Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.50 n.2 San José Jun. 2002
(Trematoda: Philophthalmidae) in Venezuela
Marcos T. Díaz 1 , Luz E. Hernández 2 and Abul K. Bashirullah 3*
1 Instituto de Investigaciones en Biomedicina y Ciencias Aplicadas
2 Laboratorio de Parasitología, Departamento de Biología, Escuela de Ciencias
3 Instituto Oceanográfico de Venezuela, Universidad de Oriente, Apartado 138, Cumaná 6101, Venezuela. Fax: 58-93-301358. e-mail: bashiru@re.udo.edu.ve
* Author for correspondence.
Received 09-VIII-2000. Corrected 06-IV-2001. Accepted 18-VI-2001.
Abstract
Descriptions of the miracidium, mother redia, daughter redia, grandaughter redia, cercaria, metacercaria and adult stages of Philophthalmus gralli Mathis and Leger,1910 recovered from experimental infections are presented. The intermediate host, Melanoides tuberculata, was collected from freshwater rivulets in Aguasanta and Yaguracual, Sucre State, Venezuela. Chicken were orally infected with cercariae and metacercariae, and metacercariae were introduced directly into the eyes by pipette. Both processes of infection produced adult worms. This is a new geographical record for P. gralli.
Key words: Trematoda, Philophthalmus gralli, chicken, life cycle, Venezuela, Melanoides tuberculata.
Looss (1899) created the genus Philophthalmus for P. palpebrarum from under the eyelids of birds from Egypt. Since then, over 36 species of this genus have been reported from Asia, Europe and America and adult P. gralli Mathis and Leger, 1910 were reported from the eyes of geese in the Azraq Oasis, Jordan by Ismael and Issa (1987). Ismael and Saliba (1985) isolated a gymnocephalous cercaria from Melanoides tuberculata snails in the same oasis, identified as P. gralli (Ismael and Issa 1987). This species has not been reported so far in Venezuela and this is the first natural occurrence of cercariae of P. gralli in a freshwater mollusc, M. tuberculata, from Aguasanta and Yaguaracal, Sucre State, Venezuela. In addition, Nasir and Díaz (1972) reported the occurrence of two more philophthalmid trematodes: P. lacrymosus and Ophthalmotrema semipalmatus in birds from Venezuela.
The present report describes all developmental stages of its life history acquired from experimental infection and compares the discrepancies and inconsistencies recorded in P. gralli in other geographical regions.
Materials and methods
A total of 250 freshwater snails of M. tuberculata was collected from rivulets of Aguasanta and Yaguaracal, Sucre State, Venezuela during 1998 and 1999, and maintained in a laboratory in aquaria filled with water from the snails natural habitat. Ten were infected with cercariae of P. gralli, which were maintained in separate glass bowls in dechlorinated water, and exposed to artificial lighting (tungsten tube light) to stimulate shedding of cercariae. Each container was examined every morning for emergent cercariae. Emerging cercariae encysted and preferably stayed at the bottom of the container.
All materials collected were deposited in the Laboratory of Parasitology, Department of Biology, School of Science, Núcleo Sucre, Universidad de Oriente, Cumaná, Venezuela.
Experimental infection of the host snail: Fifteen M. tuberculata snails cultured and grown in the laboratory were infected with miracidia of P. gralli. Miracidia were recovered from experimentally grown adult P. gralli (41 –43 days old) in chicken. Snails were infected as follows: 3 uninfected snails were placed in a small glass dish with 10 cc of water and 10 miracidia were pipetted into each. These snails were later transferred to an aquarium, and 15 days post-infection, they were transferred to bowls and examined every morning for the emergence of cercariae. Two snails produced cercariae of P. gralli after 83 days of infection and formed cysts at the bottom of the bowl.
Results
(All descriptions are based on 25 live and mounted specimens.)
Experimental infection of chicken: A total of 103 adult P. gralli was recovered from the nictitant membrane of 11 experimentally infected chicken of 3 - 4 days old. These chickens were infected either orally or directly on the eyes using a Pasteur pipette. The results of the experimental infection are presented in Table 1. Alicata and Ching (1960) and Ching (1961) recommended these methods of infection. Both cercariae and excysted metacercariae introduced in the chicken grew to sexually matured adult P. gralli. Two rats were fed orally 20 and 30 metacercariae and autopsied after 23 and 25 days, respectively. No parasites were found in the experimental hosts (Table 1). No conjunctivitis was observed in the eyes of infected chicken as mentioned by Alicata (1962).
