concomitant decrease in H. pyloriprevalence is probably partly responsible (Parkin, Bray, Ferlay, & Pisani, 2005; Bertuccio et al., 2009). The decrease in incidence depends, however, on the histological type and anatomical location of the tumor. The intestinal subtype of GC has become much less frequent, whereas diffuse GC remains more or less stable (Parkin, 2001). Also, the rate of cancer of the cardias is stable, if not increasing, while distal adenocarcinomas in the lower part of the stomach are becoming less common (Kamangar, Dores, & Anderson, 2006; Brenner et al., 2009).
With respect to mortality, GC occupies the third place on a global level, after lung and hepatic cancer (Ferlay et al., 2015). Mortality rates vary geographically and are particularly high in developing countries, although a decrease has been observed during the last decades (Forman & Burley, 2006; Bertuccio et al., 2009; Ferlay et al., 2015; Sierra et al., 2016). The five year survival rates are lower than 30 % in most countries due to the fact that the majority of cases are diagnosed at advanced stages when therapy is likely to fail (Forman & Burley, 2006). Studies in population groups with same ethnic background but dissimilar access to health services, suggest that environmental and biological factors may also play an important role in explaining differences in mortality and survival of gastric cancer between high- and low-risk countries, or developing and developed economies (Redaniel et al., 2009).
Histopathology
There are different systems for classifying GC by microscopic and histological characteristics, namely the Japanese system, World Health Organization (WHO) classification and Lauren (Lauren, 1965; Japanese Gastric Cancer, 1998; Catalano et al., 2005). Among those mentioned, Lauren classification system is the most commonly used and divides GC into two subtypes; intestinal and diffuse with important differences at the epidemiological, pathological and molecular levels (Lauren, 1965; Catalano et al., 2005; Dicken et al., 2005; Correa & Houghton, 2007; Nobili et al., 2011; Yasui et al., 2011). The reader is referred to Yakirevich and Resnick (2013) for a more detailed discussion about the main differences between intestinal and diffuse subtypes of GC, since this is out of the scope of the present review.
The anatomical location of the tumor in the stomach is also considered an important parameter in the classification of GC. Two major types can be distinguished; the tumors located in the distal region (non-cardia cancer) and in the proximal region (cardia cancer) (McColl, 2006). These two anatomical subtypes are different in their etiology and epidemiology (Hansen et al., 2007; Derakhshan et al., 2008; Bertuccio et al., 2009; Wang et al., 2013). Some of the main differences between anatomical subtypes of GC have been addressed with more detail by previously published excellent reviews (McColl, 2006; Colquhoun et al., 2015; Huang, Sun, Fang, Zhou, & Zou, 2016).
Risk factors
Among the risk factors for developing GC are diet and other features of life style, genetic predisposition of the individuals, infection with H. pyloriand the chronic and persistent inflammation that is generated in its presence (Catalano et al., 2005; Fock et al., 2008; Peek, Fiske, & Wilson, 2010; Müller, Oertli, & Arnold, 2011; Tramacere et al., 2012; Salama, Hartung, & Müller, 2013; Petrovchich & Ford, 2016).
Diet has a dual role in the etiology of GC. Some foodstuffs, such as many fruits and vegetables, contain antioxidants that will reduce the formation of, or neutralize the activity of carcinogenic substances. On the contrary, other classes of food are sources of recognized carcinogens or their precursors (Tricker & Preussmann, 1991; Larsson Bergkvist, & Wolk, 2006a; Larsson, Orsini, & Wolk, 2006b; Liu & Russell, 2008; Tsugane & Sasazuki, 2007).
Around 10 % of GC cases show familial aggregation, with two or three first grade relatives affected. This does not necessarily imply a genetic predisposition given that in addition to genetics, families share cultural and environmental influences (Brenner et al., 2000). Nevertheless, there do exist a number of inheritable germ-line mutations and well-characterized genetic syndromes that increase the risk of developing GC (Lynch, Grady, Suriano, & Huntsman, 2005; Oliveira, Seruca, & Carneiro, 2006; Petrovchich & Ford, 2016). Among the genetic variations that predispose for GC are germ-line mutations in the gene CDH1(Guilford et al., 1998; Huntsman et al., 2001; Carneiro, Oliveira, Suriano, & Seruca, 2008; Hansford et al., 2015). This gene codes for the protein E-cadherin, which is expressed by epithelial cells and functions as an adhesion molecule (cell-cell interaction) thus inhibiting cellular invasion (Nagar, Overduin, Ikura, & Rini, 1996). GC associated with mutations inCDH1exhibit an autosomal dominant inheritance pattern with a penetration of more than 70 %. Most of the GC cases attributed to mutations inCDH1are of the diffuse histological subtype, and the malignancy appears at younger age than GC cases not having germ-line mutations in this gene (Guilford et al., 1998; Huntsman et al., 2001; Carneiro et al., 2008; Carneiro, 2012; Hansford et al., 2015).
