Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.57 no.4 San José dic. 2009
Anti-inflammatory and analgesic activities of the aqueous extracts of Margaritaria discoidea (Euphorbiaceae) stem bark in experimental animal models
Adeolu A. Adedapo1; Margaret O. Sofidiya2 & Anthony J. Afolayan3
1. Department of Veterinary Physiology, Biochemistry and Pharmacology, University of Ibadan, Nigeria
2. Department of Pharmacognosy, University of Lagos, Nigeria
3. Department of Botany, University of Fort Hare, Alice 5700, South Africa; aafolayan@ufh.ac.za
Abstract: Margaritaria discoidea is a medicinal plant used for the treatment of various body pains in Central, Eastern and Southern Africa. The aqueous extract of its stem bark was investigated for its anti-inflammatory and analgesic activities in animal models. The extract at 50, 100 and 200mg/kg body weight reduced significantly the formation of oedema induced by carrageenan and histamine. In the acetic acid-induced writhing model, the extract had a good analgesic effect characterized by a reduction in the number of writhes when compared to the control. Similarly, the extract caused dose-dependent decrease of licking time and licking frequency in rats injected with 2.5% formalin. These results were also comparable to those of indomethacin, the reference drug used in this study. Acute toxicity test showed that the plant may be safe for pharmacological uses. This study has provided some justification for the folkloric use of the plant in several communities for conditions such as stomachache, pain and inflammations. Rev. Biol. Trop. 57 (4): 1193-1200. Epub 2009 December 01.
Key words: analgesic, anti-inflammatory, carrageenan, histamine, indomethacin, Margaritaria discoidea, extract, rats.
Margaritaria discoidea (Baill.) Webster syn. Phyllanthus discoideus (Baill.) Mull-Arg (Euphorbiaceae) is a tree reaching height of 30m. It is common in Senegal, western Cameroon, and the rest of tropical Africa (Burkill 1994). The stringy and fibrous bark is commonly used as a purgative in West Africa and as anthelmintic in Central Africa (Watt & Breyer- Brandwijk 1962, Kerharo & Adam 1974). The Fula of Sierra Leone use the bark for toothache while in Central Africa Republic its decoction is used for the relief of post-partum pains. In Congo (Brazzaville) the bark decoction is also used to relieve stomach and kidney complaints and to facilitate parturition. In Malawi, powdered bark-extract is applied to swellings and inflammation for quick relief (Irvine 1961).
Many alkaloids have been isolated from this plant, among which phylochrysine and securinine are the most important. Phylochrysine is a central nervous stimulant which may account in part for the plant’s stimulatory properties (Kerharo & Adam 1974, Weenen et al. 1990, Burkill 1994).
Despite the progress made in medical research during the past decades, the treatment of many serious diseases is still problematic. Chronic inflammatory diseases remain one of the world’s major health problems (Bohlin 1995, Yesilada et al. 1997, Li et al. 2003). Inflammation is the response of living tissues to injury. It involves a complex array of enzyme activation, mediator release, extravasations of fluid, cell migration, tissue breakdown and repair (Vane & Bolting 1995, Perianayagam et al. 2006). Inflammation has become the focus of global scientific research because of its implication in virtually all human and animal diseases. The conventional drugs used to ameliorate this phenomenon are either too expensive or toxic and not commonly available to the rural folks that constitute the major populace of the world (Kumara 2001, Dharmasiri et al. 2003, Li et al. 2003). This study therefore seeks to examine Margaritaria discoidea for anti-inflammatory activity and analgesic effects since pain is one of the cardinal signs of inflammation.
Materials and methods
Chemicals and drugs: Carrageenan, acetic acid, and Tween 80, all from Sigma- Aldrich Chemie Gmbh, Steinheim, Denmark were the chemicals used. The standard drugs used were indomethacin and histamine also from Sigma- Aldrich Chemie Gmbh, Steinheim, Denmark. All the chemicals and drugs used were of analytical grade.
Plant collection and extract preparation: The bark of Margaritaria discoidea was collected in January 2004 at Olokemeji Forest Reserve (7º1’41’’N, and 3º2’12’’W) Ogun State, Nigeria. The plant was authenticated at the Forestry Research Institute of Nigeria (FRIN), Ibadan, by Mr. Kola Odewo. Voucher specimens were prepared and deposited at the herbaria of FRIN and the Pharmacognosy Department of the University of Lagos, Nigeria.
