Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.49 no.2 San José jun. 2001
Received 31-I-2000. Corrected 4-X-2000. Accepted 23-X-2000
Abstract
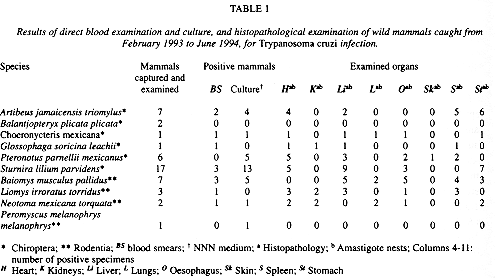
Wild vectors and reservoir hosts of Trypanosoma cruzi were surveyed from February 1993 to June 1994 in Ticumán (18°46N, 99°07W), Mexico (Deciduous Tropical Forest). Direct faeces examination showed that 87% of Triatoma pallidipennis hosted the parasite; T. cruzi forms were present in cultures inoculated with faeces of fifty 67% triatomine bugs and thirty CD-1 strain mice (10 d old) inoculated (peritoneum) with faeces of positive insects T. cruzi amastigotes were found in heart 67%, kidneys 47%, liver 80%, lungs 50%, oesophagus 60%, skin 23%, spleen 73% and stomach 60%. T. cruzi was isolated by direct blood examination from seven 21% chiropterans and five 38% rodents and T. cruzi forms were present in cultures inoculated with blood of twenty-three 68% chiropterans and seven 54% rodents and T. cruzi amastigotes were seen in the kidneys of one 3% chiropterans and four 31% rodents and only in one Pteronotus parnellii mexicanus, organisms were seen in skin 2%. There was no association between organs and T. cruzi infection (p> 0.05)
Key words
Trypanosoma cruzi, wild mammals, Triatoma pallidipennis, Mexico.
Epidemiology of American Trypanosomiasis (Chagas disease) includes the vector, triatomine bugs TB infected with Trypanosoma cruzi, and susceptible mammals (sources of infection for the humans). In rural areas, contact between T. cruzi and humans is easy because of the persistence of intra-domiciliary vectors and domestic mammals that may acquire the infection from sylvatic foci (Botero and Restrepo 1994). We determined the infection rate IR of wild vectors and reservoir hosts RH of T. cruzi from Ticumán, Mexico.
In the study area (Ticumán 18°46N, 99°07W), Tlaltizapán, Morelos, Mexico, 965 m above sea level, climate is warm and sub-humid Awo(w)(e)gw in Köeppens classification modified by García (1988) and the surrounding vegetation is characterized by Deciduous Tropical Forest. Three sylvatic sites were selected as trapping stations and from February 1993 to June 1994, specimens of TB according to Carcavallo (1985) were collected and transported to the laboratory where the Lent and Wygodzinsky (1979) identification keys were followed. To determine T. cruzi infection TCI the captured TB faeces extracted through abdominal compression were placed on slides, diluted in PBS (pH 7.2) and observed by light microscopy LM according to Rosario (1989). Trypanosomes from faeces (0.2 ml) were cultured in NNN medium and inoculated (0.2 ml) into the peritoneum of thirty CD-1 strain mice (10 d old). Wild mammals WM were caught with Mist nets, 200 x 600 cm, for chiropterans CHT and Sherman traps for rodents ROD; all nets and traps were arranged 10 m from each other. In the laboratory the WM were identified according to Ramírez-Pulido et al. (1989) and Wilson and Reeder (1993). Mammals were killed by aseptic cardiac puncture and 1 ml of heparinized blood was immediately added to sterile culture tubes with NNN medium and incubated at 26oC for 30 d according to Rosario (1989); once a week a drop was observed under a coverslip by LM. Internal organs such as heart H, kidneys K, liver LI, lungs L, oesophagus O, skin SK, spleen S and stomach ST were removed and fixed in 10% neutral formalin solution and histologically examined to determine the presence of T. cruzi amastigotes TCA according to Wisnivesky-Colli et al. (1992). Giemsa and Wright-stained blood smears BS from each mammal were prepared, and the IR was determined by prevalence according to Bush et al. (1997). Dependence between different organs of WM and the TCI was determined through chi-square goodness of fit.
