Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.47 no.3 San José sep. 1999
Abstract
A total of 98 liverwort species (43 genera, 10 families), 54 moss species (33 genera, 17 families) and one species of hornwort have been reported for Cocos Island (5°32N, 87°04W), Costa Rica. Over 60% of the bryophytes have a Neotropical or Pantropical distribution, about 10% are Caribbean, and less than 5% are endemic or subendemic. In comparison to the Galapagos Archipelago, Cocos Island harbors a more typical tropical bryoflora with foliose hepatics (e. g. Lejeuneaceae, Lepidoziaceae) constituting the bulk of diversity; fewer thallose liverworts and moss taxa as in the Archipelago were found. A richer habitat variety including wet and dry habitats, as well as its bigger area, seem to account for the higher number of bryophyte species in Galapagos Archipelago. Most bryophytes in Cocos Island are corticolous (46%), the remaining are epiphyllous (25%), saxicolous (23%) or terrestrial (12%). Bryophyte occurrence in eight plots (10 x 10 m) with 20 quadrates (30 x 30 cm) were recorded at different habitats and altitudes (0-600 m). Bryophyte distribution within the island coincides with lowland forest (0-100 m), secondary lowland forest (0-200 m) and montane forest (to 600 m). Physantholejeunea portoricensis (Hampe & Gott.) Schust. is reported as new to Costa Rica.
Key words
Bryophytes, Costa Rica, Cocos Island, Galapagos Archipelago, Island biogeography, ecology.
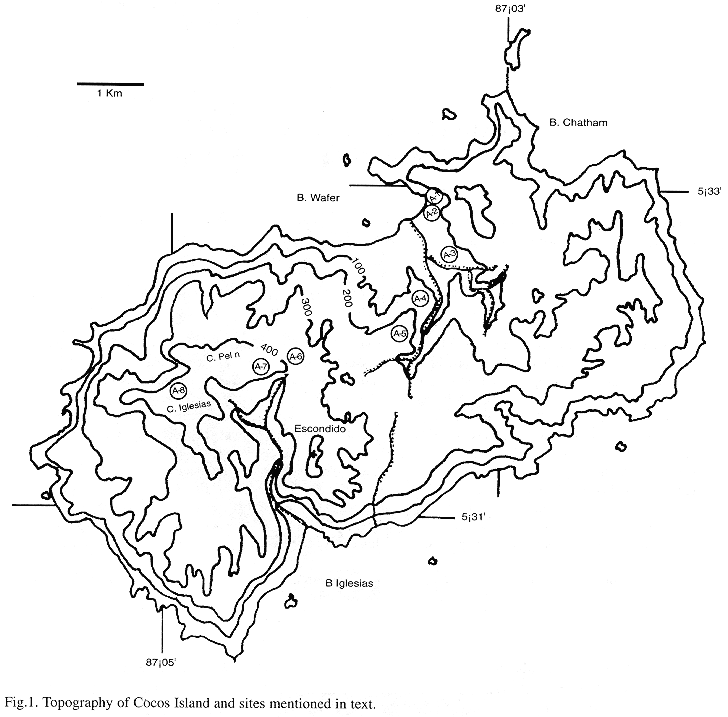
Cocos Island is an insular territory of Costa Rica, 494 km SW of Cabo Blanco, Nicoya Peninsula, 5°32N, 87°04W (Castillo et al. 1988). Declared National Park since 1970 and included in the Unesco World Heritage List since 1997 (Montoya 1990a, Quesada 1997), the island has an area of 24 km2 (Alvarado 1984, Herrera 1986) and a maximum altitude of 600 m (Figure 1). The mean annual temperature at 0 m is of 25.5 °C and the mean annual rainfall ranges from 5000 mm (Chatham Bay) to 7000 mm (Cerro Yglesias); the dryer months are between January and March. Cocos Island is a young volcano originated on a hot spot on the Cocos Plate, whose age is calculated between 1.2 and 2 millions years (Alvarado 1984, Castillo et al. 1988). Geologically, the island is composed of alkaline basalts, pyroclastic rocks, trachyte and hawaiites (Castillo et al. 1988). The island surface shows an irregular morphology, being almost inaccessible from the shore, except at Wafer and Chatham bays. There is a central and eastern area of undulate topography, between 200 and 260 m elevation, and V-shaped valleys (Alvarado 1989). The southwest side of the island has a more pendent area, with almost vertical walls to the sea.
The flora of Cocos Island has been irregularly studied (Gómez 1975). About 129 species of flowering plants have been reported, of which 12 are endemic (Montoya 1990b). The ferns and fern allies have been studied in detail by Luis Diego Gómez (1975) who recorded 74 species, including six endemic ones. The same author also reported 85 species of basidiomycetes (cited by Montoya 1990b).
A vegetation analysis was performed by Gómez (1975), who recognized two elevational belts: 1) the Littoral Zone (0-50m), located along the coast and on valleys, with two habitat types: the Annona glabra swamp and the firm terrain with various species of flowering plants. 2) the Mountainous Zone dominated by the trees Saccoglottis holdridgei, Ocotea insularis, and Euterpe macrospadix with an understory of Hypolitrum amplum. Further vegetation types are recognized by Montoya (1990b).
The byological exploration of Cocos Island is closely related with that of Galapagos Archipelago, since many scientific expeditions to the Galapagos also landed on Cocos (Weber 1966). As in The Galapagos, bryological collecting on Cocos was at first done by non-specialists, i.e. vascular plant collectors who also collected bryophytes. Early collections date from more than 200 years, when Menzies with the Vancouver Expedition collected the endemic Pilotrichum rugifolium C. M., from logs originating from Cocos Island (Crosby 1969). Pittier (1899) claimed to have been the first to do botanical collecting on Cocos during an expedition of the Costa Rican Government in 1898. He collected many vascular plants as well as some cryptogams, including Pilotrichum corrugatum Ren. & Card. (Crosby 1969) and Hygrolejeunea ocellata, described by Stephani (Stephani 1914). Robinson (1902 cited by Clark 1953) reported the occurrence of Macrolejeunea subsimplex (=M. cerina (Lehm. & Lindenb.) Gradst.) in a paper about the flora of Galapagos (cited by Clark 1953). In 1905 and 1906, Alban Stewart collected bryophytes which were identified by Williams (1924) who described the endemic Lepidopilum crassisetum Williams and Pilotrichum obtusatum (=P. corrugatum Ren. & Card.). John Thomas Howell made bryophyte collections along with the Templeton Croker Expedition in 1930. His mosses were treated by Bartram (1933), who recognized 25 species and Clark (1953) identified the liverworts. Howe (1934) described Anthoceros vegetans (=A. tuberculatus Lehm. & Lindenb.) from Cocos Island based on collections from the 1930 Astor Expedition.
Clark (1953) made the first significant contribution to the knowledge of the liverwort flora of Cocos. She newly described Ceratolejeunea lobata and Lopholejeunea cocosensis (=L. eulopha (Tayl.) Schiffn.) and listed 26 liverworts and a hornwort based on material collected by Howell and earlier reports of Howe (1939), Stephani (1914) and Robinson (1901). In 1964 collecting was done by W. A. Weber, S. Itow and R. Schuster in the frame of the Galápagos International Expedition (Weber 1993). Fosberg & Klawe (1966) reviewed the literature on the bryophytes of Cocos Island and listed 25 mosses and 27 hepatics. In 1992 R. Luecking and A. Bernecker collected epiphyllous lichens and bryophytes and reported 99 lichen species (Luecking and Luecking 1995) and recorded 37 bryophytes (Bernecker in prep.). They were also the first to collect bryophytes in the upper elevations of the island, earlier collecting having been restricted to lower elevations near Chatham and Wafer bays, due to difficulties of access other areas. However, since the latter focused mostly on epiphylls, most of the bryophyte diversity remained unknown.