The bark materials were air dried at room temperature and were later ground to powder. The ground plant material (200g) was shaken in distilled water for 48h on an orbital shaker (Digisystem Laboratory, Germany) at room temperature. The extract was filtered using a Buckner funnel and Whatman No 1 filter paper. Filtrate was concentrated to dryness under reduced pressure at 40ºC. The thick solution was lyophilized using freeze drying system for biological investigations. The extract yielded 19.2g. Graded aqueous solutions of the extract was prepared and used for the various experiments.
Animals: The animals used in this study were male Wistar rats weighing between 100 and 250g. They were maintained at the Experimental Animal House of the Agricultural and Rural Development Research Institute, University of Fort Hare, South Africa. They were kept in rat cages and fed on commercial rat cubes (EPOL Feeds, South Africa Ltd.) and allowed free access to clean fresh water in bottles ad libitum. All experimental protocols were in compliance with University of Fort Hare Ethics Committee on Research in Animals as well as internationally accepted principles for laboratory animal use and care (Working party of the Laboratory Animal Science Association 1990).
Acute toxicity test: The acute toxicity of M. discoidea aqueous extract was determined in rats according to the method of Hilaly et al. (2004) with slight modifications. Rats fasted for 16h were randomly divided into groups of six rats per group. Graded doses of the extract (200, 400, 800, 1600 and 3200mg/kg p.o.) were separately administered to the rats in each of the groups by means of bulbed steel needle. All the animals were then allowed free access to food and water and observed over a period of 48h for signs of acute toxicity. The number of deaths within this period was recorded.
Anti-inflammatory activities
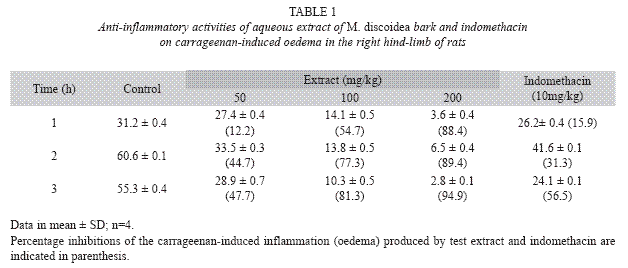
Carrageenan-induced rat paw oedema: Four group of rats containing four animals in each group received either plant extract (50, 100, 200mg/kg body weight), indomethacin (10mg/kg body weight) or vehicle control (0.9% normal saline in 3% Tween 80 (2ml/kg). These were administered orally. Acute inflammation was produced by the sub-plantar administration of 0.1ml of 1% carrageenan in normal saline that contained Tween 80 in the right paw of rats. The paw volume was measured at 0 and 3h after carrageenan injection using a micrometer screw gauge. Increases in the linear diameter of the right hind paws were taken as an indication of paw oedema. Oedema was assessed in terms of the difference in the zero time linear diameter of the injected hind paw and its linear diameter at time t (i.e. 60, 120, 180min) following carrageenan administration. The anti-inflammatory effect of the extract was calculated by the following equation: anti-inflammatory activity (%)=(1-D/C) x 100, where D represented the percentage difference in paw volume after the extract was administered to the rats and C represents the percentage difference of volume in the control groups. The percentage inhibition of the inflammation was calculated from the formula: % inhibition=D0- Dt/D0x100 where D0 was the average inflammation (hind paw oedema) of the control group of rats at a given time; and Dt was the average inflammation of the drug treated (i.e. extracts or reference indomethacin) rats at the same time (Gupta et al. 2005, Sawadogo et al. 2006, Moody et al. 2006).
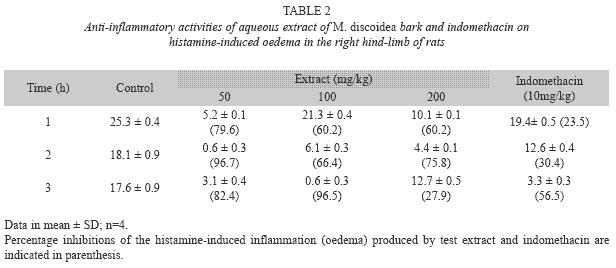
Histamine-induced rat paw oedema: Using the method of Perianayagam et al. (2006), the paw oedema was produced by subplantar administration of 0.1% freshly prepared solution of histamine into the right hind paw of rats. The paw volume was recorded before 0 and 1h after histamine injection. Different groups of animals were pretreated with different extracts (50, 100, 200mg/kg or with 2ml/ kg of 0.9% normal saline in Tween 80 (vehicle control) or 10mg/kg indomethacin (standard drug). The drug and extracts were administered orally 1h before eliciting paw oedema. The anti-inflammatory effect of the extract was calculated using the formula for carrageenaninduced paw oedema.