Most of the TB collected were the third, fourth and fifth instar nymphs and some adult males and females belonging to Triatoma pallidipennis species. Direct faeces examination showed that T. cruzi trypomastigotes TCT were present in 87% of 75 captured TB and T. cruzi forms TCF were present in cultures of 67% TB. Mice inoculated were found already infected at 7-15 d post-inoculation and parasitaemia peaks oscillated among (mean 2.3 x 105trypomastigotes/ml) observed on day 10 and (mean 4.6 x 103 trypomastigotes/ml) recorded on day 19 post-inoculation and TCA were found in H 67%, K 47%, LI 80%, L 50%, O 60%, SK 23%, S 73% and ST 60%. Most of the WM trapped wereCHT and also someROD were caught and TCT were found in BS of 21% CHT and 38% ROD. Only specimens of Balantiopteryx plicata plicata were negative and in culture medium one specimen of Peromyscus melanophrys melanophrys was positive showing recent and low parasitaemia. TCF were present in cultures of 68% CHT and 54% ROD while TCA were found in theKof 3% CHT and 31% ROD and only in one Pteronotus parnellii mexicanus, organisms were seen in SK 2% and for additional results, see Table 1. There was no association (p> 0.05)between the whole group of WM; or for CHT and for ROD .
Our finding of specimens of T. pallidipennis infected with T. cruzi from caves where CHT and other mammals live, and the 87% infection frequency was not unusual. The data were not significantly different from that of 81% obtained by Arriola (1989), but did differ clearly from Magdaleno et al. (1990), Bautista et al. (1992) and Herlindo-Jaimes (1998) who found 15%, 29% and 54% infected respectively. In Mexico, studies on CHT reservoirs of T. cruzi one few. The IR did differ significantly from that of 59% for P. parnellii obtained by Parra et al. (1992) and from that of 29% for Artibeus jamaicensis triomylus and 60% for Sturnira lilium parvidens obtained by Herlindo-Jaimes (1998), so as from that of 50% for A. jamaicensis obtained by DAlessandro et al. (1984). Our finding of TCI in bats agrees with previous researches characterizing various CHT species as RH of the parasite in America (reviewed by Pereira-Barretto 1985). Concerning ROD reservoirs in Mexico, there is insufficient epidemiological research, that is why more synantropic that wild rodents have been studied. The IR were not significantly different from that of 91% for P. melanophrys and 97% for Neotoma mexicana obtained by Parra et al. (1992), but did differ significantly from that of 43% for P. m. melanophrys and 40% for Baiomys musculus pallidus obtained by Herlindo-Jaimes (1998). The fact that some infected reservoirs were culture-negative indicates possible contamination and that they lacked a circulating parasitaemia.
Acknowledgements
Demetrio Porcayo-Tavira, Doroteo Adrian Ramírez-Ceballos and Marco Antonio Lozano-García helped us with mammal trapping and taxonomical identification. We are also grateful to Félix Herlindo-Jaimes for his assistance in the laboratory. The critical review of the manuscript by Eduardo Aranda-Escobar, Lucio Galavíz-Silva, Patricia Trujillo-Jiménez, Adriana Elizabeth Flores-Suárez, Janine M. Ramsey (English grammar), Roberto Mercado-Hernández (Biostatistical Analysis) and Luis Carlos GarcíaSierra-Cárdenas is greatly appreciated.
Resumen
Se determinó la infección por Trypanosoma cruzi en chinches triatóminas y mamíferos silvestres capturados de febrero de 1993 a junio de 1994 en Ticumán (18°46N, 99°07W), Tlaltizapán, Morelos, México. El 87% de los ejemplares de Triatoma pallidipennis tenía el parásito; T. cruzi fue aislado de la sangre de siete quirópteros y cinco roedores. No hubo dependencia significativa entre los órganos examinados de los mamíferos y la infección por T. cruzi.