The aims of this paper are to provide a comprehensive overview of the bryophyte flora of Cocos Island, incorporating new and previous reports of all bryophyte taxa, and to analyze of the origin of the flora. An analysis of the ecological distribution of the bryophytes in the island is also given.
From January to March and in June 1994, bryophytes were collected hazardously on the island in the greatest possible variety of substrates, the material was identified by the author and Maria Isabel Morales (liverworts), with help of specialists (see Acknowledgments). The samples where deposited in the Herbarium of the Universidad de Costa Rica (USJ) and in the Herbario Nacional de Costa Rica (CR), and selectively in herbaria of specialists. For the biogeographical analysis the area of distribution of each species was determined using floras, checklists and monographs. For many liverworts, data on geographical ranges were obtained from S. Rob Gradstein.
For the study of the ecological distribution of the bryophytes, eight plots (10 x 10 m) were set at different altitudes and habitats (0-600 m). In each plot, 20 quadrates (30 x 30 cm) where randomly set on different substrates, including humus, soil, rocks and trunks. Species occurrence in the squares was recorded as an estimate of the frequency of the species in the plots and areas. Similarities between areas were established through analysis of the bryophyte vegetation. The altitudinal distribution of single species was also tracked on the floristic samples all over the island.
Floristics: the bryophyte species of Cocos Island are listed in Appendix 1. Families of hepatics are according to Yano and Gradstein (1997), those of mosses according to Churchill & Linares (1995). For each species one or a few specimens and/or literature references are given. For a complete list of specimens and references, see Dauphin (1995).
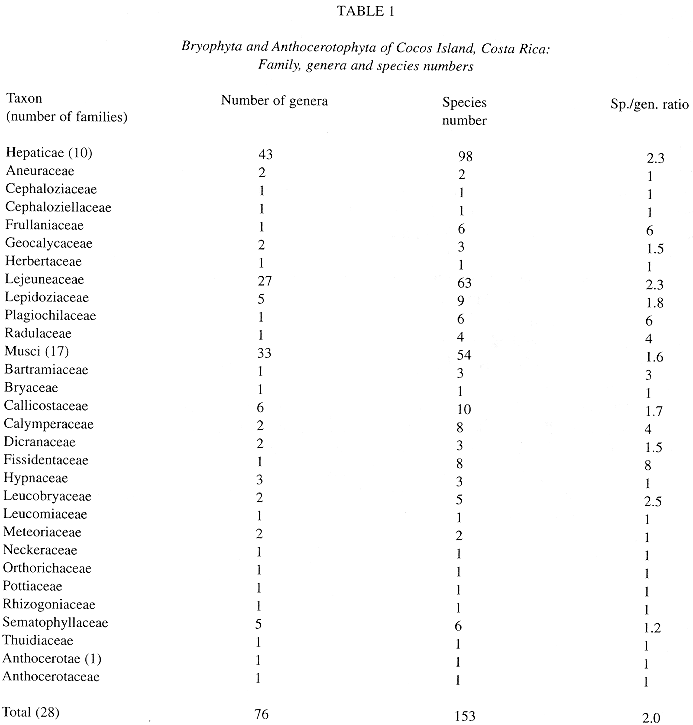
Table 1 provides a summary of the bryophyte taxa reported for the island. A total of 153 bryophyte species in 76 genera and 28 families are listed. Physantholejeunea portoricensis (Hampe & Gott.) Schust. is a new record for Costa Rica.
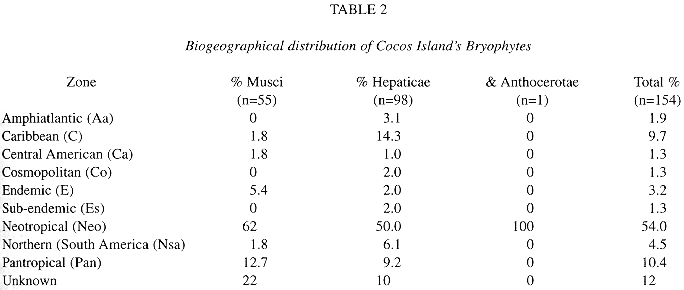
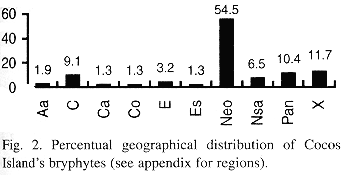
Geographical distribution: the geographical distribution of the bryophytes from Cocos Island is shown in Table 2 and Fig. 2.
a. Neotropical element: it is the best represented in the bryoflora (55%). Among liverworts (50%), most of the elements belong to the families Lejeuneaceae, Plagiochilaceae, Lepidoziaceae and Radulaceae, and some of the species e. g. Plagiochila vincentina and Bazzania hookeri conform important elements of the bryophyte flora of premontane and montane forest also in continental areas. As for mosses (62%), typical lowland element are good represented in the island by species like Syrrhopodon circinatus, Fissidens elegans and others (see altitudinal distribution).
b. Pantropical element: being the second best represented (10%), contains mostly Lejeuneaceae (e. g. Lejeunea flava and Lopholejeunea nigricans) and Arachniopsis diacantha (Lepidoziaceae) among liverworts, and common species like Octoblepharum albidum, Philonotis uncinata, Calym-peres afzellii and Pyrrhobryum spiniforme among mosses.
c. Caribbean element: better represented among hepatics (14.3%) than mosses (1.8%).
d. Northernsouth-american element (4.5%): includes species whose distributional range is not necessarily well known (e. g. Lepidolejeunea ornata and Symbiezidium dentatum).
e. Endemic element: Pilotrichum rugifolium C. M.; P. corrugatum Ren. & Card. and Lepidopilum crassisetum Williams are, so far, the only known endemics for Cocos Island; the last two are commonly found in lowland areas of the island, and P. corrugatum is more common at middle and higher elevations of Cocos.
f. Sub-endemic element (after Pócs 1988): Calypogeia rhynchophylla (Herz.) Bischler and Stenorrhipis sp. nov. are plants known only for Cocos Island and specific locations in the Guanacaste Cordillera, Costa Rica. Stenorrhipis sp. nov. has also been found in Panama (Dauphin et al. in press.).
g. Cosmopolitan element: includes 2 widespread hepatics: Aneura pinguis (L.) Dum. and Lophocolea bidentata (L.) Dum. The first one found a very suitable habitat on boulders along protected ravines.
h. Unknown: mostly material identified to genus level.
Ecology. Substrates: The island is mostly covered by forest, therefore more than 70% of the local bryophytes grow epiphytic: 46% on Bark, 25% epyphyllous. Rocks provide another important substrate for bryophyte species (23%), most of them mosses (e. g. Fissidens and Callicostaceae) and taxa associated with riparian habits. Cocos Island does not show well developed colonies of terrestrial bryophytes, even though 12% of them have been found growing on soil or humic soil. Only in higher elevation areas with exposed soil are frequently seen species of Dicranella, but otherwise the dense leaf cover on the understory of the surroundings do not allow terrestrial bryophytes to grow. On rotten logs a few well-distributed moss taxa like Octoblepharum albidum, Pyrrhobryum spiniforme, Isopterygium tenerum and Callicostella spp. can be found (4.5%).
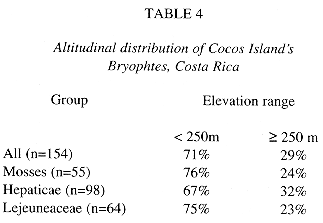
General altitudinal distribution: Table 4 includes altitudinal distribution data from plots and individual samples from different areas of the island. Only three of the reported bryophyte species have been found in the whole elevation range of Cocos, Plagiochila vincentina, Pilotrichum corrugatum and Leucomium strumosum. Most of the bryophyte species (70%) are found under 250 m elevation, corresponding to the lowland areas (Table 5).