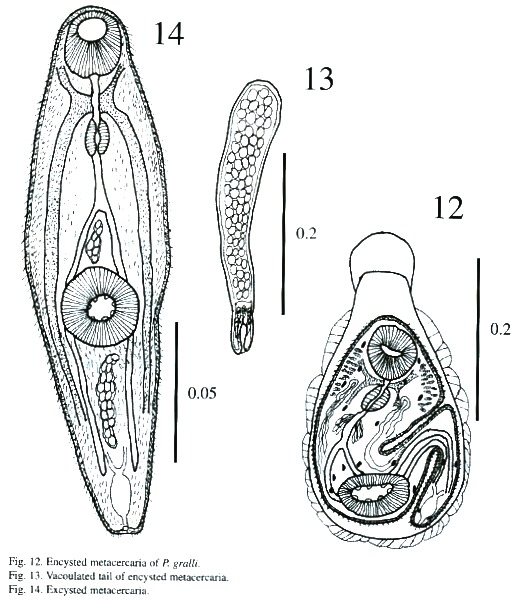
Encystation (Figs. 12-13): Freshly emerged cercariae are not active swimmers. Swimming is slow and occurs by undulations of the body and tail in the form of an "S". They hang upside down by their tail tips when they are near the surface of the water during the period of swimming up and down. Possibly, the efflux of some sticky substance from the caudal end, aids cercariae in the act of suspending themselves from the surface film of the water. While hanging upside down, cercariae perform contractile movements constantly. Sooner or later, tail tips get detached, cercariae resume their undulatory and contractile movements, and reach the bottom of the container where they crawl with the help of their suckers. At times while crawling a cercaria attaches itself to the substratum by its tail tip, stands upright and performs swaying movements. During crawling, its forebody actively moves by contraction and expansion, apparently to select a suitable site for encystation. The cercarial body contracts as soon as attachment is secured, and then the body shortens and loses its characteristic shape. The body flattens and broadens out, particularly the forebody; the tail, too, contracts and lashes sideways. Subsequently, a cystogenous secretion oozes from the body and envelopes it. The cystogenous materials, due to their continuous exudation, spread from either side towards the anterior extremity of the cercarial body, gradually forming a cyst wall. While the process continues, posterior bodies of cercariae take the shape of a bottleneck and tails detach. The process of cyst formation is completed within a period of 40 s to 1 min. The anterior part of metacercariae in recently formed cysts is directed towards the bottom of the bottle but it changes its position within a short time. Cercariae tend to encyst on whichever object they attach to. They have even encysted on the tip of dissecting needles and pipettes.
Measurements of encysted metacercaria of P. gralli: Cyst 335 - 387 x 225 –295 µm; body 217 - 295 x 135 - 182 µm; anterior part of cyst 26 - 53 x 86 - 99 µm; oral sucker 48 - 64 x 51 - 67 µm; prepharynx 5 – 21 x 5 - 5 µm; pharynx 24 - 34 x 21 - 29 µm; esophagus18 - 26 x 13 - 16 µm and acetabulum 51 - 72 x 72 - 88 µm.
