Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.49 no.2 San José jun. 2001
Abstract
We describe morphological variation (scalation and coloration) observed among eight individuas of the Panamanian lizard species Anolis casildae. This variation was not observed in the holotipe and aids in identification of this recently described species (originally describes on the basis of a single, male specimen). This species occurs only in the Reserva Forestal Fortuna (Chiriquí Province) and the adjacent Bosque Protector Palo Seco (Bocas del Toro Province) in western Panama. Anolis casildae can be distinguisbed from all other Panamanian anole species via six features: (1) two enlarged superciliary scales (the first larger than the second); (2) an anterior nasal scale in contact with the rostral scale or separated from the rostral by one scale; (3) 6-8 sublabial scales to the center of the eye; (4) 3-4 scales between the supraorbital semicircles; (5) unique coloration (4-6 oblique brown bands interspersed by blueoutlined yellow patches; dewlap is a dirty cream color with broad yellow scale rows irregularly interspersed with smaller emerald green scales) and (6) A. casildae occurs from 1 050 to 1 400 m in the Cordillera Central. We also compare our natural history observations of A. casildae to a similar large anole, A. frenatus, a species which we believe A. casildae to be elosely related.
Key words
Squamata, Polychrotidae, Anolis, A. casildae, morphology, natural history.
Since the description of Anolis casildae in 1991 (Arosemena et al.), we have collected and observed additional individuals providing information not previously reported for the species. This western Panamanian species was described on the basis of a single male (Museo de Vertebrados, Universidad de Panamá, MVUP-755) captured in the Reserva Forestal Fortuna in the Cordillera Central, Provincia de Chiriquí. Recently, we examined several females and additional males revealing variation in scalation within the species, the existence of two female color morphs, and sexual dimorphism in severas characters. In this paper we describe morphological variation in A. casildae, review comparisons of this species to other known A. latifrons species, and provide brief ecological and distributional notes for A. casildae.
Morphological Variaton
Eight specimens (Natural History Museum of Los Angeles County, LACM 146192-146197, Los Angeles, California, USA; Smithsonian Tropical Research Institute, STRI 0113, Panama; Naturhistoriches Museum Bern, NMBE 1049173-1049174, Switzerland) of A. casildae were collected, some of which exhibit variation in one to several characters not previously reported for the species. Characters that differ from those found in the holotype are describes below with the holotype's features noted in parentheses. In addition, RID reexamined the holotype, finding a few discrepancies from the original description, noted within the parentheses. Characters are describes following the external feature characterizations of Williams et al. (1995) and the format of Arosemena et al. (199l).
Head: 11-15 scales between die second canthals (11); 5-8 canthal scales (7) enlarged, keeled; 6-10 postrostral scales (7); most individuals possess a single anterior nasal either in contact with the rostral or separated from the rostral by one scale, but one individual (STRI-0113) possesses an inferior nasal in contact with the rostral scale; 6-8 rows of loreals (5, but reexamination showed 6) with 47-65 loreals (not given, but reexamination showed 47), loreals subequal in size and slightly keeled to rugose (slighdy keeled); 6-8 supralabials to the center of eye (7); 3-4 scales between the supraorbital semicircles (3); supraocular disk contains 8-18 enlarged scales (10); interparietal separated from supraorbital semicircles by 3-4 scales (3); one keeled, elongate supercilary scale extending approximately half the length of the eye, followed by one keeled scale 1/3 this length, and rarely an even smaller third scale, then a series of granular scales (one keeled and elongate superciliary followed by a subgranular series, but reexamination showed up to 2 superciliaries followed by a series of small granular series; 3-4 scales between interparietal and nape scales (3, but reexanúnation showed 4); 6-9 postmental scales (7); 1-3 sublabials (3) in contact with first two infralabials. One feature, not noted for the holotype, is the presence of several dark punctations on the tympanum of all other individuals exanúned.
Trunk: Dorsal scalation consists of approximately 2-6 rows of keeled, imbricate mid-dorsal scales that are larger than nuchal scales (2-3 rows); mid-dorsal scales grade smoothly to conical, granular flank scales as in holotype; flank scales in most specimens are juxtaposed (not given, but reexamination showed juxtaposed to slightly imbricate); ventral scales larger than mid-dorsal and flank scales, smooth, and can be juxtaposed or imbricate (identical for holotype).
Dewlap: Male dewlap as in holotype, large, extending to anterior third of venter, with broad scale rows 2-3 scales wide interspersed with smaller, single scales, scale rows separated by skin; female dewlap present, small, extending to anterior edge of shoulder, scale rows as in males (see coloration)
Limbs and digits: 21-26 lamellae under phalanges ii and iii of fourth hind toe (23, but reexamination showed 22); hind limbs as in holotype, long, with tip of fourth toe reaching anteriorly between eye and rostral scales. Forelimb extends anteriorly beyond snout level and posteriorly to thigh articulation.