Altitudinal distribution in plots: table 6 summarizes the plot result data. The bryophyte species number increase with altitude and reaches its maximum of 25 species/ 100 m2 in plot 6 (380 m). The species number decrease with further elevation, due to an increasing dominance of Bazzania hookeri and Plagiochila vincentina, which cover a whole range of microhabitats and probably displacing almost all other epiphytes.
Vegetation analysis based on bryophytes: 1. Lowland forest (plots 2-3). Plot 2 is an Annona glabra swamp that may become inundated, and has no terrestrial bryophytes and with a low canopy. Plot 3 belongs to the most common and extensive Cocos tropical forest. Saccoglottis holdridgei is the dominant tree, and on the ground grows abundant Hypolitrum amplum. The epiphyte flora is rich with Guzmania sanguinea, Marcgravia waferi and Tillandsia sp. Radula macrostachya and Symbiezidium transversale are the bryophytes most frequently found here on lower parts of the trunks (Table 5), higher branches show a different bryophyte component, where Frullania mucronata, F. riojaneirensis and sun bryophytes are found.
2. Secondary lowland forest (plots 1 & 4): located on rocky and exposed soil, often also on high pendent terrains, this forest type may reach up to 200-250 m elevation. Some of these areas where probably exploited agricultural at a time, plot 4 shows saplings of coffee plants that may have been planted somewhere near before. The vascular flora elements are Hibiscus tiliaceus at the sea level and Ocotea insularis with Cecropia pittieri which make up an open canopy on hills. Mosses dominate typically this areas, which provide above all rocks and the hard dry barks of the Ocotea and Cecropia, which are frequently covered by Lepidopilum crassisetum; Callicostella depressa and Taxithelium planum are growing on logs. Aneura pinguis, Fissidens neglectus and Callicostella depressa grow on rocks.
3. Montane forest (plots 5-8): the dominant tree in this forest type is Saccoglottis holdridgei, accompanied in higher areas by Euterpe macrospadix. Found over 200 up to 600 m, this forest is typified by an increasing abundance of corticolous hepatics, especially Bazzania hookeri and Plagiochila vincentina, that first grow conspicuously on the horizontal branches of trees and then cover down the trunk and are also important part of the mantle. This is particularly evident in the upper part of Cerro Yglesias where an overwhelming mass of bryophytes covers all trees and saplings. In a more detailed scale, some areas show different dominance patterns, for instance, Cerro Escondido (Figure 1), where Herbertus divergens is covering all trunks and branches at about 450 m.
Protected areas as creeks also provide shelter for other bryophyte associations. Particular bryophyte associations: 4.a. Pasture areas: on the eastern side of Chatham bay there are some open areas that bear some particular associations similar to those described for secondary lowland forests. Typical of these habitats are Sematophyllaceae and Callicostaceae. 4.b. Rivers and waterfalls: provide a substrate and habitat for different bryophyte species and associations, which vary depending on substrate and light availability. For instance waterfalls that are richly covered with Philonotis uncinata, and give a metallic glance as the drops reflect the sunlight.
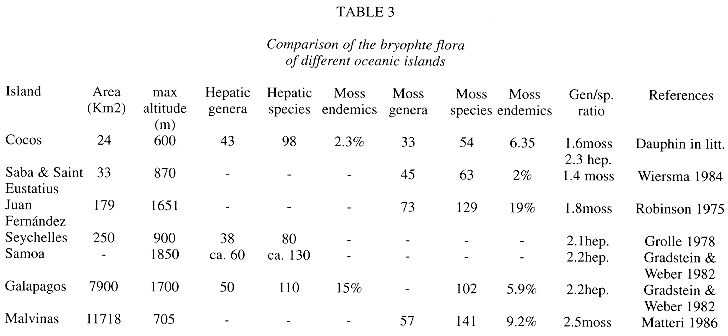
Floristics. As usual in tropical lowland rain forests (Richards 1983), liverworts overrun mosses in numbers of species and genera, but moss families are more numerous. Data for comparison with other island areas are provided by Table 3. A different situation was found in the Galapagos bryophyte flora, where the number of hepatics is almost the same as that of mosses (Gradstein & Weber 1982). This may be due to the greater diversity of habitats, including dry and wet ones, in the Galapagos which has also a larger area than Cocos Island. A similar number of mosses is found in the West Indian islands Saba and Saint Eustatius (Wiersma 1984), territory of somewhat similar geographical and climatic conditions. In Juan Fernández and Malvinas, the higher species numbers of mosses can be explained by their larger territory and more temperate climate, that seems to favor the growth of other moss taxa. The most species rich moss families in Cocos, Callicostaceae, Calymperaceae and Fissidentaceae are also families which attain the highest diversity in tropical areas (Richards 1983). Families like Pottiaceae and Bryaceae appear poorly represented in the list. This may be due to undercollecting of exposed areas of the island, e. g. Cabo Dampier, vertical risks around the island.
The liverwort diversity in Cocos Island shows the typical pattern of tropical lowland forest, where Lejeuneaceae is by far the most dominant family in terms of species number (Table 1). This dominance pattern is also typical for tropical continental and island areas (e. g. Grolle 1978, Gradstein 1995, Dauphin et al. in press). There is no poor representation of important mainland groups of other tropical Jungermaniinae as seen in Galapagos (Gradstein & Weber 1982). In comparison to Galapagos, Cocos Island shows more tropical components (see biogeography), as stated by Gradstein & Weber (1982). Thallose hepatic taxa (except Aneura and Riccardia) are absent in this list. A similar situation was found in the Seychelles (Grolle 1978). This may be due to the few natural exposed areas in the island. Also a wetter climate does not favor the growth of thallose taxa, as it happens in the dryer Galapagos Archipelago (Gradstein & Weber 1982).
Bryophyte species numbers in oceanic islands seem to increase with territory size (Table 3). Another important element influencing bryophyte diversity is the presence of different altitudinal belts, what is reflected in a greater microhabitat diversity and niches for more bryophyte taxa. This latter factor may be more important than the long-range dispersal capacity of the different taxa to explain the occurrence of a taxon in an oceanic island.
Geographical distribution. Most of the bryophyte taxa in Tropical America have a wide distribution. The dominant Neotropical element may contain more species now known only from some restricted areas. In the same way the Pantropical element may show more elements, what can be put in evidence only through world treatments of many taxa, which are usually lacking. Concerning the Caribbean element, Gradstein & Weber (1982) explained the generic resemblance of Puerto Rico and Cocos Islands in terms climatic factors. The latter authors also found a higher affinity among Galapagos and West Indian mosses.
A better knowledge of the Northernsouth-american element component of the flora may result in putting these taxa together with the Central American or Caribbean elements. Because of the situation of Cocos before the South American Coast, and its even further southern origin on the Galapagos hot spot (Castillo et al. 1988), it is to expect some affinity of both areas.
Endemic elements: the status of the two hepatic species reported as endemic is uncertain: Ceratolejeunea lobata Clark may fall into synonymy of a wide distributed tropical taxon in that genus, as commented by the author (Clark 1953); the generic and specific identity of Hygrolejeunea ocellata Steph. must be checked on the type1. This low bryophyte endemism rate (3%) compared to that of flowering plants (9.3% fide Montoya 1990b) is normal in oceanic islands (Gradstein & Weber 1982, Pócs 1997). A higher endemism of mosses is also seen in oceanic islands, as the case of Cuba, where 12% of moss endemics is shown (Pócs 1988). In respect to Galapagos, the causes of lower endemism in Cocos may be caused by the homogeneity of the area (Fournier 1966), that do not provide areas so suitable for species diversification as the dry, exposed and older areas of Galapagos Archipelago (Gradstein & Weber 1982). In the Sub-endemic element (after Pócs 1988), belong species like Calypogeia rhynchophylla and Stenorrhipis sp. nov., which have restricted distribution ranges. Even though the first taxon has been mentioned to be endangered (Gradstein 1992), this species grows abundantly on logs in the upper elevations of the island (Dauphin 1995). The species conforming the Cosmopolitan element occur in non-disturbed habitats. The absence of other cosmopolitan taxa like Bryum argenteum Hedw. can be explained by the absence of rocks in the higher elevations where it could eventually grow.