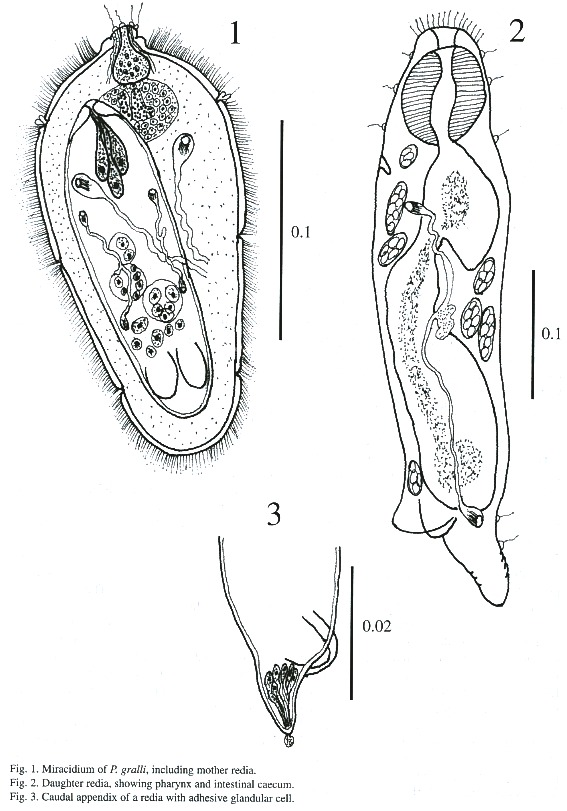
Miracidium (Fig. 1): The oval shaped miracidia measuring 94 - 156 µm long by 0.051 - 0.067 µm wide, totally ciliated except for the extreme anterior region which is occupied by apical papillae and separated by rows of epidermal plates. Cilia bundled together to form a tuft on each side of the apical papilla. Each side consists of 4 papillae with sensorial hairs. The apical gland with granulated materials opens to the apical papilla. Epidermal plates are arranged in 4 tiers. The 1st , 3rd and 4th rows consist of 6 plates and the 2 nd of 8 plates. Eye spots located between the 1st and 2 nd rows of epidermal plates, consist of one pair of dark brown pigmented bodies in the shape of an inverted "L". The nervous system is composed of one large cephalic ganglion with numerous small nuclei. Penetration glands were not detected clearly, but two glandular ducts on each side of the apical gland were observed. Two large flame cells are located at midlevel on both sides of the body. A mother redia was encased in the miracidial body cavity.
Mother redia: Mother redia within a miracidium occupies about two-thirds of the body. Anterior parts of body possess muscular structures similar to a pharynx. A young mother redia in a miracidium measures 80 - 118 µm in length and 32 - 40 µm in width. The external surface lacks papillae and other structures. The posterior part possesses two ventro-lateral processes and a caudal appendix. There are 3 nucleated glandular cells which open to the anterior margin of the body of the redia and the right cell is larger than other two cells. The granular contents of these cells are fine. The redia possesses germinal cells and balls. The protonephridial system consists of symmetrical bilateral flame cells and their ramifications. Each half of the system consists of 2 flame cells which open to a small vessel on each side of body.
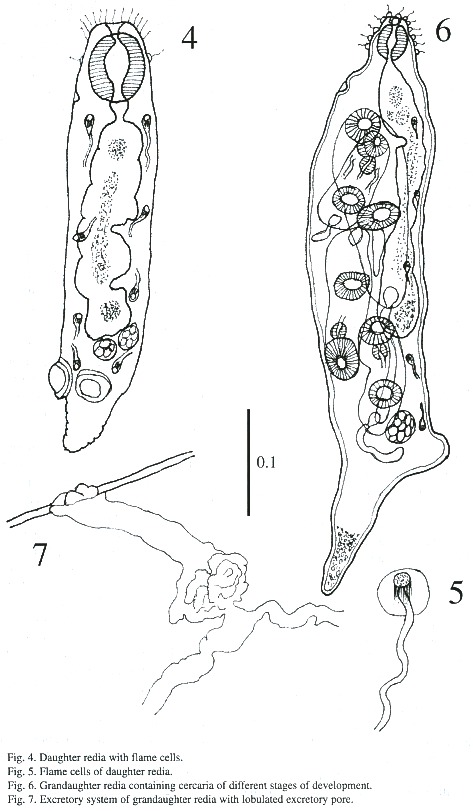
Daughter redia (Figs. 2-5): The elongated body measures 558 - 812 µm in length and 131 - 182 µm in width and its anterior margin is decorated with a row of sensory filaments.