References
Arriola, G. M. T. 1989. Frecuencia de Trypanosoma cruzi (Kinetoplastida: Trypanosomidae) en Triatoma pallidipennis (Hemiptera: Reduviidae) recolectadas en la ciudad de Cuernavaca, Morelos. Resúmenes I Encuentro Estatal sobre Entomología Médica y Veterinaria y II Jornadas Médicas de Salud en el Trabajo, Cuernavaca, Morelos, México. 109 -115 p. [ Links ]
Bautista, L. N. L., P. M. Salazar-Schettino, I. De Haro-Arteaga & A. H. U. Brailovsky. 1992. Estudio de transmisores de Trypanosoma cruzi en el Estado de Morelos, México. Resúmenes III Reunión Nacional sobre la Enfermedad de Chagas, Instituto Nacional de Cardiología Dr. Ignacio Chávez, Secretaría de Salud, México, D. F. 30 p. [ Links ]
Bush, A. O., K. D. Lafferty, J. M. Lotz & A. W. Shostak. 1997. Parasitology meets ecology on its own terms: Margolis et al. Revisited. J. Parasitol. 83: 575 -583. [ Links ]
Carcavallo, R. U. 1985. Técnicas de estudio de triatóminos en ambiente silvestre, p. 49 -52. In R. U. Carcavallo, J. E. Rabinovich & R. J. Tonn (eds.). Factores Biológicos y Ecológicos en la enfermedad de Chagas. Ministerio de Salud y Acción Social, Buenos Aires, Argentina. [ Links ]
DAlessandro, A., P. Barretto, N. Saravia & M. Barretto. 1984. Epidemiology of Trypanosoma cruzi in the oriental plains of Colombia. Amer. J. Trop. Med. & Hyg. 33: 1084 -1095. [ Links ]
García, E. 1988. Modificaciones al sistema de clasificación climática de Köeppen, para adaptarlo a las condiciones de la República Méxicana. Instituto de Geografía, Universidad Nacional Autónoma de México, México, D. F. 220 p. [ Links ]
Herlindo-Jaimes, F. 1998. Infección de triatóminos y presencia de Trypanosoma cruzi en mamíferos silvestres y peridomésticos del municipio de Jiutepec, Morelos, México. Tesis de Licenciatura, Facultad de Ciencias Biológicas, Universidad Autónoma del Estado de Morelos, Cuernavaca, Morelos, México. 95 p. [ Links ]
Lent, H. & P. Wygodzinsky. 1979. Revision of the triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas' disease. Bull. Amer. Mus. Natur. Hist. 163: 123-520. [ Links ]
Magdaleno, P. N., G. E. Magallón & D. G. Katthain. 1990. Prevalencia de Trypanosoma cruzi en triatomas de 50 municipios del estado de Jalisco, México. Resúmenes II Reunión Nacional sobre la Enfermedad de Chagas, Universidad Autónoma de Nayarit, Tepic, Nayarit, México. 4 -5 p. [ Links ]
Parra, T. R. L., F. A. Cervantes-Reza, I. De Haro-Arteaga & P. M. Salazar-Schettino. 1992. Estudio de reservorios de Trypanosoma cruzi en el Estado de Morelos, República Mexicana. Resúmenes III Reunión Nacional sobre la Enfermedad de Chagas, Instituto Nacional de Cardiología Dr. Ignacio Chávez, México, D. F. 13 p. [ Links ]
Pereira-Barretto, M. 1985. Reservorios del Trypanosoma (Schizotrypanum) cruzi Chagas-1909, p. 275 -288. In R. U. Carcavallo, J. E. Rabinovich & R. J. Tonn (eds.). Factores Biológicos y Ecológicos en la enfermedad de Chagas. Ministerio de Salud y Acción Social, Buenos Aires, Argentina. [ Links ]
Ramírez-Púlido, J., I. Lira, S. Gaona, C. Müdespacher & A. Castro. 1989. Manejo y mantenimiento de colecciones mastozoológicas. Laboratorio de Mastozoología, Universidad Autónoma Metropolitana, Unidad Iztapalapa, División de Ciencias Biológicas y de la Salud, Departamento de Biología, México, D. F. 47 -52 p. [ Links ]
Rosario, C. R. 1989. Análisis de los Esquizodemos de cuatro aislamientos de Trypanosoma cruzi de diferentes estados de la República Mexicana. Tesis de Maestría en Ciencias, Universidad Autónoma del Estado de Morelos, Cuernavaca, Morelos, México. 63 p. [ Links ]
Wilson, D. E. & D. A. M. Reeder. (eds.). 1993. Mammal Species of the World: A taxonomic and Geographic Reference. Smithsonian Institution. American Society of Mammalogists, Washington, D.C. 1206 p. [ Links ]
Wisnivesky-Colli, C., N. J. Schweigman, A. Alberti, S. M. Pietrokovsky, O. Conti, S. Montoya, A. Riarte & C. Rivas. 1992. Sylvatic american trypanosomiasis in Argentina. Trypanosoma cruzi infection in mammals from the Chaco forest in Santiago del Estero. Trans. Roy. Soc. Trop. Med. Hyg. 86: 38 -41. [ Links ]
1 Laboratorio de Mastozoología, Departamento de Biología Animal, Centro de Investigaciones Biológicas, Universidad Autónoma del Estado de Morelos. Avenida Universidad No. 1001, Colonia Chamilpa, Cuernavaca, Morelos 62210, México. Fax: +52-7-3297056. E-mails: jcvilleg@hotmail.com; jcvilleg@terra.com.mx; jcvilleg@correoweb.com; jvilleg@insp3.insp.mx.
Mailing address: Calle Venecia # 210, Fraccionamiento Santa Fe, Monterrey, Nuevo León, C.P. 64540, México.












 uBio
uBio