In general, the geographical distribution of bryophyte taxa in Cocos Island agree with that of other areas, especially with Neotropical ones with which most of species are shared. If the species/genus ratio is used as a measure of the degree of isolation (Gradstein & Weber 1982, Pócs 1997), Cocos shows similar figures as islands with comparable species numbers (2.3 hepatics,1.6 for mosses, Table 3). Low endemism is a common feature for spore-producing organisms as ferns, lichens and bryophytes, groups that also show low endemism in Cocos Island and elsewhere (Gómez 1975, Luecking & Luecking 1995). The lack of more detailed data on distribution of the different taxa do not allow to make more precise analyses of biogeographical relationship between different areas.
Vegetation analysis based on bryophytes: 1. Lowland forest (plots 2 & 3). Herrera & Gómez (1993) call this as a Tropical, Tropical very wet with one or two dry months biotic zone. These authors show similar areas in continental Costa Rica mainly in the plain of Osa Peninsula on the Pacific coast and the Northern part of the San Carlos plain. This forest shows the typical structure of a Lowland Tropical forest, with a vertical distribution of the species according to their light and moisture requirements (Cornelissen & Steege 1989, Frahm & Gradstein 1991). Secondary lowland forest (plots 1& 4): the structure of these areas is similar to secondary growth areas in continent, with the particularity of showing abundant endemic species.
Montane forest: such a forest type can be compared with the "Lower Montane Forest" described from El Darién, Panama by Gradstein and Salazar Allen (1992). Montane forests are normally found between 1800 and 2400 m in continental areas, but due to the excessive moisture in this place, the altitudinal belts are lower, what has been called "Massenerhebung effect" (Frahm & Gradstein 1991). This is particularly evident in the upper part of Cerro Yglesias where an overwhelming mass of bryophytes covers all trees and saplings. Cerro Yglesias forest can be compared with that from El Darién, between 1100 and 1200 m above sea level, as described by Gradstein and Salazar Allen (1991). Herrera & Gómez (1993) provide two biotic zone subdivisions for Cocos Islands higher elevations: Subtropical, tropical with one to two dry months and without dry months. This latter subdivision corresponds to the summit of Cerro Yglesias. These authors base this subdivision on climatic factors (more rain), but the differences seen on bryophyte vegetation, do not support this division, for a continuous species association and dominance pattern is seen in the whole mountain area. The latter results provide bryophytical evidence for Grubbs observation, who pointed out that melanges of flowering plant species of the different forest belts may be found where the altitudinal limits for the formation types are lowered (cited by Frahm & Gradstein 1991).
Further collecting and survey work of the bryophyte flora on Cocos island is needed to provide information on many more different bryophyte communities there, also as source of information on bryophyte conservation.
The present work could not have been done without the help of the staff of the Isla del Coco National Park, especially Joaquín Alvarado who gave his approval and logistic help and Wilfrido Conejo who helped me reach with skilled boat otherwise inaccessible places.
Many group specialists have kindly helped with the determination of specimens, namely (alphabetical order) B. H. Allen (Dicranaceae), A. Bernecker (Bazzania), M. Crosby (Pilotrichum), S. R. Gradstein (Plagiochila and general hepaticae), D. Griffin III (Bartramiaceae), M. I. Morales (general hepaticae), R. Pursell (Fissidens), N. Salazar Allen (Octoblepharum) and K. Yamada (Radula).
A. Bernecker & R. Luecking provided the species list from their manuscript on epiphyll bryophytes and M. Montoya his manuscript on Cocos vascular plant flora. L. Fournier kindly helped with references. I thank also Luis Diego Gómez, S. Rob Gradstein and María Isabel Morales for their valuable comments on different phases of this work.
Resumen
Allen, B. H. 1994. Dicranaceae. In B. H. Allen (ed.). Moss Flora of Central America. Monographs Syst. Bot. Missouri Bot. Gard. 49: 84-168. [ Links ]
Allen, B. H. & M. R. Crosby. 1986. Revision of the genus Squamidium (Musci: Meteoriaceae). J. Hattori Bot. Lab. 61: 423-476. [ Links ]
Alvarado I., G. E. 1984. Aspectos petrológicos-geológicos de los volcanes y unidades lávicas del Cenozoico superior de Costa Rica. Tesis de Licenciatura. Escuela Centroamericana de Geología, Universidad de Costa Rica, San José. [ Links ]
Bartram, E. B. 1933. Mosses of the Templeton Crocker Expedition, collected by John Thomas Howell, and lists of mosses known from the Galápagos Islands and from Cocos Island. Proc. Cal. Acad. Sci. 21: 75-86. [ Links ]
Bartram, E. B. 1949. Mosses of Guatemala. Fieldiana Botany 25: i-v, 1-442. [ Links ]
Bischler, H. 1964. Le genre Drepanolejeunea Steph. en Amérique Centrale et Méridionale. Rev. Bryol. Lichénol. 35: 95-137. [ Links ]
Bischler, H., C. E. B. Bonner & H. A. Miller. 1963. Studies in Lejeuneaceae VI. The genus Microlejeunea Steph. in Central and South America. Nova Hedwigia 5: 359-411. [ Links ]
Castillo, P., R. Batiza, D. Vanko, E. Malavassi, J. Barquero & E. Fernández. 1988. Anomalously young volcanoes on old hot spot traces: I. Geology and petrology of Cocos Island. Geol. Soc. America Bul. 100: 1400-1414. [ Links ]
Churchill, S. P. & E. L. Linares. 1995. Prodromus Bryologiae Novo-Granatensis, Introducción a la flora de musgos de Colombia. Instituto de Ciencias Naturales-Museo de Historia Natural Biblioteca "José Jerónimo Triana" 12, Bogotá. 926 p. [ Links ]
Clark, L. 1953. Some Hepaticae from the Galápagos, Cocos and other Pacific Coast islands. Proc. Cal. Acad. Sci. 27: 593-624. [ Links ]