Analgesic activity
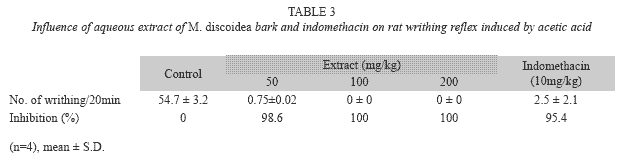
Acetic acid-induced writhing response in rats: To evaluate the analgesic effects of the plant extract, the method described by Dharmasiri et al. (2003) was used with slight modifications. Different groups of four rats each received orally normal saline solution (2ml/kg) (i.e. control), indomethacin (10mg/kg), or plant extract (50, 100, 200mg/kg). Thirty minutes later, 0.7% acetic acid (10ml/kg) solution was injected intraperitoneally to all the animals in the different groups. The number of writhes (abdominal constrictions) occurring between 5 and 20 min after acetic acid injection was counted. A significant reduction of writhes in tested animals compared to those in the control group was considered as an antinociceptic response.
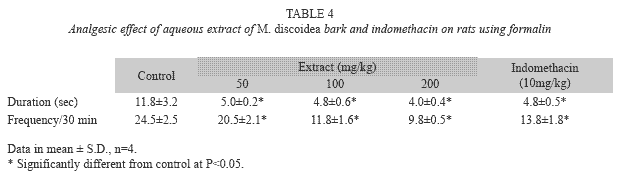
Formalin test: Formalin test was conducted as described by Dharmasiri et al. (2003). Male rats (n=4/group) were treated respectively with 50, 100 and 200mg/kg of M. discoidea extract, 10mg/kg of indomethacin and 2ml/kg of normal saline. Thirty minutes later, the rats were injected with 0.05ml of 2.5% formalin into the right hand foot pad, immediately placed in a transparent plastic cage separately; the licking time and frequency of the injected paw were recorded for 30min (Hunskaar et al. 1985).
Statistical analysis: The data were expressed as mean±S.D. Where applicable the difference in response to test drugs was determined by student’s t-test. P<0.05 was considered significant.
Results
Acute toxicity test: Oral administration of graded doses (200, 400, 800, 1600 and 3200 mg/kg p.o.) of the aqueous extract of M. discoidea to rats did not produce any significant changes in behaviour, breathing, cutaneous effects, sensory nervous system responses or gastrointestinal effects during the observation period. No mortality was recorded in any group after 72h of administering the extract to the animals.
Anti-inflammatory activity
Carrageenan-induced paw oedema: When compared with the control, the extract and indomethacin significantly reduced the paw oedema 3h after carrageenan injection. The anti-inflammatory effect of the extract and the reference drug increased with time. This was dose-dependent for the extract (Table 1).
Histamine-induced paw oedema: The effect of the extract (100mg/kg) and the reference drug on histamine-induced paw oedema was most pronounced 3h after histamine injection, while the 50 and 200mg/kg doses of the extract showed highest activity at 2h after histamine administration. The anti-histaminic activity of the extract decreased with increase in the dose of the extract (Table 2).
Analgesic activity
Acetic acid-induced writhing in rats: The aqueous of M. discoidea and indomethacin induced significant decrease in the number of writhes when compared to the control (Table 3). The extract at 50, 100, 200 and indomethacin at 10mg/kg exhibited higher antinociceptive power at 98.6, 100, 100 and 95.4% respectively indicating that the extract has slightly higher antinociceptive than the reference drug used in this study.
Formalin test in rats: Treatment with the aqueous extract at 50, 100, 200 and indomethacin at 10mg/kg caused significant decrease in licking time and frequency of licking of the formalin- injected paw of rats (Table 4). The 200mg/kg dose showed the highest effect.
Discussion
Carrageenan-induced oedema involves the synthesis or release of mediators at the injured site. These mediators include prostaglandins, especially the E series, histamine, bradykinins, leucotrienes and serotonin, all of which also cause pain and fever (Asongalem et al. 2004). Inhibitions of these mediators from reaching the injured site or from bringing out their pharmacological effects normally ameliorate the inflammation and other symptoms. This study has shown that the aqueous extract of the stem bark of M. discoidea possessed a significant anti-oedematogenic effect on paw oedema induced by carrageenan and histamine.
