Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.49 no.2 San José jun. 2001
Received 17-III-2000. Corrected 17-X-2000. Accepted 31-X-2000
Abstract
Five species of Cyprinodon in Laguna Chichancanab, Yucatan, Mexico comprise a young species flock whose ecology and evolution has not been thoroughly studied, but whose existence is threatened with extinction. Species flocks evolve in isolated areas where predators and competitors are absent. Since the description of the Chichancanab flock, Oreochromis mossambicus, a species introduced into the lake for which I examined habitat in the 1980s, has become common throughout the basin. I assessed relative abundance of flock species in the lake. examined habitat use and segregation among the three most common flock species and examined the affects of O. mossambicus upon flock species habitat use. Cyprinodon beltrani was the most abundant flock species in 1997, followed by C. maya and C. labiosus; C. verecundus and C. simus were rare. Cyprinodon beltrani was found in shallow water, nearshore, over thick beds of submerged Chara, and little emergent vegetation Cyprinodon beltrani exhibited diurnal variation in nearshore habitat use. In the field, the habitat use of C. beltrani and O. mossambicus broadly overlapped. In aquarium experiments, three flock species exhibited habitat use segregation and C. beltrani and C. labiosus showed agonistic behaviors that strengthened segregation. Cyprinodon maya differed from C. beltrani and C. labiosus by its greater dispersion of individuals and use of areas higher in the water column. The presence of O. mossambicus caused a shift in habitat use by C. maya and C. labiosus that put these species into habitat occupied by C. beltrani. The presence of introduced species has caused a significant perturbation of the conditions that fomented speciation of the Chichancanab flock 8000 years ago.
Key words
Habitat segregation, Laguna Chichancanab, Cyprinodon, species flock, Yucatan, Mexico
The Laguna Chichancanab species flock is an exceptional group of species whose study provides insights into rapid speciation events but whose existence is threatened by an introduced exotic species. Species flocks are not formed from sequential invasions, basin capture or other aggregations of species into one lake, but rather result from speciation that occurs within a lake basin boundary (Greenwood 1984) in the absence of predators or competitors (Masters and Rayner 1993, Dieckman and Doekli 1999). The five species of Cyprinodon in Laguna Chichancanab, (C. beltrani, C. labiosus, C. maya, C. simus and C. verecundus) constitute a young species flock thought to have diverged from its sister species as recently as 8000 years ago and to have speciated sympatrically within the Chichancanab basin. When Humphries and Miller (1981) described the Chichancanab species flock, the sole constituents of the lake ichthyofauna were the five flock species, four of which were very abundant, and Gambusia sexradiata. The recent introduction of Oreochromis mossambicus (Strecker et al. 1996, Schmitter-Soto and Caro 1997) threatens the continued existence of the flock.
Extinction of endemic flock species caused by the introduction of exotic species is well documented (Witte et al. 1992). Over 200 species were lost in Lake Victoria after the introduction of the Nile perch and an entire species flock in Lake Lanao went extinct in the wake of a number of introductions, including O. mossambicus (Witte et al. 1992, Cohen 1994). The status of the Chichancanab flock since the introduction of O. mossambicus in the 1980s, and the impacts of the introduction upon the flock species have not been determined. The impacts of O. mossambicus upon habitat segregation among flock species are of particular interest because habitat segregation may have played a important role in the evolution of the Chichancanab flock (Humphries 1981). If O. mossambicus overlaps in habitat use with flock species, detection of shifts in habitat use by flock species may provide insights into the mechanisms originally driving segregation among the species.
I surveyed fishes in Laguna Chichancanab in December, 1996 to: describe nearshore habitat use by flock species (pupfishes) and O. mossambicus, determine the degree to which these species overlapped in habitat use, and describe the status of the flock after the introduction of O. mossambicus. The results from this initial survey provided the framework for aquarium experiments that I conducted in November, 1997 to determine differences in microhabitat use among three of the flock species and to investigate the effects of O. mossambicus upon microhabitat use by these flock species.