Cornelissen, J. H. C. & H. Ter Steege. 1989. Distribution and ecology of epiphytic bryophytes and lichens in dry evergreen forest of Guyana. J. Trop. Ecol. 5: 131-150. [ Links ]
Crosby, M. R. 1969. A revision of the tropical moss genus Pilotrichum. Bryologist 72: 275-343. [ Links ]
Crum, H. 1994. Various families and genera treatments. In A. J. Sharp, H. Crum & P. Eckel (eds.). The Moss Flora of Mexico. Mem. New York Bot. Gard. 69: 1-1113. [ Links ]
Crum, H. & W. R. Buck. 1994. Thuidiaceae. In A. J. Sharp, H. Crum & P. Eckel (eds.). The Moss Flora of Mexico. Mem. New York Bot. Gard. 69: 873-886. [ Links ]
Dauphin, G. 1995. Briófitos de la Isla de Cocos: Diversidad y Ecología. Tesis de Licenciatura. Escuela de Biología, Universidad de Costa Rica, San Pedro. [ Links ]
Dauphin, G., S. R. Gradstein, A. Bernecker-Luecking & M. I. Morales. 1998. Additions to the Hepatic flora of Costa Rica II. Lindbergia 23: 74-80. [ Links ]
Delgadillo, C., B. Bello & A. Cárdenas. 1994. Latmoss, a catalogue of neotropical Mosses. Monographs Syst. Bot. Missouri Bot. Gard. 56: 1-191. [ Links ]
Fosberg, F. R. & W. I. Klawe. 1966. The Galápagos International Scientific. Preliminary list of plants from Cocos Island, p. 187-189. In R. S. Bowman (ed.). The International Scientific Project, Berkeley & Los Angeles. [ Links ]
Fournier, L. A. 1966. Botany of Cocos Island, Costa Rica, p. 183-186. In R. S. Bowman (ed.). The International Scientific Project, Berkeley & Los Angeles. [ Links ]
Frahm, J. P. & S. R. Gradstein. 1991. An altitudinal zonation of rain forests using bryophytes. J. Biogeography 18: 669-678. [ Links ]
Fulford, M. H. 1945. Studies on American Hepaticae IV. Ceratolejeunea. Brittonia 5: 368-403. [ Links ]
Fulford, M. H. 1962. Leafy Hepaticae of Latin America. Part I. Mem. New York Bot. Gard. 11: 1-172. [ Links ]
Fulford, M. H. 1966. Leafy Hepaticae of Latin America. Part II. Mem. New York Bot. Gard. 11: 173-276. [ Links ]
Fulford, M. H. 1968. Leafy Hepaticae of Latin America. Part III. Mem. New York Bot. Gard. 11: 277-392. [ Links ]
Fulford, M. H. 1976. Leafy Hepaticae of Latin America. Part IV. Mem. New York Bot. Gard. 11: 393-535. [ Links ]
Gómez, L. D. 1975. Contribuciones a la pteridología costarricense. VII. Pteridófitos de la Isla de Cocos. Brenesia 6: 33-48. [ Links ]
Gómez, L. D. 1986. Vegetación de Costa Rica, vol. ii. In L. D Gómez (ed.). Vegetación y Clima de Costa Rica. EUNED, San José. [ Links ]
Gradstein, S. R. 1992. Threatened bryophytes of the neotropical rain forest: a status report. Trop. Bryol. 6: 83-93. [ Links ]
Gradstein, S. R. 1994. Lejeuneaceae: Ptychantheae, Brachiolejeuneae. Flora Neotropica Monograph 62. New York Botanical Garden, New York. 216 p. [ Links ]
Gradstein, S. R. 1995. Diversity of hepaticae and Anthocerotae in montane forests of the tropical Andes, p. 321-334. In S. P. Churchill et al. (eds.). Biodiversity and Conservation of Neotropical Montane Forests. The New York Botanical Garden, New York. [ Links ]
Gradstein, S. R. 1997. A guide to the bryophytes of Tropical America I. Liverworts and Hornworts. Brussels. [ Links ]
Gradstein, S. R. & W. H. A. Hekking. 1979. A catalogue of the Hepaticae of Colombia. J. Hattori Bot. Lab. 45: 197-230. [ Links ]
Gradstein, S. R., A. Luecking, M. I. Morales & G. Dauphin. 1994. Additions to the hepatic flora of Costa Rica. Lindbergia 19: 73-86. [ Links ]
Gradstein, S. R. & N. Salazar Allen. 1992. Bryophyte diversity along an altitudinal gradient in Darién National Park, Panamá. Trop. Bryol. 5: 61-71. [ Links ]
Gradstein, S. R. & W. A. Weber. 1982. Bryogeography of the Galápagos Islands. J. Hattori Bot. Lab. 52: 127-152. [ Links ]
Griffin III, D. 1994. Bartramiaceae. In A. J. Sharp, H. Crum & P. Eckel (eds.). The Moss Flora of Mexico. Mem. New York Bot. Gard. 69: 537-574. [ Links ]
Grolle, R. 1956. Revision der Clasmatocolea Arten. Rev. Bryol. Lichenol. 25: 288-303. [ Links ]
Grolle, R. 1978. Die Lebermoose der Seychellen. Wiss. Zeitschr. Friedrich-Schiller Univ. Jena, Math-Nat. R. 27: 7-17. [ Links ]
Haarbrink, P. 1981. Studies on Colombian Cryptogams XI. High Andean Species of Frullania subg. Chonantelia (Hepaticae). Lindbergia 7: 47-57. [ Links ]
Haessel de Menéndez, G. A. 1990. Las especies de Anthoceros y Folioceros (Anthocerotophyta) de América del Norte, Sur y Central: la ornamentación de sus esporas taxonomía. Candollea 45: 201-220. [ Links ]
He, X. L. 1996. Type studies on Pycnolejeunea (Lejeuneaceae: Hepaticae), II. Ann. Bot. Fennici 33: 51-58. [ Links ]
Herrera, W. 1986. Clima de Costa Rica, vol.2. In L. D. Gómez (ed.). Vegetación y Clima de Costa Rica. EUNED, San José. [ Links ]
Herrera, W. & L. D. Gómez. 1993. Mapa de Unidades Bióticas de Costa Rica, escala 1: 685 000. The Nature Conservancy et al., Costa Rica. [ Links ]
Hertlein, L. G. 1963. Contribution to the biogeography of Cocos Island, including a bibliography. Proc. Cal. Acad. Sci. 32: 129-289. [ Links ]
Howe, M. A. 1934. The Hepaticae (chiefly Riccia and Anthocerotae) of the Galápagos Islands and the coast Islands of Central America and Mexico. Proc. Cal. Acad. Sci. 21: 199-210. [ Links ]
Ireland, R. R. 1992. The moss genus Isopterygium (Hypnaceae) in Latin America. Trop. Bryol. 6: 111-132. [ Links ]
Jovet-Ast, S. 1953. Le genre Colura. Rev. Bryol. Lichenol. 22: 206-312. [ Links ]
Lichénd, R. & A. Lücking. 1995. Foliicolous lichens and bryophytes from Cocos Island, Costa Rica, a taxonomical and ecogeographical study. I. Lichens. Herzogia 11: 143-174. [ Links ]
Magill, R. 1994a. Acroporium Mitt. In A. J. Sharp, H. Crum & P. Eckel (eds.). The Moss Flora of Mexico. Mem. New York Bot. Gard. 69. 989-993. [ Links ]
Magill, R. 1994b. Sematophyllum Mitt. In A. J. Sharp, H. Crum & P. Eckel (eds.). The Moss Flora of Mexico. Mem. New York Bot. Gard. 69. 993-1000. [ Links ]
Matteri, C. M. 1986. Los Musci (Bryophyta) de las Islas Malvinas, su habitat y distribución. Nova Hedwigia 43: 159-89. [ Links ]