Infection withH. pyloriis one of the most prevalent bacterial infections. It is estimated that about half of the world’s population is infected (Parkin, 2006).H. pylorihas been decisively linked to several gastric pathologies, such as atrophic gastritis, gastric and duodenal ulcers, MALT-lymphomas and GC. In recent years, the infection has also been inversely associated with a range of extra-gastric pathologies, like asthma, allergies and adenocarcinoma of the esophagus (Blaser, 2008; Blaser, Chen, & Reibman, 2008; Chen & Blaser, 2008; Imamura et al., 2010; Kyburz & Müller, 2017). In spite of the aforementioned, the vast majority of infected people remain asymptomatic throughout their life. It is the combination of bacterial, host and environmental factors that determine the ultimate clinical picture.
H. pyloriis genetically highly variable and some strains are more strongly associated with GC. The most prominent are those that express the virulence factors VacA and Cag pathogenicity island (Cag-PAI) (Yamaoka, 2010; Cover, 2016). VacA is a cytotoxic protein which is expressed by the genevacA. It induces the formation of vacuoles that generate intracellular damage in the gastric epithelium (Odenbreit et al., 2000; Polk & Peek, 2010; Wroblewski, Peek, & Wilson, 2010). Although all strains ofH. pyloriproduce VacA, the level of expression varies as a function of polymorphisms at three specific sites of the gene vacA: the signal region (s), the middle region (m) and the intermediate region (i) (Cover & Blanke, 2005; Rhead et al., 2007). Certain haplotypes of these polymorphic sites have been associated with GC (Rhead et al., 2007). The Cag-PAI-positive strains possess a functional genetic region which contains approximately 30 genes that code for proteins that together make up a type IV secretion system. On average, functional Cag-PAI is expressed by about 60 % ofH. pyloristrains but there are considerable variations in the prevalence of these strains between populations. The secretion system introduces a number of molecules, including the virulence factor CagA, into the cytoplasm of epithelial cells of the gastric mucosa (Shaffer et al., 2011). Studies performed with transgenic mice have demonstrated that CagA is an oncoprotein capable of inducing cancer in the gastric epithelium (Ohnishi et al., 2008). Once in the cytoplasm, CagA is phosphorylated and this triggers downstream intracellular events such as cytoskeletal rearrangement, alterations in cellular polarity, expression of inflammatory mediators and activation of signaling pathways that promote cellular proliferation (Tan, Tompkins, & Amieva, 2009; Polk & Peek, 2010; Hatakeyama, 2014; Naumann, Sokolova, Tegtmeyer, & Backert, 2017).
Other Cag-PAI-related pathogenicity mechanisms, independent of translocation and phosphorylation of CagA, have been described (Backert & Blaser, 2016; Naumann et al., 2017). There is no known physical or functional relation betweenvacAandcag-PAI. Nevertheless, strains that express virulent VacA usually contain functional Cag-PAI (Rhead et al., 2007; Basso et al., 2008). In addition to VacA and CagA, other, less well-characterized virulence factors ofH. pylorihave been associated to gastric pathology, for example BabA, SabA, OipA and DupA (Wroblewski et al., 2010; Cover, 2016; Naumann et al., 2017).
Pathogenesis
The pathogenesis of GC is a complex and multifactorial process (Correa, 1992; Correa, 2004; Hayakawa, Sethi, Sepulveda, Bass, & Wang, 2016). Infection with H. pylori, in combination with environmental factors, results in superficial gastritis that, when persisting, develops into chronic gastritis, in many cases subclinical. The majority of infected people never progress to severe pathology and many remain asymptomatic, whereas others develop gastric diseases that are not related to GC, such as duodenal ulcers. However, in some individuals, a severe and persistent inflammation evolves into chronic atrophic gastritis, characterized by the disappearance of glands and specialized cells in the gastric mucosa (Correa, 1992; Correa, 2004). Chronic atrophic gastritis is considered to be a precancerous lesion, both for the intestinal and diffuse subtypes (Correa, 1992; Correa, 2004; Yuasa, 2003; Fox & Wang, 2007). The biological mechanisms underlying such diverse clinical outcomes resulting from infection with H. pyloriare far from understood. It has been suggested that factors involved are the genetics of the infecting strain, polymorphic variants of genes regulating the host inflammatory response and the anatomical location of the tissue affected by chronic gastritis (El-Omar et al., 2000a; Alpízar-Alpízar, Pérez-Pérez, Une, Cuenca, & Sierra, 2005; Kodaman et al., 2014; Suerbaum &Michetti, 2002).