Materials and Methods
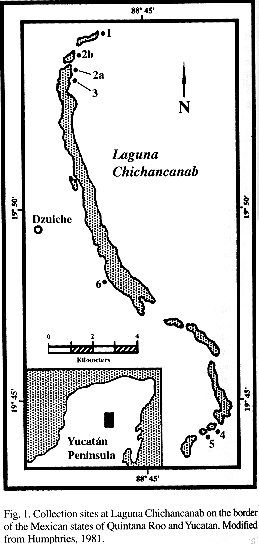
Laguna Chichancanab is located in the interior of the Yucatan peninsula on the border of the Mexican states of Quintana Roo and Yucatan (Figure 1). Covich and Stuiver (1974) and Humphries (1981) provide detailed descriptions of the lake basin. The following experimental methods have been abbreviated but a detailed description of methods is available from the author.
To assess relative abundances of lake fishes, I fished unbaited minnow traps at sites 1 - 4 sampled by Humphries (1981; Figure 1). To assess abundance of C. simus, a limnetic dweller, I seined, dip-netted and snorkeled areas at site 2a and b. To describe differences in nearshore habitat use among fishes, I fished 24 unbaited minnow traps, stacked to fill the vertical water column, at 0.5-m intervals on transects stretching from shore into the lake to a depth of 1 m. I checked traps every 3 - 4 hours throughout a 24-hr period then moved the transects to another location, and repeated the 24 hr survey at five different locations at station 2b (Figure 1). The following variables were recorded for each trap set: water depth at trap opening, presence of emergent and submerged vegetation, and distance to nearest shore.
Aquarium Experiments Experiments using Cyprinodon beltrani, C. labiosus, C. maya, and O. mossambicus were conducted in a continuous-flow system of four fiberglass tanks with an observation window marked with a 2-dimensional grid. Each trial and tank contained a different group of randomly selected fishes. I used O. mossambicus between 28 mm and 69 mm total length because this size was within the range of O. mossambicus most commonly captured with pupfishes in the lake. I conducted two trials of each of five experimental treatments to examine habitat use by single species of pupfishes, by a group of congeneric pupfishes and by flock species in the presence of O. mossambicus. A trial consisted of a 15-minute observation period, during which a count of the number of fishes of each species within each tank grid location was recorded every minute. As a measure of dispersion, I recorded the number of different areas in which fish of the same species were found at the 1-minute observation mark. The treatments were as follows: the first treatment was a single-species control designed to show that fishes did not exhibit a tank-side preference. Only gravel substrate was present in a tank that included five individuals of a single species. Treatment 2 was a single-species treatment used to examine microhabitat use for the species and provided a control with vegetation present with which to compare multi-species treatments. There were five individuals of the same species in a tank with half gravel and half submerged vegetation (SV) substrate. Treatment 3 was an intruder treatment where five individual pupfish of the same species and one O. mossambicus were in a tank with half gravel and half SV substrate. Treatment 4, a multiple-intruder treatment, included five conspecific cyprinodontids and five O. mossambicus.
Analyses - I used SAS for all statistical analyses (SAS Institute 1988) and adjusted the probability of acceptance to 0.05 divided by the number of tests examined. I used a general linear model (PROC GLM) for an unbalanced ANOVA and least squared means comparisons to identify differences in habitat use by fishes collected on lake transects. For aquarium experiments, I used t-tests to determine if fishes showed a tank side preference. I used analyses of covariance (ANCOVA) with time of day as a covariable and least-squared means to compare average locations of species within the tanks and to examine microhabitat preferences for the species. I used the log transformed values of the average number of occurrences of each species in each tank location for each trial for seven of the eight possible tank locations, and dispersion in an ANCOVA. The area nearest the water surface, on the non-vegetated tank side was omitted from analyses so the possible tank positions would be independent. I examined planned comparisons based on a priori hypotheses. I predicted that C. beltrani and C. labiosus would use areas nearest, or within vegetation more often when O. mossambicus were present, with the highest use coinciding with the highest density of O. mossambicus. I predicted that C. maya would use areas closer to the bottom of the tank on either tank side when O. mossambicus were present because C. maya were not commonly encountered in nearshore minnow traps.
Agonistic BehaviorsIn separate aquarium trials, I quantified the numbers of interspecific and intraspecific agonistic encounters between two male-female pairs of C. beltrani and C. labiosus in tanks with gravel substrate that included two small stones (10 x 5 cm) as focal points (Kodric-Brown 1995). A charge was defined as an aggressive approach by one individual that resulted in the receiver moving away from the charger. I used a 2x2 contingency analysis and Chi-square distribution to compare the numbers of interspecific and intraspecific charges.