Development of oedema induced by carrageenan is commonly correlated with early exudative stage of inflammation (Ozaki 1990, Silva et al. 2005). Carrageenan oedema is a multimediated phenomenon that liberates diversity of mediators. It is believed to be biphasic; the first phase (1h) involves the release of serotonin and histamine while the second phase (over 1h) is mediated by prostaglandins, the cyclooxygenase products, and the continuity between the two phases is provided by kinins (Silva et al. 2005, Perianayamgam et al. 2006). Since carrageenan-induced inflammation model is a significant predictive test for anti-inflammatory agents acting by the mediators of acute inflammation (Mossai et al. 1995, Sawadogo et al. 2006), the results of this study are an indication that M. discoidea can be effective in acute inflammatory disorders.
The extract also caused pronounced reduction in the oedema produced by histamine. This result tends to suggest that the anti-inflammatory activity of the extract is possibly backed by its anti-histamine activity. The antihistaminic effect of the extract decreased with increase in the dose of the extract. Histamine is an important inflammation mediator, potent vasodilator substance and also increases the vascular permeability (Cuman et al. 2001, Linardi et al. 2002, Vasudevan et al. 2007). Since the extract effectively suppressed the oedema produced by histamine, it showed that the extract exhibited anti-inflammatory actions by inhibiting the synthesis, release or action of inflammatory mediators such as histamine, serotonin and prostaglandins.
With respect to the acetic acid-induced abdominal writhing which is the visceral pain model (Vyklicky 1979), the result has shown that all the doses produced significant analgesic effect. This could be attributed, partly, to its anti-inflammatory effect as, in the visceral pain model, the processor releases arachidonic acid via cyclooxygenase and prostaglandin biosynthesis which plays a role in the nociceptive mechanism (Franzotti et al. 2002, Sawadogo et al. 2006). Thus the results obtained for the writhing test are similar to those obtained for the oedematogenic test using carrageenan. Therefore, an anti-inflammatory substance may also be involved in the peripheral analgesic activity because inhibition of the acute inflammation by this extract led to their inhibitory effect on pain development.
In formalin test, the pain in the early phase was due to the direct stimulation of the sensory nerve fibres by formalin while the pain in the late phase was due to inflammatory mediators, like histamine, prostaglandins, serotonin and bradykinins (Murray et al. 1988, Tjolsen et al. 1992, Dharmasiri et al. 2003). Formalin test is believed to be a more valid analgesic model which is better correlated with clinical pain (Tjolsen et al. 1992, Ghannadi et al. 2005). In this study, the extract caused a dose-dependent decrease in licking time and licking frequency by the rats injected with formalin signifying the analgesic effect of the extract.
Phytochemically, the bark of M. discoidea has been reported to yield about 10% tannin (Watt & Breyer-Brandwijk 1962, Burkill 1994). Tannins are important compounds known to be potent cyclooxygenase-1 inhibitors and with anti-phlogistic activity (Wagner 1989, Xu 1996). The mechanisms of anti-inflammatory activity may be related to the antiphlogistic action of the tannins. Non-steroidal anti- inflammatory drugs (NSAID) such as indomethacin used in this study are known to inhibit cyclooxygenase enzymes I and II which are implicated in the production of inflammation- mediating agent prostaglandin E2 (PGE2) from arachidonic acid (Dhara et al. 2000, Wu 2003, Moody et al. 2006). The pattern of anti-inflammatory and analgesic activities exhibited by this extract was similar to that of indomethacin which suggests that the plant’s activity may be mediated by cyclooxygenase I and II inhibition.
In conclusion, since the plant extract reduced significantly the formation of oedema induced by carrageenan and histamine, as well as reduced the number of writhes in acetic acidinduced writhing models and formalin test, the stem bark of M. discoidea exhibited anti-inflammatory and analgesic activities. Again, no mortality was recorded in the acute toxicity test, it showed that the plant is safe for use. The study has thus provided some justification for the folkloric use of the plant in several communities for conditions such as stomachache, pain and inflammations.
Acknowledgment
The authors wish to acknowledge the financial support of the National Research Foundation, South Africa in carrying out this study.