The pharynx is muscular, measures 56 -67iµm in length and 40 - 48 µm in width, followed by a short esophagus. The long intestinal caecum almost reaches the posterior part of the body of the redia, and contains brown-yellow granular materials. Birth pore is inconspicuous, and is located on one side of body just below the level of the pharynx. A pair of protrusive locomotory appendices are located at posterior part of body with a transverse pore, similar to ventral sucker. A prominent caudal appendix is present, measuring 80 - 215 µm long and 40 - 48 µm wide. Both the locomotory and caudal appendices are used for adhering to tissues of snail. The body cavity of rediae contains a maximum of 8 germ cells. Protonephridial system in some daughter rediae is similar to mother redia (2 flame cells) but in others varies between 4 - 8 flame cells in each redia.
Grandaughter redia (Figs. 6-7): The majority of these were found in abundance free in the digestive gland but some were found attached to tissues of snail. The structure of grand daughter rediae is somewhat similar to daughter rediae but much more developed. These appear subcylindrical with a blunt anterior end and a conical posterior end in live, and measures 548 - 1i268 µm in length and 182 –243 µm in breadth. The terminal mouth is surrounded by sensory filament and papillae. A birth pore is located anteriorly a little behind the pharynx. The pharynx is muscular and measures 51 - 64 X 45 - 56 µm; the gut is long and extends beyond mid-body. The caudal process is well developed and the caudal appendix measures 18 - 269 X 13 - 88 µm. Besides germ cells, germ balls, 1 - 5 cercariae of different stages are found in these rediae. A protrusive excretory pore is situated on opposite to intestinal caecum and are observed only 5 flame cells.
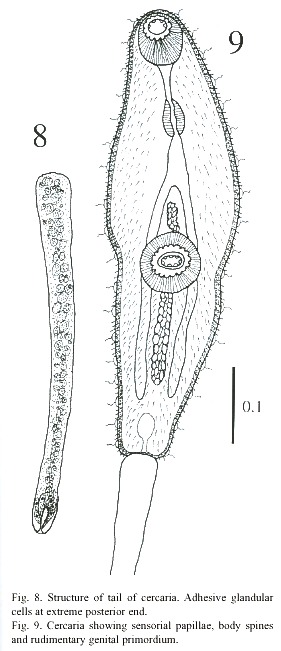
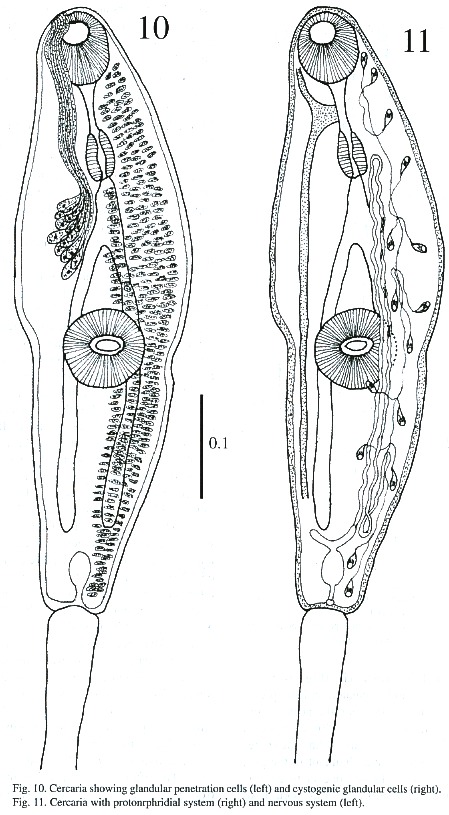
Cercariae (Figs. 8-11): The body is elongated, the preacetabular region is wider than the postacetabular, beset with fine spines. It measures 456 - 598 µm in length and 101 – 162 µm in maximum width. The tegument is thick, beset with papillae with sensory filament. A tail terminal with small spine-like points measures 294 - 446 x 30 - 50 µm. Parenchymetic cells of different sizes and forms with nucleus are observed in the center of the tail. A short invagination observed at the end of the tail may function as an adhesive organ. It contains glandular cells with fine granular materials which secretes viscous substances, probably for adhesiveness of cercariae. Longitudinal and transverse muscles are observed in the tail of live cercariae, which may be responsible for undulation and expansion. An oral sucker subterminal measures 55 - 64 x 51 - 61 µm. The external border of the mouth is surrounded with papillae, with only 4 papillae on the inside border. The alimentary canal includes a prepharynx, measuring 10 - 61 x 5 - 10 µm and strong muscular pharynx, measuring 26 - 40 x 18 - 28 µm, followed by long esophagus, measuring 45 - 94 x 18 - 37 µm in mounted specimen. Esophageal bifurcation occurs near to the anterior third of the body and intestinal caeca extend posteriorly up to the hind end of the body. Acetabulum slightly posts equatorial and its internal and external borders are lined with papillae.A mass of gland cells organized in two groups of 16 each are on either side of the esophagus. The ducts of these gland cells on each side run forward in a compact bundle and open independently at the anterior border of body. Genital primordium is composed of 2 masses of undifferentiated cells, localized immediately anterior and posterior to acetabulum. Nervous system consists of a transverse commissure, situated between oral sucker and pharynx, which give rise to 2 anterior and 2 posterior nerves. The short anterior nerves pass lateral to an oral sucker and longer posterior nerves pass posteriorly up to the end of the intestinal caecum.