Results
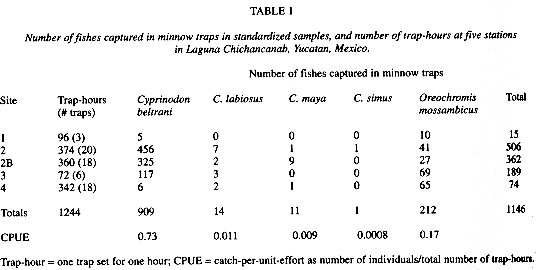
Relative abundanceCyprinodon beltrani was the most abundant flock species, C. labiosus,the second and C. maya, was the third most abundant flock species in minnow trap samples from stations 1 - 4 (Table 1). Oreochromis mossambicus outnumbered all flock species except C. beltrani. Only one individual C. simus was collected in a minnow trap at site 2; a site where Humphries (1981) collected all five species in 1978. No C. simus were observed during snorkeling surveys or in seine hauls and no definitively identified C. verecundus were collected in systematic samples. The most abundant fishes observed in snorkeling surveys were O. mossambicus and large schools (30 - 80 individuals) of Astyanax aeneus. Both G. sexradiata and A. aeneus were underrepresented in minnow trap samples, but abundant in seine hauls. I considered A. aeneus the species of Astyanax present in the lake based on Schmitter-Soto (1998).
Nearshore habitat use Transects were trapped for 1244 trap-hours and 120 traps were fished. There were no C. labiosus, C. simus, C. verecundus, G. sexradiata or A. aeneus collected in traps on transects.Cyprinodon beltrani was found in shallow water, nearshore, over thick beds of SV, and little emergent vegetation. This species inhabited these areas early in the morning but moved out of the nearshore area in the evening. Traps that contained C. beltrani were: (1) in areas with less emergent vegetation (P=0.003); (2) were checked earlier in the day (P=0.004); (3) were closer to the nearest shore (P=0.01); and (4) had more O. mossambicus present in the same trap (P=0.01) compared to traps without C. beltrani. There were no differences in habitat characteristics in areas where C. beltrani was present versus where it was absent for two variables: presence of submerged Chara and trap depth.
Habitat use of adult C. beltrani and O. mossambicus between 30 - 81 mm total length broadly overlapped. Of the total traps set, twenty-seven percent contained both C. beltrani and O. mossambicus. Forty percent of the traps containing C. beltrani also contained O. mossambicus individuals, and both C. beltrani and O. mossambicus were observed together during snorkeling surveys. Low capture rates of flock species other than C. beltrani prevented the quantification of habitat use for most of the flock species. There were significantly more O. mossambicus captured in traps with C. maya (P=0.0001), but this was likely an artifact of small sample size because only 15 traps contained C. maya.
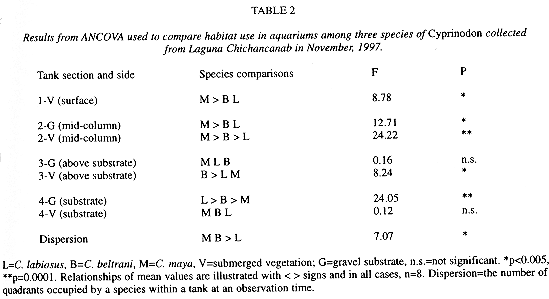
Single-species treatments There was no difference in tank side use for either of the three species(P > 0.20). Presence of submerged vegetation affected pupfish habitat use in single-species trials and species dispersed differently within tanks (Table 2). Cyprinodon maya used areas nearer the water surface more than the other two flock species and used the area just above the vegetation less often than C. beltrani. Both C. labiosus and C. beltrani were found more often on the unvegetated substrate and just above the SV substrate more often than C. maya (P < 0.006). Cyprinodon maya tended to remain more widely dispersed within a tank than C. labiosus (P = 0.005) and C. beltrani, though not significantly in the latter case.