The subsequent step in the cascade of events leading towards intestinal GC is the transformation of the gastric epithelium intoa morphology and histology that resembles intestinal epithelium, known as intestinal metaplasia (Correa, 1992; Correa, Piazuelo, & Wilson, 2010; Goldenring, Nam, & Mills, 2011; Suh et al., 2012). This new environment is not a favorable niche forH. pylori, which tends to be spontaneously eradicated from the transformed tissue (Valle, Kekki, Sipponen, Ihamaki, & Siurala, 1996; Kokkola et al., 2003). The continued progression of the lesions involves dysplasia, characterized by histological alterations of the epithelium such as nuclear atypia, loss of polarity and irregular shape of the gastric epithelial cells. In advanced stages, the dysplastic cells acquire the capacity to migrate across basal membranes and surrounding tissue, thus completing the malignant transformation that culminates with the formation of early invasive carcinomas (Correa, 1992; Correa, 2004).
As for the diffuse subtype, no readily detectable gastric preneoplastic lesions following chronic atrophic gastritis have been described and many aspects about its etiology remain unknown. As already mentioned, a small number of cases of this particular subtype are attributed to mutations in the gene coding for E-cadherin, a genetic syndrome known as hereditary diffuse GC (Carneiro, 2012; Hansford et al., 2015).
The remodeling of the extracellular matrix
A key feature of malignant transformation and progression is the invasion of malignant cells to the adjacent stroma and, in advanced stages, to distant sites (metastasis) (Hanahan & Weinberg, 2011; Lambert, Pattabiraman, & Weinberg, 2017). Metastasis is the result of a stepwise sequence involving the individual or collective invasion of neoplastic cells locally, their entry into the vascular system (intravasation) and survival in circulation, the extravasation at distant sites and, finally, the establishment and colonization by disseminated tumor cells in new tissues and organs (Valastyan & Weinberg, 2011; Lambert et al., 2017). The degradation of the extracellular matrix, or remodeling of tissue, is essential in several phases of this process. This remodeling is mediated by proteases that are capable of degrading virtually any component of the extracellular matrix (Danø et al., 1999; Kessenbrock, Plaks, & Werb, 2010). These proteases belong to two main groups: the MMPs and the PA system. This review is focused on the PA system and its relation togastric carcinogenesis.
The plasminogen activation system
This system comprises a few proteins that by acting in sequence lead to the conversion of zymogenic plasminogen into its active enzymatic form, plasmin. Plasmin is a serine-protease capable of breaking down components of the extracellular matrix such as fibrin, fibronectin, laminins and vitronectin (Liotta et al., 1981). Other substrates of plasmin are TGFβ, the zymogenic form of its own activator (pro-uPA) and some pro-MMPs (Lamarre, Vasudevan, & Gonias, 1994; Lijnen, 2002). Plasminogen is activated after proteolytic cleavage by either of the two well-characterized serine-proteases: urokinase-type and tissue-type plasminogen activators (uPA and tPA, respectively). tPA is relevant in generating plasmin for vascular fibrinolysis, whereas uPA plays a crucial role in processes involving tissue remodeling, both in homeostasis and pathologies like cancer (Kristensen et al., 1984; Andreasen, Egelund, & Petersen, 2000); uPA is, therefore, responsible for the conversion of plasminogen into plasmin in the context of tumor invasion. This occurs after binding of pro-uPA to its cognate membrane receptor, uPAR, which results in uPA activation and, subsequently, plasminogen activation in the surface of uPAR expressing cells (Ploug, 2003). The activity of tPA and uPA is mainly controlled by two endogenous inhibitors, PAI-1 and PAI-2, the former being the primary regulator of the plasminogen activation in cancer (Andreasen, Georg, Lund, Riccio, & Stacey, 1990; Behrendt, List, Andreasen, & Danø, 2003). The expression levels of uPA, uPAR and PAI-1 under normal homeostatic conditionsis almost undetectactable, however in cancer and other pathologies, their expression increase significantly (Kriegbaum et al., 2011). The stepwise process leading to plasminogen activation is summarized in Figure 1.