Cytogenous cells are numerous through-out the body except in the region occupied by suckers and excretory bladder. The excretory bladder is single chambered and located at posterior end of body. The primary excretory duct arises from a short stem from the excretory vesicle. It runs forward up to the level of the pharynx where it recurves and continues posteriorly as secondary excretory ducts with three tufts of preacetabular cilia and one postacetabular, reaching the level of the excretory bladder where it forms a loop and gives rise to the tertiary excretory duct. Then, between the esophageal bifurcation and the ventral sucker, it bifurcates into an anterior and posterior collecting canal; of these two, the former runs forward up to the pharyngeal region, while the latter up to the caecal termination. Each is connected to the canaliculus of flame cells. The excretory pore is terminal.The flame cell formula is 2 [(3 + 3 + 3) + (2 + 2 + 2)] = 30.
Excysted metacercaria (Fig. 14): The body is spinose and elongated and the preacetabular region is wider than the postacetabular region. It measures 153 - 190 µm in length and 36 - 47 µm in maximum width. The spines are more prominent and abundant in the post acetabular regions. There appears to be fine spines on the border of anterior portion of body. The suckers are more developed than in the cercaria. Oral sucker measures 56 - 61 µm x 43 - 51 µm and ventral sucker, 40 - 72 µm x 56 - 72 µm.
The mouth leads through a long prepharynx measuring 29 - 45 x 5 - 8 µm and into a muscular pharynx measuring 29 - 34 x 21 -24lµm. The esophagus is long, measuring 40 –64 x 8 - 13 µm, and bifurcates near the ventral sucker. The intestinal caecum is long and narrow, extending backwards up to the excretory bladder. The rudimentary reproductive organs appear as two undifferentiated mass of cells on and below the ventral sucker. A mass of round gland cells with granular cytoplasm of unidentified number is located on either side of esophagus. About 20 - 22 gland cell pores with their respective ducts are observed on each side, close to the body margin. The nervous system is slightly better developed than in cercariae, but the posterior nerves are finer. The excretory bladder is increased in size but the arrangement of flame cells remained same as cercaria.