Intruder treatmentsContrary to my predictions, neither C. beltrani nor C. labiosus used areas in or near the SV more often in the presence of O. mossambicus. Cyprinodon labiosus was found more often on the gravel substrate in intruder trials compared to single-species trials and had higher dispersion values in the single-intruder compared to single-species and multiple-intruder treatments (F = 3.83, r2 = 0.31, P = 0.03). Cyprinodon beltrani showed no differences in dispersion pattern in single species and intruder treatments.
Cyprinodon maya shifted its habitat use from lower in the water column in single-species treatments to higher in the water column in the single-intruder treatment, and changed its dispersion pattern. Cyprinodon maya was more often at the water surface above the gravel substrate in the multi-intruder treatment compared to the single-intruder and single-species treatments (F = 7.07, r2 = 0.49, P = 0.005) and less often at the water surface above the vegetation in single-species compared to single-intruder treatments. Cyprinodon maya also had significantly lower dispersion values in the multi-intruder treatment compared to its single-species and single-intruder treatments (F = 7.11, r2 = 0.43, P = 0.005).
Agonistic behaviorsMore charges (59.9%) were initiated by C. labiosus (¯x = 11.5 per trial) and received by C. beltrani than any other combination of interactions between and within species in 19 trials. The Chi-square statistic was significant for the 2 x 2 contingency analysis (X2 = 11.142, P = 0.001) and indicated there were more interspecific charges (n = 264) delivered compared to intraspecific (n = 112) charges.
Discussion
The introduction of O.mossambicus and its subsequent dispersal throughout the Chichancanab basin has significantly negatively impacted the flock of species of Cyprinodon in the basin. Compared to descriptions of large schools of flock species observed in 1978 (Humphries and Miller 1981, Stevenson 1992), four of the five flock members have decreased in abundance since their initial description. Humphries and Miller (1981) described schools of ranging from 50-1000 adult C. simus at station 2b in 1978. In contrast to my difficulty in collecting most flock species, in 1978 in one 10-m seine haul at station 2b, Humphries (1981) collected 33 C. beltrani, 26 C. labiosus, 11 C. maya and 2 C. simus. Of the four readily identifiable cyprinodontids, C. beltrani was abundant during my study, but C. labiosus and C. maya and C. simus were rare. Very few C. verecundus were confidently identified in this study but their abundance was assumed to also have declined. I quantified abundance of fishes in only one season but because flock species breed year-round (Strecker and Kodric-Brown 1999) and additional, unpublished visits to the lake yielded the same low numbers, I assume that the low abundances are not seasonal in nature. The pattern of decreased abundance of Chichancanab flock species parallels that observed in Lake Victoria (Witte et al. 1992) where the less abundant species disappeared first (Cohen 1994). In Chichancanab, the originally less abundant species have experienced declines since 1978 whereas Cyprinodon beltrani remained abundant.
The nature of the negative impacts of O. mossambicus upon flock species was investigated here by examining microhabitat use of pupfishes alone and in the presence of O. mossambicus. Initial characterization of habitat use by flock species was important because hypotheses of flock speciation involve ecological segregation. Although Humphries and Miller (1981) and Stevenson (1992) qualitatively described habitat used by flock species, they did not quantify microhabitat differences among them. Results of my field surveys and laboratory experiments indicated that species of Cyprinodon in Chichancanab were segregated by microhabitat and behavioral differences and that the presence of O. mossambicus caused a shift in microhabitat use by C. maya and C. labiosus.
In nearshore habitats of the lake, O. mossambicus overlapped in habitat use with C. beltrani and possibly C. maya. Characters that typified habitat used by C. beltrani in nearshore areas included presence of submerged vegetation, proximity to shore and time of day. Diel variation in nearshore habitat use by C. beltrani was likely related to water temperature fluctuations (Barlow 1958, Humphries and Miller 1981) and may also correspond to foraging periods (Stevenson 1992).
In aquarium experiments, C. maya was most distinct in microhabitat use compared to C. beltrani and C. labiosus. Generally, C. maya used areas higher in the water column rather than just above or within vegetation beds. This species exhibited higher dispersion compared to C. beltrani and C. labiosus, which tended to travel in groups. These results were consistent with observations made by Humphries and Miller (1981) who described C. maya as a lone forager compared to the other species of Cyprinodon that moved in large schools.