Mizutani, M. 1970. Lejeuneaceae, subfamilies Lejeuneoideae and Cololejeuneoideae from Sabah (North Borneo). J. Hattori Bot. Lab 33: 225-265. [ Links ]
Montoya, M. 1990a. Plan de manejo, Parque Nacional Isla del Coco. Documento preparado para el Sistema de Parques y Reservas Marinas (SIPAREMA) de Costa Rica, San José. [ Links ]
Montoya, M. 1990b. La Flora y Vegetación de la Isla del Coco, Costa Rica. Escuela de Biología Universidad de Costa Rica, San José. [ Links ]
Morales Z., M. I. 1991. Las hepáticas comunicadas para Costa Rica. Trop. Bryol. 4: 25-57. [ Links ]
Piippo, S. 1986. A monograph of the genera Lepidolejeunea and Luteolejeunea (Lejeuneaceae, Hepaticae). Ann. Bot. Fennici 132: 1-69. [ Links ]
Pittier, H. F. 1899. Apuntamientos preliminares sobre la Isla de Cocos, posesión costarricense en el Océano Pacífico. Memoria de la Secretaría de Fomento, San José. [ Links ]
Pócs, T. 1984. Present knowledge on Aphanolejeunea Evans. J. Hattori Bot. Lab. 55: 307-313. [ Links ]
Pócs, T. 1988. Biogeography of the Cuban bryophyte flora. Taxon 37: 615-621. [ Links ]
Pócs, T. 1997. The distribution and origin of the foliicolous bryophyta in the Indian Ocean islands. Abstracta Botanica 21: 123-134. [ Links ]
Pursell, R. A. 1994. Fissidentaceae. In B. H. Allen (ed.). Moss Flora of Central America. Monographs Syst. Bot. Missouri Bot. Gard. 49: 40-80. [ Links ]
Quesada Chanto, B. 1997. Isla del Coco declarada patrimonio mundial. "La Republica" 5.XII. 1997: 4A. [ Links ]
Reese, W. D. 1994. Calymperaceae. In B. H. Allen (ed.). Moss Flora of Central America. Monographs Syst. Bot. Missouri Bot. Gard. 49: 192-225. [ Links ]
Richards, P. W. 1983. The ecology of tropical rain forest bryophytes, p. 1233-1270. In R. M. Schuster (ed.). New Manual of Bryology. The Hattori Botanical Laboratory, Japan. 1295 p. [ Links ]
Robinson, H. 1975. The Mosses of Juan Fernandez Islands. Smithsonian Contributions to Botany No.27. [ Links ]
Salazar Allen, N. 1994. Octoblepharum. In B. H. Allen (ed.). Moss Flora of Central America. Monographs Syst. Bot. Missouri Bot. Gard. 49: 182-189. [ Links ]
Schuster, R. M. 1982. The Hepaticae and Anthocerotae of North America, East of the Hundreth Meridian. Vol. IV. Columbia University Press, New York. 1331 p. [ Links ]
Schuster, R. M. 1992. The Hepaticae and Anthocerotae of North America, East of the Hundreth Meridian. Vol. V. Field Museum of Natural History, Chicago. 937 p. [ Links ]
Smith, D. K. 1994. Neckeraceae. In A. J. Sharp, H. Crum & P. Eckel (eds.). The Moss Flora of Mexico. Mem. New York Bot. Gard. 69: 741-765. [ Links ]
Stephani, F. 1914. Species Hepaticarum, vol. V: 565-566. Complement to Bulletin de l Herbier Boissier, Geneva. [ Links ]
Stotler, R. E. 1969. The genus Frullania subg. Frullania in Latin America. Nova Hedwigia 18: 397-555. [ Links ]
Vitt., D. H. 1994. Orthotrichaceae. In A. J. Sharp, H. Crum & P. Eckel (eds.). The Moss Flora of Mexico. Mem. New York Bot. Gard. 69: 590-656. [ Links ]
Weber, W. A. 1966. Lichenology and bryology in the Galápagos Islands, with checklists of the Lichens and Bryophytes thus far reported. In R. J. Bowman (ed.). The Galapagos. University of California Press, Berkeley. [ Links ]
Weber, W. A. 1993. Additions to the Galápagos and Cocos Islands lichen and bryophyte floras. Bryologist 96: 431-434. [ Links ]
Wiersma, P. 1984. Moss Flora and the vegetation of St. Eustatius (West Indies). Proceedings C 87: 337-364. [ Links ]
Williams, R. S. 1924. Galápagos and Cocos Island mosses collected by Alban Stewart in 1905-1906. Bryologist 27: 37-44. [ Links ]
Yamada, K. 1988. The genus Radula from Cuba. J. Hattori Bot. Lab. 65: 379-390. [ Links ]
Yano, O. & S. R. Gradstein. 1997. Genera of Hepatics. Systematisch-Geobotanisches Institut Universitaet Goettingen, Germany. 29 p. [ Links ]
1 Albrecht von Haller Institute of Plant Sciences, Dept. of Systematic Botany, University of Goettingen, Untere Karspuele 2, D-37073 Goettingen, Germany. Fax 49 551 392329. gdauphi@gwdg.de
| Class1 | Family | Genus | Species | Sub2 | Sex3 | Distr4 | |
| A | Anthocerotaceae | Anthocerus | tuberculatus Lehm. & Lindenb. | r | X | Neo | Dauphin 1122/ Hässel de Menéndez 1990 |
| H | Aneuraceae | Aneura | pinguis (L.) Dum. | r | Di | Co | Dauphin 1096/ Schuster 1992 |
| H | Aneuraceae | Riccardia | sp. | s | x | X | Dauphin 966, 984, 111, 1145 |
| H | Calypogeiaceae | Calypogeia | cyclostipa (Spruce) Steph | lb | Di | Nsa | Dauphin 963/Fulford 1968 |
| H | Calypogeiaceae | Calypogeia | rhynchophylla (Herz.) Bischler | b,h | Di | Es | Dauphin 965, 982, 1212/Gradstein et. al. 1994 |
| H | Cephaloziaceae | Cephalozia | crassifolia (Lindenb.& Gott.) Fulford | h | Di | Neo | Dauphin 965, 1159/ Gradstein et. al. 1994 |
| H | Cephaloziellaceae | Stenorrhipis | sp nov. | r | Au | Es | Dauphin 1024/Dauphin et al 1998 |
| H | Jubulaceae | Frullania5 | caulisequa (Nees) Nees | b | Au | Neo | Morales 1991/Gradstein pers. Comm. |
| H | Jubulaceae | Frullania | ecuadorensis Steph. | x | Di | Neo | Morales 1991/ Stotler 1969 |
| H | Jubulaceae | Frullania | intumescens (Lehm. & Lindenb) Lindenb. & Lindenb. | x | Di | Neo | Fosberg & Klawe 1966, Morales 1991/ Stotler 1969 |
| H | Jubulaceae | Frullania | mucronata Lehm. & Lindenb. | lb | Di | Nsa | Dauphin 947, 9526/ Stotler 1969 |
| H | Jubulaceae | Frullania | riojaneirensis(Raddi) Aongstr. | lb | Au | Neo | Dauphin 944, Morales 1991/ Haarbrink 1981 |
| H | Geocalycaceae | Leptoscyphus | gibbossus (Tayl.) Mitt. | b | Di | Neo | Dauphin 1141/ Fulford 1976 |
| H | Geocalycaceae | Leptoscyphus | ovatus (Spruce) Grolle | lb | Di | C | Dauphin 967/ Fulford 1976 |
| H | Geocalycaceae | Lophocolea | cf. bidentata (L.) Dumm | b | Au | Co | Dauphin 1210/ Gradstein, pers. Comm. |
| H | Herbertaceae | Hertbertur | divergens (Steph.) Herz. | b | Di | Neo | Dauphin 1146/ Fulford 1963 |