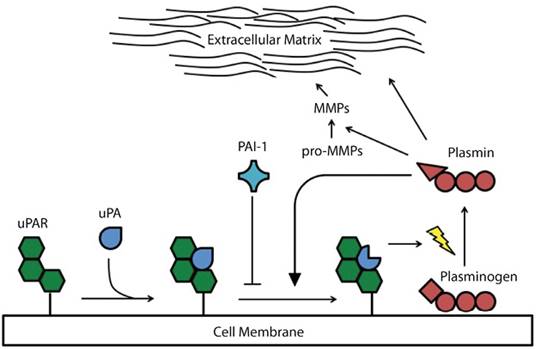
Figure 1 The members of the plasminogen activation system and the mechanism of plasminogen activation: Binding of pro-uPA to its specific receptor, uPAR, causes the conversion of plasminogen into plasmin, which is achieved by proteolytic cleavage. Newly-formed plasmin molecules further catalyze the conversion of pro-uPA into uPA, and this, in turn, accelerates plasminogen activation. PAI-1 inhibits this sequential series of events by binding to the uPA-uPAR complex. Plasmin can degrade several components of the extracellular matrix, but it can also activate some MMPs.
The PA system is involved in several key aspects of cancer development and progression, including local invasion, metastasis, tumor growth and angiogenesis (Ossowski, Russo-Payne, & Wilson, 1991; Bugge et al., 1998; Bajou et al., 2001; Binder & Mihaly, 2008). Some members of the system may also elicit important cellular and physiological effects independent of their role in plasminogen activation. By interacting with integrins, uPAR induces activation of cell signaling pathways related to increased proliferative, invasive and metastatic potential (Yu, Kim, & Ossowski, 1997; Blasi & Carmeliet, 2002; Liu, Aguirre-Ghiso, Estrada, & Ossowski, 2002; Smith & Marshall, 2010). PAI-1 plays a seemingly important role in cancer cell detachment, which is mediated by its binding to extracellular matrix proteins such as vitronectin and type-1 collagen that may ultimately lead to enhanced dissemination of these cells (Czekay, Aertgeerts, Curriden, & Loskutoff, 2003). In general, uPA, uPAR and PAI-1 are mainly expressed by non-neoplastic cells of the peritumoral stromal compartment and, to lesser extent, by cancer cells located at the invasive front of the malignant growth (Lund, Illemann, Thurison, Christensen, & Høyer-Hansen, 2011), being there where their pro-invasive and pro-metastatic effects are exerted.
The expression of the three components of the PA system is generally increased in cancer and this has proven to be of prognostic relevance. The association between high levels of uPA, uPAR and PAI-1 and poor prognosis has been convincingly demonstrated in colorectal, breast, ovarian, prostatic and gastroesophageal cancer (Duffy et al., 1990; Grøndahl-Hansen et al., 1993; Pedersen et al., 1994a; Pedersen et al., 1994b; Grøndahl-Hansen et al., 1995; Stephens et al., 1999; Foekens et al., 2000; Høyer-Hansen & Lund, 2007; Almasi, Høyer-Hansen, Christensen, & Pappot, 2009; Almasi et al., 2011; Lund et al., 2011; Brungs et al., 2017). Using immunohistochemistry, it was demonstrated that uPAR is a factor that predicts patients overall survival in gastroesophageal cancer (Laerum et al., 2012). The relevance of uPAR as prognostic biomarker has also been suggested, by using immunohistochemical means, in oral squamous cell carcinoma (Lindberg, Larsson, & Nielsen, 2006; Bacchiocchi et al., 2008). Moreover, it has been shown that presence of uPAR-expressing neoplastic cells in the bone marrow of GC patients is a prognostic parameter (Heiss et al., 1995a; Heiss et al., 2002). Therefore, it is clear that members of the PA system are potentially interesting candidates to be used in clinical practice as biomarkers to evaluate the prognosis of cancer patients.
The plasminogen activation system and gastric cancer
Several investigations have studied the expression pattern of the members of PA system in GC, as well as the mechanisms of induction and their clinical relevance as prognostic biomarkers. Most of these reports have focused on uPAR, since this receptor is crucial for the initiation of the sequential series of events that ultimately result in the activation of plasminogen. Interestingly, it has been suggested thatH. pylorimay induce the expression of uPAR in non-neoplastic gastric epithelial cells (Alpízar-Alpízar et al., 2010).
The expression pattern of the components of PA system in GC is very similar to what has been reported in other cancer types. The three members are mainly expressed by accessory (non-neoplastic) cells within the stroma surrounding the tumor growth, both in intestinal and diffuse subtype (Figure 2) (Alpízar-Alpízar et al., 2010). Macrophages located at the invasive front of the tumors are the cell population with the strongest contribution to the uPAR expression in GC. Myofibroblasts and endothelial cells at the same location also express uPAR, but their expression is not as pronounced as that of macrophages (Alpízar-Alpízar et al., 2010). Neutrophils are the fourth accessory cell type of the stromal compartment of the tumors that is positive for uPAR (Alpízar-Alpízar et al., 2010). Neutrophils, however, express this receptor constitutively (Plesner et al., 1994a; Plesner et al., 1994b), in contrast to macrophages, myofibroblasts and endotelial cells in which uPAR is induced by not yet identified factors that are present in the tumor microenvironment. The upregulation of uPAR in this area often generates an “intensity gradient”, which irradiates from the very front of the invasive tumor and dilutes out from this location (Figure 2). This phenomenon can also be seen in other cancer types, in particular colorectal cancer (Kriegbaum et al., 2011; Lund et al., 2011). Although the induction of uPA and PAI-1 in GC is not as well-documented as that of uPAR, these two molecules are primarily expressed by myofibroblasts at the invasive front of the malignant growth (Figure 2), similar to what has been observed in other cancer sites, including colorectal neoplasm (Illemann et al., 2009; Lund et al., 2011).