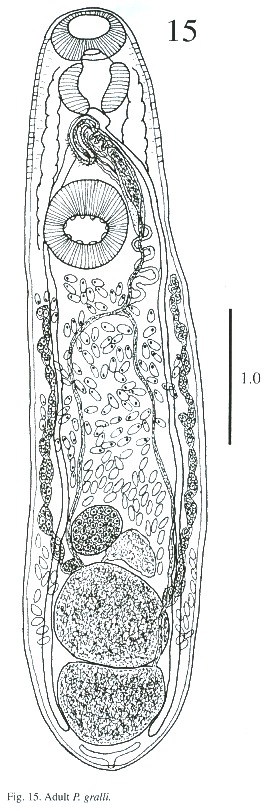
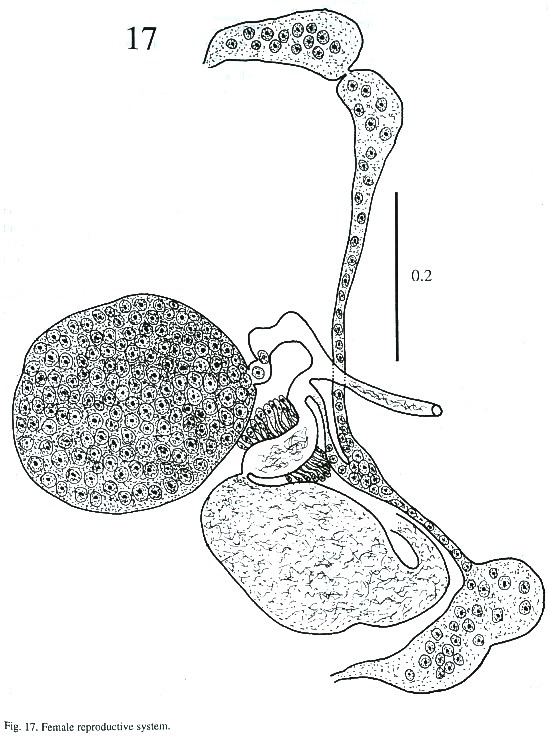
Description of adult (Figs. 15-17): The body is elongated, slightly attenuated at the anterior end and rounded posteriorly. Its length is 2.564 - 3.384 mm and its width is 0.512 -1.205 mm. The tegument in the anterior part is covered with minute spines and, in some, it is difficult to observe. The oral sucker is subterminal, 237 - 297µm long and 287 - 378 µm wide. The prepharynx is visible in the live worm. The pharynx is muscular, 227 - 323 µm long and 227 - 333 µm wide. The esophagus is 65 - 151 µm long and 80 - 141 µm wide, and bifurcates anteriorly to the acetabulum. The caecum is conspicuous, ending blindly in the posterior part of body at the level of excretory bladder. The acetabulum is pre-equatorial and very muscular, 388 - 544 µm long and 409 –505 µm wide and the internal margin is surrounded by receptor sensorial papillae.
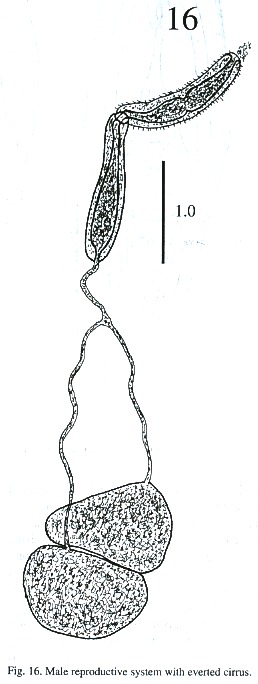
The testes are located one behind the other in the posterior part of the body, smooth or lobulated, measuring 272 - 378 x 424 –530 µm long and 272 - 464 x 404 - 505 µm wide. The efferent ducts rise antero-laterally from each testes and unite to form a single duct which opens to the cirrus pouch. The seminal vesicle may be elongate or globular. The seminal vesicle varies in its location depending on whether the cirrus extrudes from the cirrus pouch. The cirrus pouch is 717 - 868 µm long and 106 - 186 µm wide. The genital pore is close to esophageal bifurcation. The ovary is subglobular, 126 - 222 µm long and 151 –252 m wide, and is located in front of the anterior testes. The oviduct is muscular, rising from the dorsal posterior surface of ovary. It extends posteriorly to the Laurel canal. The oviduct extends to join with the principal vitellarian duct which later forms an ootype surrounded by the gland cells of the Mehlis gland. The vitellaria is tubular, long and bilaterally symmetrical. The uterus is elongated, coiled and covers the space between the ventral sucker and the testes. The first part of uterus serves as a uterine seminal receptacle. The uterus is filled with eggs in different stages of development. The eggs measure 74 - 80 x 25 - 35 µm. The excretory bladder elongates transversally, with an excretory pore terminal which opens subterminally. The main excretory duct extends up to the level of pharynx. The number of flame cells increase in adult worms and count 32 flame cells on each side of one adult worm.