| H | Lejeuneaceae | Aphanolejeunea | angustissia Steph. | e | X | X | Bemecker & Lücking in prep |
| H | Lejeuneaceae | Aphanolejeunea | camilli (Lehm.) R.M. Schust. | e | X | X | Bemecker & Lücking in prep |
| H | Lejeuneaceae | Aphanolejeunea | costariensis Bernecker | e | X | X | Bemecker & Lücking in prep |
| H | Lejeuneaceae | Aphanolejeunea | sicaefolia (Gott.) A. Evans | b,e | X | Neo | Dauphin A-8/ Pócs 1984. |
| H | Lejeuneaceae | Archilejeunea | fuscescens (Hampe ex Lehm.) Fulford | x | Di | Nsa | Morales 1991, Weber 19937/ Gradstein 1994. |
| H | Lejeuneaceae | Ceratolejeunea | cubensis (Mont.) Schiffn. | b | Au | Neo | Dauphin 1078, 1106/ Fulford 1945. |
| H | Lejeuneaceae | Ceratolejeunea | lobata Ciark | x | X | E | Clark 19538 |
| H | Lejeuneaceae | Ceratolejeunea | maritima (Spruce) Steph | b,e | Au | Neo | Dauphin 971/ Fulford 1945 |
| H | Lejeuneaceae | Ceratolejeunea | plumula (Spruce) Steph. | e | Au | Neo | Bernecker & Lücking in pre/ Fulford 1945 |
| H | Lejeuneaceae | Ceratolejeunea | ruginosa Steph. | b,e | Au | C | Dauphin 1055, 1076/ Fulford 1945 |
| H. | Lejeuneaceae | Cheilolejeunea | adnata (Kunze) Grolle | e | Di | Neo | Lücking 92-284 (USJ), Morales 1991/ Schuster 1980, Gradstein, pers comm. |
| H | Lejeuneaceae | Cheilolejeunea | decurviloba (Steph.) X. L. He | b,e | Di | Neo | Dauphin 1068/ He 1996. |
| H | Lejeuneaceae | Cheilolejeunea | rigidula (Mont.) Schust. | b,e | Di | Aa | Dauphin 1057/ Schuster 1980 |
| H | Lejeuneaceae | Cololejeunea | cadiocarpa (Mont.) Steph. | e | Mo | Neo | Bernecker & Lücking in prep./ Schuster 1980 |
| H | Lejeuneaceae | Cololejeunea | submarginata P. Tix. | e | Mo | Neo | Bemecker & Lücking in prep/ Gradstein, pers. Comm. |
| H | Lejeuneaceae | Colura | clavigera Gott. ex Jovet-Ast | b | Di | C | Dauphin 1142/ Pócs. 1988. |
| H | Lejeuneaceae | Colura | tortifolia (Moilt.) Steph. | e | Di | Neo | Bernecker & Lücking in prep./ Jovet-Ast 1953 |
| H | Lejeuneaceae | Cycloejeunea | accedens (Gott.) A. Evans | e | Mo | Neo | Bemecker 92-328, Bernecker & Lücking in prep. |
| H | Lejeuneaceae | Cycloejeunea | convexistipa (Lehm. & Lindenb.) A. Evans | lb,e | Di | Neo | Dauphin 926, 979/ Gradstein, pers. Comm. |
| H | Lejeuneaceae | Cycloejeunea | luteola (Spruce) Grolle | e | Di? | Neo | Lücking 92-317 (USJ) Gradstein et al. 1994 |
| H | Lejeuneaceae | Cycloejeunea | peruviana Lehm. & Lindenb. | lb,e | Di | Neo | Dauphin 969/ Gradstein, pers. Comm. |
| H | Lejeuneaceae | Cycloejeunea | lineata & Lehm. & Lindenb.) Herz. | lb,e | Di | C | Dauphin 972/ Gradestein, pers. Comm. |
| H | Lejeuneaceae | Diplasiolejeunea | pellucida (Meissn.) Schiffn. | e | Di | Neo | Bernecker & Lücking in prep./ Gradestein, pers. Comm. |
| H | Lejeuneaceae | Drepanolejeunea | crucianella (Tayl.) A. Evans | h,e | Di? | Neo | Dauphin 965/ Bischler 1964 |
| H | Lejeuneaceae | Drepanolejeunea | inchoata (Meissn.) A. Evans | e | Di? | Neo | Bernecker & Lücking in prep./ Bischler 1964 |
| H | Lejeuneaceae | Drepanolejeunea | lichenichola (Spruce) Steph. | b | Di? | Neo | Dauphin A-7/ Bischler 1964 |
| H | Lejeuneaceae | Echinocolea | dilatata (A. Evans) Schust. | r | Mo | C, Ca | Dauphin 1003/ Gradestein et al. 1994. |
| H | Lejeuneaceae | Hygrolejeunea | ocellata Steph. | x | X | E | Stephani 1914, Clark 1953/9 |
| H | Lejeuneaceae | Lejeunea | cf. glaucescens Gott. | lb | Mo | Pan | Dauphin 1046/ Schuster 1980. |
| H | Lejeuneaceae | Lejeunea | cladogyna A. Evans | x | Mo | neo | Morales 1991/ Schuster 1980. |
| H | Lejeuneaceae | Lejeunea | flava (Sw.) Nees | e | Mo | pan | Morales 1991/ Schuster 1980. |
| H | Lejeuneaceae | Lejeunea | laetevirens Nees & Mont. ex Schiffn. | e | Di | Neo | Bernecker & Lücking in prep./ Schuster 1980. |
| H | Lejeuneaceae | Lejeunea | phyllobola Nees & Mont. | b | Au | Neo | Dauphin 1056/ Pócs 198810 |
| H | Lejeuneaceae | Lejeunea | setiloba Spruce | x | Mo? | Neo | Morales 1991/ Schuster 1980. |
| H | Lejeuneaceae | Lejeunea | sp. 1 | r | X | X | Dauphin s.n., Chatham. |
| H | Lejeuneaceae | Lejeunea | sp. 2 | e | X | X | Bernecker & Lücking in prep. |
| H | Lejeuneaceae | Lepidolejeunea | involuta (Gottsche) Grolle | e | Di | Neo | Dauphin 992/ Gradstein, pers. Comm. |
| H | Lejeuneaceae | Lepidolejeunea | ornata(Robins.) R.M.Schust. | b,e | Di | Nsa, Ca | Dauphin A-7/ Piippo 1986, Gradstein et al. 1994) |
| H | Lejeuneaceae | Leptolejeunea | elliptica (Lehm. & Lindenb.) Schiffn. | e | Di, Au | Neo | Bernecker & Lücking in prep./ Gradstein, pers. Comm. |
| H | Lejeuneaceae | Leptolejeunea | radicosa (Nees & Mont.) Grolle | e | Di | C, Ca | Bernecker & Lücking in prep./ Bischler 1969 |
| H | Lejeuneaceae | Lopholejeunea | eulopha (Tayl.) Schiffn. | r, lb | Au | Pan | Dauphin 978, 1139, Gradstein el al. 1994/ Gradstein 199411 |
| H. | Lejeuneaceae | Lopholejeunea | nigricans (Lindenb.) Schiffn. | x | Au, Di | Pan | Gradstein 199412 |
| H | Lejeuneaceae | Lopholejeunea | subfusca (Nees) Schiffn. | r | Au | Pan | Dauphin s.n./ Gradstein 1994. |
| H | Lejeuneaceae | Macrolejeunea | cerina (Lehm. & Lindenb.) Gradst. | x | Di | Neo | Morales 1991/ Grolle 1956. |
| H | Lejeuneaceae | Microlejeunea | acutifolia Steph. | e | Di | C | Bernecker & Lücking in prep./ Bischler et al. 196?. |
| H | Lejeuneaceae | Microlejeunea | bullata Tayl. | e | Di | Neo | Dauphin 1187/ Gradstein & Weber 1982. |
| H | Lejeuneaceae | Microlejeunea | epiphylla Bischi. | e | Di | C | Bernecker & Lücking in prep/. Gradstein, pers. Comm |
| H | Lejeuneaceae | Odontolejeunea | lunulata (F. Weber) Schiffn. | e | Mo | Aa | Bernecker & Lücking in prep/. Gradstein 1994. |
| H | Lejeuneaceae | Omphalanthus | filiformis (Sw.) Nees | e | Di | Neo | Bernecker & Lücking in prep/ Gradstein & Webr 1982. |
| H | Lejeuneaceae | Physantholejeunea | portoricensis (Hame & Gott. ) Schust. | Lb | ? | Ca | Dauphin 943a13/ |
| H | Lejeuneaceae | Pictolejeunea | picta (Grolle ex Steph.) Grolle | lb | Mo | Neo | Dauphin 1099/ Gradstein et al. 1994. |