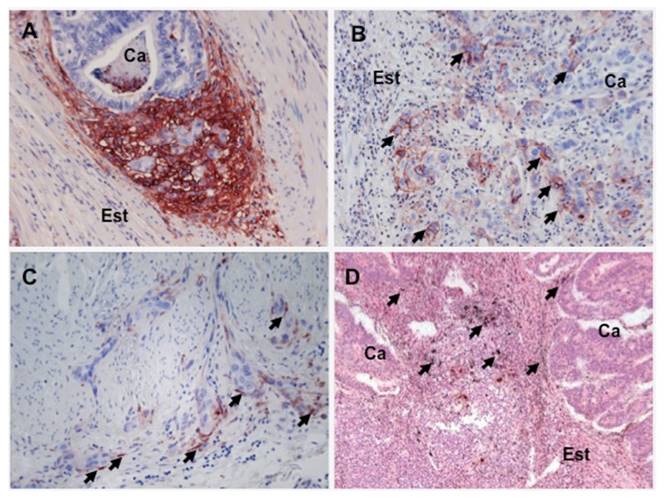
Figure 2 uPAR, PAI-1 and uPA expression in gastric cancer: uPAR expression at the invasive front of the tumor growth in GC is illustrated by immunohistochemical staining with a polyclonal antibody against human uPAR (panelsAandB). uPAR expression is strongly induced in the tumor stroma bordering the invasive front of tumor growth (panelA: Ca: cancer, Est: stroma). In GC lesions it is possible to observe uPAR expression in cancer cells (arrows in panelB), especially those localized at the invasive front of malignant growth, both in the intestinal (not shown in the figure) and in the diffuse subtype of GC (panelB). PAI-1 is also expressed in GC lesions, as evidenced by carrying out immunohistochemistry with a polyclonal anti-human PAI-1 antibody (panelB). PAI-1 is expressed by elongated stromal cells, similar to fibroblasts (arrows in panelC), which in colon cancer have been identified as myofibroblasts. PAI-1 positive cell are localized in close proximity or in direct contact with cancer cells invading the stroma (panelB). uPA expression is seen by performingin situhybridization in GC lesions with specific antisense probes against uPA mRNA, which is observed as black granular deposits (arrows in panelD). uPA expression is also prominent in the tumor microenvironment, specifically in accessory cells found in the tumor stroma bordering tumor growth (panelD).
uPAR expression in cancer cells has also been reported in GC (intestinal and diffuse subtype), particularly in detached cancer cells found within the tumor stromal microenvironment (Figure 2) (Migita et al., 1999; Alpízar-Alpízar et al., 2012; Alpízar-Alpízar et al., 2010). These uPAR-positive malignant cells are especially interesting in the context of tumor progression, since studies in colorectal cancer show that some of these cells co-express uPAR and its cognate ligand (uPA), as well as other molecules associated to increased invasive potential such as laminin-5 (Pyke et al., 1995; Illemann et al., 2009). It is speculated that these cells are particularly prone to invade and metastasize, which is also facilitated because of their increased potential to migrate (Chaurasia et al., 2006; Gårdsvoll & Ploug, 2007; Madsen & Sidenius, 2008). In fact, studies performed in GC indicate that the expression of uPAR in malignant cellsper seemerges as a feature of more aggressive tumors (Heiss et al., 1995a; Heiss et al., 2002; Alpízar-Alpízar et al., 2012). For example, studies performed in bone marrow aspirates obtained preoperatively from gastric cancer patients reveal that the presence of disseminated uPAR-positive tumor cells in bone marrow is strongly associated to poor overall survival, compared to patients with either uPAR-negative disseminated cells or no metastatic cells at all in bone marrow (Heiss et al., 1995a; Heiss et al., 2002). Also, Alpízar-Alpízar et al. (2012) studied the contribution of the different uPAR-expressing cell types that are found in the malignant growth to the overall survival of GC patients, revealing that the presence uPAR-positive cancer cells at the invasive front of the tumors can predict the survival of patients (Alpízar-Alpízar et al., 2012). Less that 10 % of the cancer cells show expression of uPAR in GC (Alpízar-Alpízar et al., 2010; Alpízar-Alpízar et al., 2012), however, as already mentioned, this fraction probably represents a more malignant subpopulation of cancer cells. The prevalence of uPAR-positive cancer cells may then be regardedas an indication of more aggressive gastric malignant tumors, highlighting the potential prognostic usefulness of this parameter.