Tail: Weakly compressed (subcylindrical, but reexamination showed weakly compressed); males possess two enlarged postanal scales, females lack enlarged postanal scales; tail length 2.1 to 3.0 times body length; mean tail length/snout-vent length of males slighty higher than that of females (male mean = 2.8; female mean = 2.5).
Color: Male color, with some additions, as in holotype: dorsal ground color emerald green with approximately 4-6 oblique brown bands; yellow patches that are frequently outined by light blue present between brown bands; limbs green with alternating brown bands; venter green to yellow green with gray spots, or mottled yellow and green; dewlap of males dirty cream with broad yellow scale rows irregularly interspersed with smaller emerald green scales; female body coloration can be as in males, or displays a second morphotype of solid forest green ground color with a broad, dark brown, mid-dorsal stripe extending from the nape scales to the proximal portion of the tail. Female dewlap is either similarly colored as male dewlap, or is green with yellow scale rows irregularly interspersed with blue spots. Iris is tan-green.
Sexual Dimorphism: Discrete differences between the sexes include dewlap size (male dewlap large, extending to the sternum; female dewlap small, extending only to anterior edge of shoulder), mid-dorsal crest (present in males extending from nuchal area; absent in females), and enlarged postanal scales (present in males; absent in females). Some females exhibit the mid-dorsal brown stripe, a pattern observed in several Anolis species, however not all females possess this stripe. There is no sexual size dimorphism in SVL or mass for captures of A. casildae (Fig. 1).
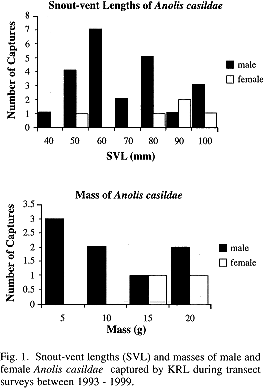
Distribution: The holotype of A. casildae was collected along the margins Quebrada Frank in the Reserva Forestal Fortuna, Chiriquí Province, western Panama. All observations of sleeping A. casildae by KRL were within 20 m of a stream, although KEN found animals in areas away from streams. The Reserva Forestal Fortuna contains the headwaters of the Río Chiriquí, so most parts of the reserve are within severas hundred meters of a strearn. Additional specimens have been collected or observed throughout the Reserva Forestal Fortuna, between approximately 1 050 and 1 400 m elevation, and within the adjacent primary forest of the Bosque Protector Palo Seco at 1 400 m elevation, Bocas del Toro Province. The specimens (NMBE 1049173-1049174) from the Bosque Protector Palo Seco represent a range extension for the species, from the Pacific to the Atlantic versant of the Cordillera Central. All localities are within cloud forest (sensu Myers 1969).
Comparisons with other Panamanian Anolis: Arosemena et al. (1991) discussed the relafionships of A. casildae to other anoles based on their similarities, but variation in characters presented here warrants a revised review and clarification. Anolis casildae is allied with the A. latifrons group (according to Arosemena et al. 1991) because it possesses the characteristic features of the group: these are giant (snout-vent length > 100 mm) mainland alpha anoles (Etheridge 1959; Dactyloa of Guyer and Savage 1986, 1992) that possess wide toe lamellae under phalanges ii and iii, have moderately-sized head scales, lack enlarged mid-dorsal scales relative to their flank scales, and lack a prohoscis and axillary pockets (Williams 1976, 1988). Anolis casildae is distinguished from other A. latirfrons group anoles (A. apollinaris, A. danieli, A. fraseri, A. insignis, A. microtus and A. propinquus) by possessing longer hind limbs (Arosemena et al. 199 l).
Anolis casildae was reported previously to possess a single, keeled and elongate superciliary scale extending the length of the eye followed by a series of subgranular scales, and this feature was used to distinguish it from a number of other species. Individuals exarnined and discussed in this paper differ in having a second enlarged superciliary scale behind the first, and sometimes even a third small superciliary scale. The second superciliary scale varies among individuals in its shape and extent of elongation, but it is never as long as the first enlarged superciliary scale. A small granular series follows the posterior - most superciliary scale. This condition distinguishes A. casildae from A. latifrons which has a series of swollen and rugose superciliaries, and from A. squamulatus which has approximately four short superciliary scales followed by a subgranular series. However, the superciliary condition of A. casildae is similar to that of A. frenatus, A. princeps, and A. purpurescens.