Cyprinodon beltrani and C. labiosus overlapped in habitat use more so than either overlapped with C. maya, but distinctions in habitat use between C. beltrani and C. labiosus were evident. Cyprinodon labiosus used areas higher in the water column less than C. beltrani, and C. beltrani individuals showed greater dispersion than did C. labiosus.
Segregation among species of the Chichancanab flock may have a behavioral component as well as a habitat use component. Cyprinodon beltrani and C. labiosus displayed more interspecific agonistic behaviors compared to intraspecific encounters. Humphries and Miller (1981) observed males of both C. beltrani and C. labiosus guarding territories and described C. beltrani defense of feeding pits. The construction and defense of feeding pits has been observed in other cyprinodontids (Barlow 1961) and may result in spatial segregation of individuals.
In the presence of O. mossambicus, C. beltrani did not differ in its habitat use or dispersion in the tank. However, C. labiosus and C. maya both shifted habitat use via increased dispersion, and use of areas near the substrate, respectively. These shifts forced C. labiosus and C. maya out of preferred habitat an in closer proximity to C. beltrani, which was found more often in groups near the substrate.
Differences in microhabitat use among flock species that I observed are concordant with genetic (Strecker et al. 1996) and dietary (Stevenson 1992) differences among the species. Cyprinodon labiosus and C. beltrani shared a mtDNA haplotype whereas, C. maya exhibited a unique haplotype (Strecker et al. 1996). The genetically most distinct flock species, C. maya, was also distinct in its ability to recognize congeneric mates (Strecker and Kodric-Brown 1999), its diverse diet (Stevenson 1992) and among congeners in its microhabitat use. This species consumed less detritus than C. labiosus or C. beltrani and uniquely included large arthropods and fish in its diet (Stevenson 1992). Cyprinodon beltrani fed mainly on blue-green algae and slightly less so on those organisms that constituted the largest percentage of the diet of C. labiosus.
If habitat segregation was integral to the evolution of the Chichancanab flock, displacement of flock species into marginal habitats may impede the divergence process by disrupting life history characteristics involved in maintenance of segregation of the species. This is not to suggest habitat use was the only factor that contributed to divergence of the flock species. Behaviors related to species recognition and postmating isolation are also implicated as driving divergence of sympatric species. Small scale spatial segregation, home territory affinity (Owen et al. 1990, Ribbink 1991) and cues involved in sexual selection (Seehausen et al. 1997) were considered influential in the speciation processes that led to the divergence of numerous trophic morphs within the African Great Lakes. In Chichancanab, C. beltrani showed little interspecific mate recognition (Strecker and Kodric Brown 1999). If O. mossambicus forces C. maya and C. labiosus into habitat occupied by C. beltrani, this shift may result in genetic introgression and loss of rare phenotypes through the breakdown of premating segregation mechanisms.
Other changes in the Chichancanab basin not studied here may contribute to the decline in abundance of flock species outside of the habitat displacement caused by O. mossambicus. First, O. mossambicus, if not a scavenger on, may be direct predator of the flock species. I found remains of cyprinodontids in gut contents of O. mossambicus collected in seine samples from the lake and observed agonistic encounters between C. beltrani and O. mossambicus during snorkeling surveys. Secondly, compared to alcohol preserved specimens from 1978, over 90% of all flock species collected in 1997 were heavily infested with nematode parasites. Finally, the presence of A. aeneus may be involved in shifts of habitat use or other interactions that affect segregation among flock species. This species is common in interior cenotes of Yucatan (Scholz et al. 1995, Schmitter-Soto and Gamboa-Pérez 1996) and was likely artificially introduced by local aquarists (Schmitter-Soto, pers. comm.).
In the Chichancanab basin, precarious conditions of an ichthyofauna devoid of predators and competitors that fomented the evolution of the species flock has been significantly altered. Based on impacts caused by exotic species in similar systems (Stiassny 1996), further declines in cyprinodontid populations in Laguna Chichancanab are likely.