The factor(s) inducing the expression of the members of PA system have not been identified, but some studies, most of them performedin vitro, have proposed a number of mechanisms by which this can occur. Several signaling pathways lead to the activation of transcription factors that recognize specific sequences within the promoter region of the gene coding for uPAR (PLAUR), thus inducing its expression in cancer lesions. Ras-ERK MAPK pathway is among the ones frequently active in cancer (Bamford et al., 2004). A transcription factor often activated by ERK kinase is AP-1, which has been implicated in the transcriptional regulation of uPAR (Lengyel et al., 1996b). In addition, the expression of uPAR seems to be upregulated in response to hypoxia, and this is associated to the binding of hypoxia-inducible factor-1α (HIF1α) to a hypoxia-response element (HRE) that is present in the promoter ofPLAUR(Krishnamachary et al., 2003). The nuclear factor-κB (NF-κB) has also been associated to the activation of uPAR expression by either, an indirect mechanism that involves transcriptional activation of the gene coding for HIF1α (Rius et al., 2008), or direct binding of NF-κB to specific sequences of the promoter ofPLAUR(Wang et al., 2000). The promoter ofPLAURalso has sequences that are specifically recognized by the transcription factors SP1 and AP2, which have been implicated in the induction of uPAR expression in human cancers, including GC (Schewe et al., 2003; Schewe et al., 2005; Maurer et al., 2007). Finally, some studies indicate that the expression of uPAR, uPA and PAI-1 in accessory cells of the tumor stroma is induced by growth factors and cytokines, among them several members of the fibroblast growth factor (FGF) family, TNFαand TNFβ(Yoshida et al., 1996; Sieuwerts, Martens, Dorssers, Klijn, & Foekens, 2002).
Studies performed with GC cell lines coincide with most of the above-mentioned mechanisms of induction for the components of the PA system in cancer. An example of this are the investigations concluding that both epidermal growth factor (EGF) and macrophage stimulating protein (MSP) induce the expression of uPAR via signaling pathways involving ERK-1/2, AP-1 and NF-κB (Baek et al., 2008; Park, Park, Khoi, Joo, & Jung, 2011). The activation of ERK, as a result of the EGF binding to its membrane receptor, c-MET, is also implicated in the upregulation of uPA in GC cell lines (Lee et al., 2006). Other molecules that have been studied in relation to the induction of uPAR and uPA in GC cell lines are interleukin-1β(IL-1β), lysophosphatidic acid (LPA) and macrophage inhibitory cytokine (MIC-1), although the evidence is less concluding in these cases (Iwamoto et al., 2003; Lee et al., 2003;Kim et al., 2008).
The relation between expression of components of the PA system, especially uPAR, and clinico-pathological aspects has been explored in GC (Heiss et al., 1995b; Kawasaki et al., 1998; Zhao, Wang, Qu, Huang, & Wang, 2002; Kaneko, Konno, Baba, Tanaka, & Nakamura, 2003; Lee et al., 2004; Zhang, Zhao, Ru, & Ma, 2006; Kita et al., 2009; Alpízar-Alpízar et al., 2012; Guo, Ling, Ma, Zhou, & Zhao, 2015). One of the parameters consistently correlated with the expression of the members of this system is patient survival, which is generally shorter in patients with elevated expression, particularly of uPAR (Table 1). This has been determined at the mRNA and protein level, using different types of biological material and methodological approaches (Table 1). In addition to patient survival, the expression of members of the PA system has been correlated to tumor size, differentiation grade, vascular and lymphatic invasion and lymph node involvement, among others (Kawasaki et al., 1998; Kaneko et al., 2003; Zhang et al., 2006; Guo et al., 2015). In most of these studies, the relation between expression of components of the PA system and clinico-pathological variables is analyzed regardless of the type and localization of the cells expressing uPAR, uPA or PAI-1 in the tumor microenvironment. This is mainly because most of the methodologies employed for the experimental quantification of the components do not allow this type of stratification. However, finely-tuned analysis of the relation between clinico-pathological parameters and subpopulations of cells that express uPAR, uPA and PAI-1 is highly desirable, because not all the cells within the tumor microenvironment make the same contribution to cancer growth and progression. This idea is supported by immunohistochemistry studies showing that uPAR-expressing cancer cells are particularly informative to predict patient survival in GC (Heiss et al., 2002; Alpízar-Alpízar et al., 2012). Overall, according to the existing evidence, uPAR emerges as the member of the PA system with more potential to be used in the clinical practice as prognostic biomarker in GC.