Resumen
Margaritaria discoidea es una planta medicinal usada para el tratamiento de varios dolores corporales en la parte sur, central y oriental de África. Se investigaron las propiedades analgésicas y antiinflamatorias de la extracción acuosa de la corteza de su tallo en modelos animales. Los extractos de 50, 100 y 200mg/kg de peso corporal redujeron significativamente la formación del edema inducido por la carragenina y la histamina. En el modelo de contracción abdominal inducida por ácido acético, el extracto mostró un buen efecto analgésico caracterizado por la reducción en el número de contracciones en comparación con el grupo control. El extracto causó una disminución dependiente de la dosis del tiempo y la frecuencia de lamido en las ratas inyectadas con formalina al 2.5%, lo cual evidencia su efecto analgésico. Estos resultados fueron comparables con los de la indometacina, la droga de referencia usada en este estudio. La prueba de toxicidad aguda mostró que la planta podría ser segura para usos farmacológicos. Este estudio proporciona justificación para el uso folclórico de esta plata en varias comunidades, con el objetivo de tratar padecimientos tales como dolor de estómago, dolor e inflamaciones.
Palabras clave: analgésico, antiinflamatorio, carragenina, histamina, indometacina, Margaritaria discoidea, ratas.
Received 20-XI-2008. Corrected 10-IV-2009. Accepted 13-V-2009.
References
Asongalem, E.A., H.S. Foyet, S. Ekoo, T. Dimo & P. Kamtchouing. 2004. Anti- inflammatory, lack of central analgesia and antipyretic properties of Acanthus montanus (Ness) T. Anderson. J. Ethnopharmacol. 95: 63-68. [ Links ]
Bohlin, L. 1995. Structure-activity studies of natural products with anti- inflammatory effects, p.137-161. In: Hostettmann, K. (Ed.). Phytochemistry of plants used in traditional medicine. Clarendon. Oxford, UK. [ Links ]
Burkill, H.M. 1994. The useful plants of west Tropical Africa. Royal Botanical Gardens, Kew. London, UK. [ Links ]
Cuman, R.K.N., C.A. Bersani-Amadio & Z.B. Fortes, Z.B. 2001. Influence of type 2 diabetes on the inflammatory response in rat. Inflammation Res. 50: 460-465. [ Links ]
Dhara, A.K., V. Suba, T. Sen, S. Pal & A.K.N. Chaudhuri. 2000. Preliminary studies on the anti-inflammatory and analgesic activity of the methanol fraction of the root extract of Tragia involucrata Linn. J. Ethnopharmacol. 72: 265-268. [ Links ]
Dharmasiri, J.R., A.C. Jayakody, G. Galhena, S.S.P. Liyanage & W.D. Ratnasooriya. 2003. Antiinflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J. Ethnopharmacol. 87: 199-206. [ Links ]
Franzotti, E.M., C.V.F. Santos, H.M.S.L. Rodriques, R.H.V. Mourao, M.R. Andrade & A.R. Antoniolli. 2002. Anti-inflammatory, analgesic, and acute toxicity study of Sida cardiafolia. J. Ethnopharmacol. 72: 273-278. [ Links ]
Ghannadi, A., V. Hajhashemi & H. Jafarabadi. 2005. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J. Medicinal Food 8: 488-493. [ Links ]
Gupta, M., U.K. Mazunder, R. Sambath Kumbar, P. Gomath, Y. Rajeshwar, B.B. Kakoti & V. Tamil Selven. 2005. Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhina racemosa stem bark in animal models. J. Ethnopharmacol. 98: 267-273. [ Links ]
Hilaly, J.E., Z.H. Israili & B. Lyoussi. 2004. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J. Ethnopharmacol. 91: 43-30. [ Links ]
Hunskaar, S., O.B. Fasmer & K. Hole. 1985. Formalin test in mice: a useful technique for evaluating wild analgesics. J. Neurosci. Methods 4: 69-76. [ Links ]
Irvine, F.R. 1961. Woody plants of Ghana. Oxford University. London, UK. [ Links ]
Kerharo, J. & J.G. Adam. 1974. La Pharmacopie Senegalese traditionelle. Plants medicinales et Toxiques. Vigot Freres. Paris, France. [ Links ]