Acknowledgements
This project was supported by grants from: the University of Kansas Natural History Museum (KUNHM), Panorama Society, the Tinker Foundation, The Explorers Club, the American Museum Theodore Roosevelt Memorial Fund, and the National Security Education Program. The University of Kansas Department of Systematics and Ecology contributed supporting funds; W. Dimmick and E.O. Wiley provided facilities, supplies, and transportation to Mexico in 1996. Peter Runyan and J. Penrose conducted field work in 1996. Julian Humphries made his field notes field and specimens available. Kevin Tang produced figures, S. Stevenson helped with field logistics. Victor Vidal and M. E. Vega-Cendejas provided assistance in all aspects of my lab work including laboratory facilities, supplies, transportation and collection permits in Mexico. CINVESTAV parasitology students provided assistance with aquarium investigations and 1997 collections and J. J. Schmitter-Soto and H. Y. Yan provided helpful review of the manuscript.
Resumen
Cinco especies de Cyprinodon en la Laguna Chichancanab, Yucatán, México comprenden un cardumen jóven de especies cuya ecología y evolución no ha sido estudiada a fondo, pero cuya existencia está siendo amenazada con la extinción. Los cardúmenes de peces evolucionan en áreas aisladas donde los depredadores y los competidores están ausentes. Desde la descripción del cardumen de Chichancanab, Oreachromis mossambicus, una especie introducida al lago de la cual yo examiné el hábitat en los 80´s, se ha vuelto común en toda la laguna. Yo evalué la abundancia relativa de especies del cardumen en el lago y uso de hábitat y segregación entre la especies del cardumen más comunes y examiné los efectos de O. mossambicus en el uso de hábitat de las especies del cardumen. Cyprinodon beltrani fué la especie de cardumen más abundante en 1997, seguida por C. maya y C. labiosus; C. verecundus y C. simus fueron muy raras. Cyprinodon beltrani fue encontrada en aguas someras, cerca de la costa, sobre lechos densos de Chara y poca vegetación emergente. Cyprinodon beltrani exhibió variación diurna en el uso del hábitat cerca de la costa. En el campo, el uso de hábitat de C. beltrani y O. mossambicus se traslapaban ampliamente. En experimentos en acuarios, tres especies de cardumen exhibieron segregación de uso de hábitat y C. beltrani y C. labiosus mostraron comportamientos agonísticos que fortalecen la segregación. Cyprinodon maya se diferencia de C. beltrani y C. labiosus por su mayor dipersión de individuos y uso de áreas más altas en la columna de agua. La presentación de O. mossambicus causó un cambio en el uso de hábitat de C. maya y C. labiosus que puso a estas especies dentro del hábitat ocupado por C. beltrani. La presencia de esta especie introducida ha ausado una perturbación significante de las condiciones que fomentaron especiación de cardumen de Chichancanab hace 8 000 años.
References
Barlow, G. W. 1958. Daily movements of desert pupfish, Cyprinodon macularius, in shore pools of the Salton Sea, California. Ecology 39: 580-587. [ Links ]
Barlow, G. W. 1961. Social behavior of the desert pupfish, Cyprinodon macularius, in the field & in the aquarium. Am. Midl. Naturalist 65: 339-359. [ Links ]
Cohen, A.S. 1994. Extinction in ancient lakes: biodiversity crises and conservation 40 years after J.L. Brooks. Arch. Hydrobiol. Beih. Ergebn. Limnol. 44: 451-479. [ Links ]
Covich, A. & M. Stuiver. 1974. Changes in oxygen 18 as a measure of long-term fluctuations in tropical lake levels and molluscan populations. Limnol. Oceanogr. 19: 682-691. [ Links ]
Dieckmann, U. & M. Doebeli. 1999. On the origin of species by sympatric speciation. Nature 400: 354-357. [ Links ]
Greenwood, P.H. 1984. What is a species flock?, p. 10-19. In A. Echelle & I. Kornfield (eds.). Evolution of Fish Species Flocks. University of Maine, Orono. [ Links ]