Discussion
Looss (1899) created the genus Philophthalmus to accommodate P. palpebrarum, a non-spinous trematode found in the eyes of a bird in Egypt. The trematode, obtained experimentally from below the nictitant membrane of chicken from Venezuela, is very similar to P. gralli. Sugimoto (1928) redescribed P. gralli from chicken in Formosa. Alicata and Noda (1960) suggested that the Philophthalmus species found in Hawaii is very similar to P. gralli but with the difference of localization of the seminal vesicle. Ching (1961) reported the development of P. gralli in Hawaii, from metacercaria to sexually matured adult found in chicken, rats and rabbits, but observed variations in reproductive organs due to age and type of host. The morphology of the different developmental stages of this study is very closely comparable with the studies of Ching in Hawaii. A difference was in the flame cell formula which is included in the present study as 2 [(3 + 3 + 3) + (2 + 2 + 2)] = 30, whereas Ching (1961) observed 2 [(3 + 3 + 3) + (3 + 3 + 3)] = 36.
Ching (1961) and Alicata (1962) confirmed the existence of 3 generations of rediae in circulatory system and digestive glands of snails. Same number of generations of rediae is found in the present studies in the digestive glands of the snail M. tuberculata. Saxena (1984) observed this for P. lucknowensis. A precercarial description of P. gralli given by Alicata (1962) coincides very closely with the present studies. He did not report the existence of 3 nucleated gland cells with fine granular contents in the mother redia, duct of which opens to the anterior body of rediae. The cavity in caudal appendix of both daughter and grandaughter rediae are filled with nucleated gland cells with fine granular contents, probably for adhesion. Various drops of these gland cells are found on the slide and rediae attached to it. In addition, a rosette of papillae around mouth is found in the grandaughter rediae as well as in cercariae. Kalantan et al. (1997) reported a gymnocephalid cercariae encysting on surface of water after it emerged from the freshwater snail, M. tuberculata, which later developed experimentally to the adult stage and they identified it as P. gralli. According to them the cyst is pear-shaped, while the present study describes it as the shape of a bottleneck. Kalantan et al. (1997) did not describe an excretory system, penetrating gland cells and cystogenic gland cells. It is difficult to compare taxonomically the two parasites to find out the validity of P. gralli in Saudi Arabia. Nasir and Díaz (1972) described P. lacrymosus Baun, 1902 and O. semipalmatus n. sp. from the optical cavity of the definitive host Catoptrophorus semipalmatus in Venezuela. P. lacrymosus differs from P. gralli in size of body, pharynx, acetabulum, ovary and testes and, markedly, in the extension of vitelline glands. Penner and Fried (1963) suggested synonymy of the genus Ophthalmotrema to Philophthalmus and emended the generic characteristics of the genus Philophthalmus to include the species of Ophthalmotrema. Philophthalmus semipalmatus differs clearly from P. gralli in the position of seminal vesicle, length of prepharynx, type and length of vitellaria.
Six cases of human ocular infection by Philophthalmus species were reported earlier, and one of them was caused by P. gralli in U.S.A. (Gutiérrez et al. 1987). Lang et al (1993) reported a case of conjunctivitis in the right eye due to an infection of P. palpebrarum. The patient suffered from irritation in his eyes and photophobia and the symptoms disappeared with the removal of the parasites. These authors believe that human ocular infection by Philophthalmus is rare and caused by accidental zoonosis.
The introduction of thiarid snails into different habitats in Valencia, Venezuela resulted in the elimination of Biomphalaria sp., the intermediate host of Schistosoma (Pointier et al.1994). M.ltuberculata appeared in Venezuela in 1972 and has adapted rapidly in all rivers and rivulets of the littoral and central region of the country (Chrosciechowski 1973). Centrocestus formosanus Nishigori, 1924 and Haplorchis pumilio were recorded from this snail in two localities of this State (pers. comm.). P. gralli has been reported from human elsewhere and there may exist the possibility of human infection in Venezuela unless preventive measures are taken.
Resumen
Se describe el miracidio, redia madre, redia hija, redia nieta, cercaria y el adulto de Philophthalmus gralli Mathis y Leger, 1910, obtenidos de infecciones experimentales. El hospedero intermediario Melanoides tuberculata fue recolectado en las localidades de Aguasanta y Yaguaracual, estado Sucre,Venezuela. Varios pollos fueron infectados oralmente con cercarias y metacercarias y colocando metacercarias directamente en los ojos con una pipeta. En ambos procesos de infección, se obtuvo parásitos adultos. Se amplía la distribución geográfica de P. gralli.