The holotype of A. casildae was reported as having as a diagnostic feature an anterior nasal scale in contact with the rostral scale (distinguishing it from A. princeps and A. purpurescens and in part from A. frenatus). However, we found that A. casildae exhibits variation in this character such that the anterior nasal scale may be in contact with the rostral or separated from the rostral by one scale. Thus, this character does not distinguish A. casildae from A. princeps, A. purpurescens or A. frenatus, but other characters may be used. Anolis princeps has a greater number of sublabials to the center of the eye than does A. casildae (9-12 for A. princeps; 6-8 for A. casildae). A. purpurescens, still known only from the holotype, possesses two scales between the nasal and rostral (A. casildae has 0-1 scale intervening), has 2 scales between the supraorbital semicircles (A. casildae has 3-4), and the dorsal scales are not keeled (see above for dorsal condition of A. casildae).
On the basis of overall similarity, A. casildae seems most closely related to A. frenatus as evidenced by the nearly complete overlap in meristic characters; they seem to differ only in coloration and in geographic range. The dorsum of A. frenatus is covered with several dark green ocelli (most oudined with yellow) that are closely arranged and from dark oblique bands and has a dull, cream-colored dewlap (occasionally with black shading) that appears somewhat translucent. In contrast, A. casildae has approximately 4-6 oblique brown bands (not formed of ad acent ocelli) interspersed by blueoutlined yellow patches, and a dewlap of dirty cream color with broad yellow scale rows irregularly interspersed with smaller emerald green scales. Anolis frenatus is primarily a lowland inhabitant (up to approximately 800 m elevation) ranging from Costa Rica southward to Colombia, whereas A. casildae has been reported only from 1 050 to 1 400 m in the Cordillera Central in the highlands of Chiriquí and Bocas del Toro Provinces, western Panama.
Discussion
Anolis casildae was regularly seen during herpetofaunal surveys at Fortuna. KRL made 34 observations of A. casildae individuals during surveys (n=174) of stream and terrestrial transects between 1993 and 1999. The annual average of A. casildae captures per transect was 0.27 + 0.34 (range = 0 - 0.30, n = 7 visits), annual average of captures/minute was 0.0014 + 0.0012 (range = 0 - 0.0031, n = 6 visits), and annual average of captures/km surveyed was 0.39 + 0.53 (range = 0 - 1.45, n = 6). During one collecting trip targeting anoles, KEN observed 9 individuals over 32 daytime collecting hours along two transects 0.00468 captures/minute).
All KRL sightings occurred at night along streams when lizards were found sleeping on large-leafed plants (e.g., tree ferns, palms, aroids) along stream courses. This species was usually observed at a height of 1 m or more in trees or vegetation adjacent to or within 100 m of a strem. Sleeping females were captured at 1.50 + 0.43 m height (range = 1 - 1.75 m, n = 3 individuals), while males were captured at a height of 1.62 + 0.95 m (range = 0 - 4 m, n = 17 individuals). Adults were capturad at 1.58 + 0.698 m (range = 1 - 3 m, n = 10 individuals), while juveniles were captured at 1.79 + 1.22 m (range = 0 - 4 m, n = 13 individuals). There were no significant differences between male or female or between adult and juvenile capture heights (Mann-Whitney U one tailed tests because adult > juvenile and male > female is expected; Losos et al. 199 l).
During the day individuals were usully observed on tree trunks at 2 m or less in height in the characteristic head down posture of Anolis, but were occasionally observed on trunks in a head up position. Individual A. casildae were captured easily by hand and allowed close approach showing only mild wariness. lf wary when approached, they moved to a position on the trunk opposite the captor as other anole species do when attempting to "hide". KEN observed one large male capturing a katydid from the ground and then quickly leaping to a trunk perch approximately 0.5 m in height to consume the prey (Nicholson 1999). It is unknown whether this species regularly forages on the ground.
Anolis casildae shares some similarities to another large Panamanian anole, Anolis frenatus. Both have been observed to assume a head-down posture when perched during daytime, and are known to forage on the ground (pers.obs., KEN). While females of both species were captured at the same height (Losos et al. 1991), male A. frenatus were found one meter higher on average than those of A. casildae (Losos et al. 1991). Little work has been done on either species, but they have been captured with varying frequencies at different sites (A. casildae: Fortuna: 0.00468/min by KEN, 0.00140/min by KRL; A. frenatus: "Lutz" in Barro Colorado Island: 0.00329/min by Losos et al. 1991; "AVA" in Barro Colorado Island: 0.00033/min by Losos et al. 1991; several sites in Barro Colorado Island: 0.00076/min by Losos et al. 1991; six sites in the lowlands of central Panama: 0.00002/min by Ibáñez et al. "1995"[19971). Thus, these species may both appear to occur at low densities, or are seen less frequently than other species due to cryptic coloration or more common associations higher in the trees.