Hodell, D. A., J. H. Curtis & M. Brenner. 1995. Possible role of climate in the collapse of classic Maya civilization. Nature 375: 391-394. [ Links ]
Humphries, J. M. 1981. The evolution of a species flock in the genus Cyprinodon (Pisces: Cyprinodontidae). Ph.D. Thesis, University of Michigan, Ann Arbor, Michigan. 233 p. [ Links ]
Humphries, J. M. 1984. Genetics of speciation in pupfishes from Laguna Chichancanab, Mexico, p. 129-139. In A. Echelle & I. Kornfield (eds.). Evolution of Fish Species Flocks. University of Maine, Orono. [ Links ]
Humphries, J. M. 1984b. Cyprinodon verecundus, n. sp., a fifth species of pupfish from Laguna Chichancanab. Copeia 1984: 58-68. [ Links ]
Humphries, J. M. & R. R. Miller. 1981. A remarkable species flock of pupfishes, genus Cyprinodon, from Yucatan, Mexico. Copeia 1981 : 52-64 [ Links ]
Kodric-Brown, A. 1995. Does past reproductive history predict competitive interactions and male mating success in pupfish? Anim. Behav. 50: 1433-1440. [ Links ]
Masters, J.C. & R.J. Rayner. 1993. Competition and macroevolution: the ghost of competition yet to come? Biol. J. Linn. Soc. 49: 87-98. [ Links ]
Owen, R. B., R. Crossley, T. C. Johnson, D. Tweddle, I. Kornfield, S. Davison, D. H. Eccles & D. E. Engstrom. 1990. Major low levels of Lake Malawi and their implications for speciation rates in cichlid fishes. Proc. Roy. Soc. London, B 240: 519-553. [ Links ]
Ribbink, A. J. 1991. Distribution and ecology of the cichlids of the African Great Lakes. p. 36-59. In M.H.A. Keenleyside (ed.) Cichlid fishes, behaviour, ecology and evolution. Chapman & Hall, London, England. [ Links ]
SAS Institute1988. SAS/Stat users guide, release 6.03. SAS Institute. Cary, North Carolina. 1028 p. [ Links ]
Schmitter-Soto, J. J. 1998. Diagnosis of Astyanax altior (Characidae), with a morphometric analysis of Astyanax in the Yucatan Peninsula. Ichthyol. Explor. Freshwaters 8: 349-358. [ Links ]
Schmitter-Soto, J. J. & C. Caro. 1997. Distribution of tilapia, Oreochromis mossambicus (Perciformes: Cichlidae) and water body characteristics in Quintana Roo, Mexico. Rev. Biol. Trop. 45: 1257-1261. [ Links ]
Schmitter-Soto, J. J. & H. C. Gamboa-Pérez. 1996. Composición y distribución de peces continentales en el sur de Quintana Roo, Península de Yucatan, Mexico. Rev. Biol. Trop. 44: 199-212. [ Links ]
Scholz, T., J. Vargas-Vásquez, F. Moravec, C. Vivas-Rodríguez & E. Mendoza-Franco. 1995. Cenotes (sinkholes) of the Yucatan Peninsula, Mexico, as a habitat of adult trematodes of fish. Folia Parasit. 42: 37-47. [ Links ]
Seehausen, O., van Alpen, J..M. & Witte, F. 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277: 1808-1811. [ Links ]
Stiassny, M. L., 1996. An overview of freshwater biodiversity with some lessons from African fishes. Fisheries 21: 7-13. [ Links ]
Stevenson, M. M. 1992. Food habits within the Laguna Chichancanab Cyprinodon (Pisces: Cyprinodontidae) species flock. Southwest. Naturalist 37: 337-343. [ Links ]
Strecker, U. & A. Kodric-Brown. 1999. Mate recognition systems in a species flock of Mexican pupfish. J. Evol. Biol. 12: 927-935. [ Links ]
Strecker, U., C. G. Meyer, C. Sturmbauer & H. Wilkens. 1996. Genetic divergence and speciation in an extremely young species flock in Mexico formed by the genus Cyprinodon (Cyprinodontidae, Teleostei). Molec. Phyl. Evol. 6: 143-149. [ Links ]
Witte, F. T., Goldschmidt, J. H. Wanink, M. J. P. van Oijen, P. C. Goudswaard, E. L. M. Witte-Maas & N. Bouton. 1992. The destruction of an endemic species flock: quantitative data on the decline of the haplochromine species of the Mwanza Gulf of Lake Victoria. Environ. Biol. Fishes 34: 1-28. [ Links ]
1 The University of Kansas Department of Systematics and Ecology, Lawrence, KS 66045 USA
(Present address: University of Kentucky, Center for Ecology, Evolution and Behavior, Lexington, KY 40506 USA FAX: 606-257-1717 E-mail: lcfuse0@pop.uky.edu)












 uBio
uBio