Table 1: Studies performed in gastric cancer to assess the prognostic value of uPAR, uPA and PAI-1 and their relation to other clinico-pathological parameters
| Author | n (patients) | Biologic Material | Method of measurement | Main finding |
|---|---|---|---|---|
| Alpízar-Alpízar et al., 2012 | 95 | Paraffin-embedded tissue | Immunohistochemistry | uPAR expression in cancer cells is associated to poor overall survival. It is a parameter independent of clinical variables. |
| Heiss et al., 2002 | 156 | Bone marrow | Immunohistochemistry | Presence of uPAR-positive cancer cells in bone marrow is associated to shorter progression-free and overall survival. It is a parameter independent of clinical variables. |
| Heiss et al., 1995b | 203 | Paraffin-embedded tissue | Immunohistochemistry | Elevated expression of uPAR, uPA and PAI-1 in neoplastic tissue is associated to shorter recurrence-free and overall survival, as well as to other clinico-pathological variables. PAI-1 expression is a parameter independent of clinical variables. |
| Kaneko et al., 2003 | 101 | Paraffin-embedded tissue | Immunohistochemistry | High uPAR and uPA expression in neoplastic tissue is associated to shorter overall survival. uPA is a parameter independent of clinical variables. |
| Kawasaki et al., 1998 | 91 | Paraffin-embedded tissue | Immunohistochemistry andin situhybridization | High uPAR and PAI-1 expression, simultaneously, correlates with venous and lymphatic invasion, lymphatic metastasis and depth of invasion (advanced cancer). |
| Kita et al., 2009 | 846 | Bone marrow and peripheral blood | qRT-PCR | Elevated uPAR mRNA levels in peripheral blood are an independent prognostic parameter for distant metastasis. |
| Lee et al., 2004 | 35 | Fresh tissue | RT-PCR y Northern blot | High uPAR expression in neoplastic tissue is associated to lower overall survival. |
| Zhang et al., 2006 | 105 | Paraffin-embedded tissue | in situhybridization | Elevated uPAR and uPA expression is associated to shorter overall survival. |
| Zhao et al., 2002 | 76 | Plasma | ELISA | High uPAR expression correlates to lymph-node involvement and distant metastasis. |
Plasminogen activation system and Helicobacter pylori
H. pylori has been related to induction of uPAR. Severalin vitrostudies have found that the expression of uPAR is upregulated in cancer and gastric epithelial cell lines when co-cultured with H. pylori(Guillemin, Salama, Tompkins, & Falkow, 2002; Sepulveda et al., 2002; El-Etr, Mueller, Tompkins, Falkow, & Merrell, 2004; Iwamoto et al., 2005; Kim et al., 2005; Kim et al., 2007; Iwamoto, Mizokami, Takahashi, Matsuoka, & Matsuzaki, 2008). This phenomenon has also been documented in vivo, specifically in non-neoplastic tissue (adjacent to the tumor growth) obtained from GC patients (Alpízar-Alpízar et al., 2010), as well as in gastric biopsies from patients that have no cancer but are infected with the bacterium (Kenny et al., 2008). In addition, one study reported a significant correlation between uPAR expression in neoplastic tissue and presence of H. pylori in adjacent non-neoplastic tissue (Beyer et al., 2006). More recently, the possible link between H. pylori and uPAR was systematically investigated in a mouse model of H. pylori-induced gastritis (Alpízar-Alpízar et al., 2013). According to this study, uPAR expression in foveloar epithelial cells (mucus-producing cells) is upregulated very early in response to the infection and increases progressively during the course of infection. The study also indicates that eradication of H. pylori infection by antimicrobial therapy causes a regression of uPAR expression to its physiological baseline levels, comparable to what is observed in non-infected mice (Alpízar-Alpízar et al., 2013). Additional experiments in this experimental model suggest that uPAR expression is directly induced by the bacterium (Alpízar-Alpízar et al., 2013 and Alpízar-Alpízar, unpublished). Nevertheless, it cannot be totally excluded the possibility that uPAR induction in the gastric epithelium occurs as a secondary event, resulting from the inflammatory reaction mounted in response to H. pylori infection.