Acknowledgements
We thank the personnel of the Instituto de Recursos Hidraúlicos y Electrificación, Fortuna E.G.E., and the Smithsonian Tropical Research Insfitute for pemission to work in the reserve, and for help in obtaining collecting and research permits. KEN especially thanks Stan Rand for encouragement. We thank Brian
Crother, Randy McCranie, Paul Richards, and Brian Warren for editing previous drafts of this paper. RID thanks Ulrich Hofer for permission to examine and publish information on specimens collected in Bocas del Toro.
Resumen
Nosotros describimos la variación morfológica (escamación y coloración) observada entre ocho individuos de la especie de lagartija panameña Anolis casildae. Esta variación no fue observada en el holotipo y ayuda en la identificación de esta especie recientemente descrita (originalmente descrita con base en un único espécimen macho). Esta especie ocurre solo en la Reserva Forestal Fortuna (Provincia de Chiriquí) y en el Bosque Protector Palo Seco (Provincia de Bocas del Toro) adyacente, en el oeste de Panamá. Anolis casildae se puede distinguir de las demás especies panameñas de Anolis por seis características: (1) dos escamas superciliares agrandadas (la primera más grande que la segunda); (2) una escama nasal anterior en contacto con la escama rostral o separada de ésta por una escama; (3) seis-ocho escamas sublabiales hacia el centro del ojo; (4) tres-cuatro escamas entre los semicírculos supraorbitales; (5) coloración única (cuatro-seis bandas café oblicuas interespaciadas por parches amarillos bordeados de azul; la papada es de color crema sucio con filas anchas de escamas amarillas interespaciadas irregularmente con escamas verde esmeralda más pequeñas) y (6) A. casildae ocurre de 1 050 a 1 400 m en la Cordillera Central. Nosotros también comparamos nuestras observaciones de historia natural de A. casildae con un Anolis similar grande, A. frenatus, una especie a la que nosotros creemos está A. casildae cercanamente relacionada.
References
Arosemena, F.A., R. Ibáñez D. & F. de Sousa. 1991. Una especie nueva de Anolis (Squamata: Iguanidae) del grupo latifrons de Fortuna, Panamá. Rev. Biol. Trop. 39: 255-262. [ Links ]
Etheridge, R.E. 1959. The relationships of the anoles Reptilia: Sauria: Iguanidae): an interpretation based on skeletal morphology. Ph.D. Dissertation, University of Nfichigan, Ann Arbor. 236 p. [ Links ]
Guyer, C. & J.M. Savage. 1986. Cladistic relationships arnong anoles (Sauria: Iguanidae). Syst. Zool. 35: 509-531. [ Links ]
Guyer, C. & J.M. Savage. 1992. Anole systematics revisited. Syst. Biol. 41: 89-110. [ Links ]
Ibáñez D., R., C.A. Jaramillo, M. Arrunátegui, Q. Fueninayor & F.A. Solís. " 1995"[1997]. II. Inventario biológico del Canal de Panamá. Estudio Herpetológico. Scientia (Panamá), Número Especial 2: 1-281. [ Links ]
Losos, J.B., R.M. Andrews, O.J. Sexton, & A.L. Schuler. 1991. Behavior, ecology, and locomotor performance of the giant anole, Anolis frenatus. Carib. J. Sci. 27: 173-179. [ Links ]
Myers, C.W. 1969. The ecological geography of cloud forest in Panama. Amer. Mus. Novit. 2369: 1-52. [ Links ]
Nicholson, K.E. 1999. Anolis casildae foraging. Herp. Rev. 30: 96. [ Links ]
Williams, E.E. 1976. South American anotes: the species groups. Pap. Avuls. Zool. S. Paulo 29: 259-268. [ Links ]
Williams, E.E. 1988. New or problematic Anolis from Colombia. V. Anolis danieli, a new species of the latifrons species group and a reassessment of Anolisapollinaris Boulenger, 1919. Breviora 489: 1-25. [ Links ]
Williams, E.E., H. Rand, A.S. Rand & J.S. O'Hara. 1995. A computer approach to the comparison and identification of species in difficult taxonomic groups. Breviora 502: 1-47. [ Links ]
1 Department of Biology, University of Miami, PO. Box 249118, Coral Gables, Florida 33124 USA; fax: 305-284-3039, email: kirsten@fig.cox.niianii.edu
2 Smithsonian Tropical Research Institute, Apartado 2072, Balboa, Ancón, República de Panamá; fax: 507-212-8148, email (RI): ibanezr@tivoli.si.edu, email (CJ): jaramilc@tivoli.si.edu
3 Círculo Herpetológico de Panamá, Apartado 10762, Estafeta Universitaria, Panamá, República de Panamá
4 Department of Zoology, Southem Illinois University, Carbondale, Illinois, 62901 USA; fax: 618-453-2806,
email: klips@zoology.siu.edu












 uBio
uBio 