References
Alicata, J.E. 1962. Life cycle and developmental stages of Philophthalmus gralli in the intermediate and final hosts. J. Parasitol. 48: 47-54. [ Links ]
Alicata, J.E & K. Noda. 1960. Observations on the life history of Philophthalmus gralli a species of eye fluke of birds in Hawaii, pp. 67-73. In M. Bravo-Hollis (ed.). Libro homenaje al Dr. Eduardo Caballero y Caballero. Universidad Autónoma de México, México City. [ Links ]
Alicata, J.E. & H.L. Ching. 1960. On the infection of birds and mammals with the cecaria and metacercaria of the eye fluke, Philophthalmus. J. Parasitol. 46: 16. [ Links ]
Ching, H.L. 1961. The development and morphological variation of Philophthalmus gralli Mathis and Leger, 1910 with a comparison of species of Philophthalmus Loos 1899. Proc. Helminthol. Soc. Wash. 28: 130-138. [ Links ]
Chrosciechowski, P. 1973. Un caracol en busca de nueva residencia. El Lago 30: 813-814. [ Links ]
Gutiérrez,Y., H.E. Grosniklaus & W.L. Annable. 1987. Human conjuntivitis caused by the bird parasite Philophthalmus. Amer. J. Ophthalmol. 104: 417-419. [ Links ]
Ismael, N.S. & I.M. Issa. 1987. Life cycle of Philophthalmus gralli (Mathis and Leger, 1910) (Trematoda: Philophthalmidae) in Azraq Oasis, Jordan. Jap. J. Parasitol. 36: 53-62. [ Links ]
Ismael, N.S. & E.K. Saliba. 1985. Studies on the larval stages of digenetic trematodes of Melanoides tuberculata (Muller) snails from Azraq Oasis, Jordan. Riv. Parassitol. 46: 263-271. [ Links ]
Kalantan, A.M.N., M. Arfin, H.A. Al-Arefi, H.I. Bobshait, S.A. Hamadah, F.H. Al-Thawab & A.A. Al-Shamarani. 1997. Occurrence of larval Philophthalmus gralli (Mathis and Leger,1910) in freshwater snail, Melanoides tuberculatus (Muller) from Al-Hafuf, Saudi Arabia and its development into adult in various experimental hosts. Parasitol. Internat. 46: 127-136. [ Links ]
Lang, Y, Y. Weiss, H. Garzozi, D. Gold & J. Lengy. 1993. A first instance of human philophthalmosis in Israel. J. Helminthol. 67: 107-111. [ Links ]
Looss, A. 1899. Weitere Beitrage zur Kenntniss der Trematoden-Fauna Aegyptens, zugleich Versuch einer Naturlichen Gliederung des Genus Distomum. Zool. Jahrb. Syst. 12: 521-784. [ Links ]
Nasir, P. & M.T. Díaz. 1960. Avian flukes of Venezuela. Riv. Parassitol. 33: 245-276. [ Links ]
Penner, L.R. & B. Fried. 1963. Philophthalmus hegeneri n. sp., an ocular trematode from birds. J. Parasitol. 49: 971-977. [ Links ]
Pointier, J.P., R.N. Incami, C. Balzan, P. Chrosciechowski & S. Prychan. 1994. Invasion of the rivers of the littoral central region of Venezuela by Thiara granifera and Melanoides tuberculata (Mollusca: Prosobranchia: Thiaridae) and the absence of Biomphalaria glabrata, snail host of Schistosoma mansoni. Nautilus 107: 124-128. [ Links ]
Saxena, S.K. 1984. Studies on the life history of Philophthalmus lucknowensis Baugh, 1962. III. Rediae and cercariae. Rev. Iber. Parasitol. 44: 291- 307. [ Links ]
Sugimoto, M. 1928. On the trematode parasite Philophthalmus in the eyes of the domestic fowl. Dobutu Gaku Zassi. 40: 343-351. [ Links ]
Sugimoto,M. 1928. On the trematode parasite Philophthalmus in the eyes of the domestic fowl. Dobutu Gaku Zassi. 40: 343- 351. [ Links ]