The mechanisms underlying the induction of uPAR in gastric epithelium in response to H. pylori infection are far from being clear; however, several studies have shed some light in relation to this. It is well known thatH. pyloriinfection can lead to the activation of the transcriptional regulator NF-κB, by several mechanisms (Rieke, Papendieck, Sokolova, & Naumann, 2011; Sokolova, Maubach, & Naumann, 2014; Naumann et al., 2017). On the other hand, NF-κB is regarded as a very likely candidate for inducing the expression of uPAR in cancer (Wang et al., 2000; Baek et al., 2008). Thus, NF-κB activation in response to H. pylori infection could at least partially explain the upregulation of uPAR expression that is observed in epithelial cells ofH. pylori-colonized mucosa, both in human and mouse. Despite the existence of somein vitrostudies indicating that NF-κB is implicated in theH. pylori-driven induction of uPAR (Kim et al., 2007), no experimental evidence has been generatedin vivoto substantiate this finding. AP-1, another transcription factor commonly associated to the induction of uPAR (Lengyel et al., 1996b; Baek et al., 2008), is activated as a result ofH. pyloriinfection (Naumann et al., 2017). This means that AP-1 may also be a factor implicated in the induction of uPAR in response to this infection (Kim et al., 2005). A commonality of the induction of uPAR mediated by NF-κB or AP-1 is that ERK signaling pathway can lead to activation of both transcriptional regulators (Lengyel et al., 1996a; Baek et al., 2008). If taking into consideration that ERK signaling pathway is often manipulated byH. pylori(Naumann et al., 2017), this pathway becomes an obvious study target to gain further insight about the mechanismof induction of uPAR in the gastric epithelium colonized withH. pylori.
Conclusions and perspectives
In GC, the expression of members of the PA system is elevated, particularly for uPAR, therefore, their potential clinical utility as prognostic biomarkers in this cancer is evident. The clinical use of some components of this system has been validated in other cancer types, as it is the case for uPA and PAI-1, which has been recommended by the American Society of Clinical Oncology (ASCO) since 2007, for the identification of lymph node-negative breast cancer patients that would benefit from adjuvant treatment (Jänicke et al., 2001; Look et al., 2002; Harris et al., 2007). Given that uPAR is a factor that promotes local and distant dissemination of malignant cells, its utilization as prognostic parameter could contribute to the identification of GC patients with more aggressive phenotypes of the disease and, consequently, higher risk of either recurrence or metastasis. GC patients with elevated uPAR expression may benefit from adjuvant therapies that compensate for their higher risk of relapse and metastasis, especially those in which the disease is diagnosed at an early stage.
In spite of the above-mentioned, most of the investigations carried out thus far about uPAR and its prognostic value in GC are small and retrospective. Therefore, studies, ideally prospective ones, with large sample size are needed to validate the prognostic impact of uPAR in this malignancy. This is especially relevant when taking into account that the mortality attributed to GC is very high and only a few parameters have proven to have prognostic value, most of them are clinical variables not related to the biology of the disease.
The suitability of some members of the PA system as cancer diagnostic biomarkers has been proposed (Lund et al., 2011). Studies in prostatic and ovarian cancers demonstrate that the measurement of uPAR protein levels in blood can discriminate very well between benign and malignant tumors (Piironen et al., 2006; Henic, Borgfeldt, Christensen, Casslén, & Høyer-Hansen, 2008). The diagnostic value of uPAR has not been explored at all in GC, but this evaluation is important, especially in early stage tumors, because this may translate into a better chance of curing the disease and, consequently, in a reduction in the mortality ratesfor this neoplasm.
The potential link between H. pylori and uPAR induction may represent a novel finding in the genesis of GC and other pathologies associated to this bacterial infection. More specifically, this observation may contribute to explain, from a molecular and cellular perspective, how it is that H. pylori infection is associated to several clinical outcomes. Particularly, interesting is the fact that this bacterium is inducing in non-neoplastic gastric mucosa a protein that until now has been implicated in processes related to late stages of cancer development and progression and, in addition, has been correlated to the prognosis of cancer patients in general. It is, however, necessary to clarify a series of questions that arise, some related to the cellular and molecular mechanisms by which H. pylori induces uPAR, others regarding the implications of this phenomenon in the pathological context. First, it is important to determine whether uPAR expression in the apical membrane of gastric epithelial cells is related to strains harboring certain virulence factors or particular features. Second, it is relevant to clarify the signaling mechanisms that govern this phenomenon. Finally, is it necessary to understand whether the presence of uPAR in the surface of gastric epithelial cells confers some survival benefit to H. pyloriand to what extent this affects the development of cancer or other gastric pathologies. Addressing these aspects is relevant since the upregulation of uPAR in non-neoplastic gastric mucosa could imply that these people have an increased risk of developing GC, so it would have clinical utility as parameter for prevention and early detection, or to decide whether a patient requires H. pylorieradication therapy.












 uBio
uBio 