Kumara, N.K.V.M.R. 2001. Identification of strategies to improve research on medicinal plants used in Sri Lanka, p. 12-14. In WHO Symposium. University of Ruhuna, Galle, Lanka. [ Links ]
Li, R.W., S.P. Myers, D.N. Leach, G.D. Lin & G. Leach. 2003: A cross-cultural study: anti-inflammatory activity of Australian and Chinese plants. J. Ethnopharmacol. 85: 25-32. [ Links ]
Linardi, A., S.K.P. Costa, G.R. DeSilva & E. Antunes. 2002. Involvement of kinins, mast cells, and sensory neurons in the plasma exudation and paw edema induced by staphylococcal entrotoxin B in the mouse. Euro. J. Pharmacol. 399: 235-242. [ Links ]
Moody, J.O., V.A. Robert, J.D. Connolly & P.J. Houghton. 2006. Anti-inflammatory activities of the methanol extracts and an isolated furanoditerpene constituent of Sphenocentrum jollyanum Pierre (Menispermaceae). J. Ethnopharmacol. 104: 87-91. [ Links ]
Mossai, J.S., S. Rafatullah, A.M. Galal & M.A. Al-Yahya. 1995. Pharmacological studies of Rhus retinorrhea. Int. J. Pharmacol. 33: 242-246. [ Links ]
Murray, C.W., F. Porreca & A. Cowan. 1988. Methodological refinements in the mouse paw formalin test an animal model of tonic pain. J. Pharmacolog. Methods 20: 175-186. [ Links ]
Ozaki, Y. 1990. Anti-inflammatory effects of Curcuma xanthorrhiza Roxb, and its active principle. Chem. Pharmaceut. Bull. 38: 1045-1048. [ Links ]
Perianayagam, J.B., S.K. Sharma & K.K. Pillai. 2006. Antiinflammatory activity of Trichodesma indicum root extract in experimental animals. J. Ethnopharmacol. 104: 410-414. [ Links ]
Sawadogo, W.R., R. Boly, M. Lompo & N. Some. 2006. Anti-inflammatory, analgesic and antipyretic activities of Dicliptera verticillata. Int. J. Pharmacol. 2: 435-438. [ Links ]
Silva, G.N., F.R. Martins & M.E. Matheus. 2005. Investigation of anti-inflammatory and antinociceptive activities of Lantana trifolia. J. Ethnopharmacol. 100: 254-259. [ Links ]
Tjolsen, A., D.G. Berge, S. Hunskaar, J.H. Rosland & K. Hole. 1992. The formalin test: an evaluation of the method. Pain 51: 5-17. [ Links ]
Vane, J.R. & R.M. Bolting. 1995. New insights into the mode of action of anti- inflammatory drugs. Inflammation Res. 44: 1-10. [ Links ]
Vasudevan, M., K.K. Gunman & M. Parle. 2007. Antinociceptive and anti- inflammatory effects of Thespesia populnea bark extract. J. Ethnopharmacol. 109: 264-270. [ Links ]
Vyklicky, L. 1979. Techniques for the study of pain in animals, p.773-778. In: Bonica, JJ, Liebeskin & Albe- Fessard, DG (Eds), Advances in Pain Research and Therapy. Raven . New York, USA. [ Links ]
Wagner, H. 1989. Search for new plant constituents with potential anti-phlogistic and anti-allergic activity. Planta Medica 55: 235-241. [ Links ]
Watt, J.M. & B.N. Breyer-Brandwijk. 1962. Medicinal and poisonous plants of Southern and Eastern Africa. Churchill Livingstone. London, UK. [ Links ]
Weenen, H., M.H. Nkunya, D.H. Bray, L.B. Mwasumbi, L.S. Kinabo, V.A. Kilimali & J.B. Wijnberg. 1990. Antimalarial compounds containing an alpha, betaunsaturated carbonyl moiety from Tanzanian medicinal plants. Planta Med. 56: 371-373. [ Links ]
Working party of the Laboratory Animal Science Association. 1990. The assessment and control of the severity of scientific procedures on laboratory animals. Lab. Anim. Sci. 24: 97-130. [ Links ]
Wu, K.K. 2003. Aspirin and other cyclooxygenase inhibitors: new therapeutic insights. Semin.Vasc. Med. 3: 107-112. [ Links ]
Xu, G.J. 1996. The Chinese Materia Medica. Volume 1. Chinese Medicine and Technology. Beijing, China. [ Links ]
Yesilada, E., O. Ustun, E. Sezik, Y. Takishi, Y. Ono & G. Honda. 1997. Inhibitory effects of Turkish folk remedies on inflammatory cytokines: interleukin- 1α, interleukin-1ß and tumor necrosis factor-α. J. Ethnopharmacol. 58: 59-73. [ Links ]












 uBio
uBio