| H | Lejeuneaceae | Prionolejeunea | cf. innovata A. Evans | h | X | X | Dauphin 965 |
| H | Lejeuneaceae | Prionolejeunea | denticulata (Web.) Schiffn. | x | Di | Neo | Dauphin A-7/ Gradstein et al. 1994. |
| H | Lejeuneaceae | Prionolejeunea | sp. | e | X | X | Dauphin 976. |
| H | Lejeuneaceae | Pycnolejeunea | sp. | X | X | X | Weber 1993 |
| H | Lejeuneaceae | Rectolejeunea | berteroana (Gottsche) A. Evans | b,e | Di | Neo | Dauphin 1143/ Schuster 1980. |
| H | Lejeuneaceae | Rectolejeunea | cf. emarginuliflora (Gott.) A. Evans | b | Di | C | Dauphin 1107/ Gradstein, pers. Comm. |
| H | Lejeuneaceae | Schiffneriolejeunea | polycarpa (Nees) Gradst.? | b | Au, Di | Pan | Dauphin A-5/ Gradstein 1994. |
| H | Lejeuneaceae | Stictolejeunea | squamata (Willd. ex Web.) Schiffn. | r | Di | Neo | Dauphin 1001, 1021/ Gradstein 1994. |
| H | Lejeuneaceae | Symbiezidiuml4 | barbiflorum (Lindenb. & Gott) A. Evans | b | Mo | Neo | Dauphin 918, Morales 1991/ Gradstein 1994. |
| H | Lejeuneaceae | Symbiezidium | dentatum Herz. | lb | Au, Di | Nsa | Dauphin 952/ Gradstein 1994. |
| H | Lejeuneaceae | Symbiezidium | transversale (Sw.) Trev. | b, e | Mo,Di | Neo | Dauphin 919/ Gradstein 1994. |
| H | Lejeuneaceae | Taxilejeunea | pterigonia (Lehm & Lindenb.) Schiffn. | x | Mo | Neo | Morales 1991/ Gradstein & Weber 1982. |
| H | Lejeuneaceae | Taxilejeunea | sp. | b | X | ---- | Dauphin 956, 1025. |
| H | Lepidoziaceae | Arachniopsis | diacantha (Mont.) Howe | H | X | Pan | Dauphin A-5, 960pp/ Gradstein et al. 1994. |
| H | Lepidoziaceae | Bazzania | affinis (Lindenb.) Trev. | b | Di | Ca, Nsa | Dauphin 1063/ Fulford 1962. |
| h | Lepidoziaceae | Bazzania | cubensis (Gott.) Pagán | b | Di | C | Dauphin 1190/ Fulford 1962. |
| H | Lepidoziaceae | Bazzania | cuneistipula (Gott. & Lindenb.) Trev. | b | Di | Neo | Dauphin A-6/ Fulford 1962. |
| H | Lepidoziaceae | Bazzania | hookeri (Lindenb.) Trev. | b | Di | Neo | Dauphin 970, 974/ Fulford 1962. |
| H | Lepidoziaceae | Bazzania | stolonifera (Swarz Trev. | b | Di | C | Dauphin 1063, 1105/ Gradstein, pers. Comm. |
| H | Lepidoziaceae | Kurzia | capillaris (Swarz) Grolle | s | Di | Neo | Dauphin 1059/ Gradstein et al. 1994. |
| H | Lepidoziaceae | Micropterygium | carinatum Steph. | b | Di | C,Nsa | Dauphin 961/ Fulford 1966. |
| H | Lepidoziaceae | Zoopsidella | antillana (Steph.) Schust. | b | Di | C,Ca | Dauphin 960/ Gradstein et al. 1994 |
| H | Plagiochilaceae | Plagiochila | bursata (Desv.) Lindenb. | x | Di | Neo | Morales 1991/ Gradstein, pers. Comm. |
| H | Plagiochilaceae | Plagiochila | guilleminiana Nee & Mont. | x | DI | Neo | Morales 199115/ Gradstein, pers. Comm. |
| H | Plagiochilaceae | Plagiochila | gymnocalycina (L. & L.) Mont. | b | Di | Neo | Dauphin 987, Gradstein, pers. Comm. |
| H | Plagiochilaceae | Plagiochila | martiana Nees | lb | Di | Neo | Clark 1953/ Gradstein, pers. Comm. |
| H | Plagiochilaceae | Plagiochila | rutilans Lindenb. | x | Di | Neo | Dauphin 914, 925/ Gradstein, pers. Comm. |
| H | Plagiochilaceae | Plagiochila | vincentina Lindenb. | h | Di | Neo | Dauphin 1104/ Gradstein, pers. Comm. |
| H | Radulaceae | Radula | afinis Lindenb. & Gott. | X | Di | X | Morales 1991. |
| H | Radulaceae | Radula | flaccida Lindenb. | e | Di | Aa | Dauphin 1231 / Schuster 1980. |
| H | Radulaceae | Radula | macrostachya Lindenb. & Gott. | b,lb | Di | Neo | Dauphin 916, 923/ Yamada 1988. |
| H | Radulaceae | Radula | pusilla Spruce | r | Di | Nsa | Dauphin 1013/ Gradstein, pers. Comm. |
| M | Bartramiaceae | Philonotis | elongata (Dism.) Crum & Steere | r | Di | Neo | Dauphin 1008, 11931 Griffin 1994. |
| M | Bartramiaceae | Philonotis | sphaericarpa (Hedw.) Brid. | r | Di | Neo | Dauphin 1134/ Griffin 1994. |
| M | Bartramiaceae | Philonotis | uncinata (Schwaegr.) Broth. | r | Di | Pan | Dauphin 1023, 112316/ Griffin 1994. |
| M | Bryaceae | Bryum subg. Bryum | sp. | r | Di? | X | Dauphin 1060. |
| M | Callicostaceae | Pilotrichum | corrugatum Ren. & Card. | h,r,lb | Di? | E | Dauphin 973, 997, 1027, 928, 943, 1028,17/ Crosby b 1969 |
| M | Callicostaceae | Pilotrichum | rugifolium C. M. | lb | Di? | E | Dauphin 943/ Crosby 1969. |
| M | Callicostaceae | Callicostella | depressa (Sw.) Jaeg. | rb | Au? | C,Ca, Nsa | Dauphin 937pp, 1016pp, 1125/ Delgadillo et al. 1995. |
| M | Callicostaceae | Callicostella | oerstediana (C. M. ) Jaeg. | rb | Au, Syn | Ca | Dauphin 1016pp/ Delgadillo et al. 1995. |
| M | Callicostaceae | Callicostella | sp. | r | X | X | Dauphin 1040, 1004, 1007, 1019. |
| M | Callicostaceae | Crossomitrium | epiphyllum (Mitt.) C. M. | e | Di | Neo | Weber 199318/ Delgadillo et al. 1995. |
| M | Callicostaceae | Crossomitrium | patrisiae (Brid.) C.M. | lb,e | Di | Neo | Dauphin 929/ Delgadillo et al. 1995. |
| M | Callicostaceae | Hookeriopsis | sp. | r | X | X | Dauphin 1095, 1098. |
| M | Callicostaceae | Hypnella | pallescens (Hook.) Jaeg. | x | X | Ca,C, Nsa | Fosberg & Klawe 1966, Weber 1993/ Delgadillo et al. 1995. |
| M | Callicostaceae | Lepidopilum | crassisetum Willims | lb, b, r | Au? | E | Dauphin 994/ Williams 1924. |
| M | Callicostaceae | Thamniopsis | cruegeriana (C. M.) Buck | s | X | X | Weber 1993. |
| M | Calymperaceae | Calymperes | afzeliii Sw. | b | Di,V | Pan | Dauphin s. n.19/ Reese 1994. |
| M | Calymperaceae | Calymperes | erosum C. M. | b | Di | Pan | Weber 1993/ Reese 1994. |
| M | Calymperaceae | Syrrhopodon | circinatus (Brid.9 Mitt. | b,lb,r | Di | Neo | Dauphin 915, 1188/ Reese 1994. |
| M | Calymperaceae | Syrrhopodon | incompletus Schwaegr. var berteroanus (Brid.) Reese | b | Di | Neo | William 1924, Weber 1993/ Reese 1994. |
| M | Calymperaceae | Syrrhopodon | lycopodioides (Brid.) C.M. | lb,b | Di | Neo | Dauphin A-9, 1211/ Reese 1994 |
| M | Calymperaceae | Syrrhopodon | prolifer Schwaegr. var. scaber (Mitt.) Reese | rb | Di | Neo | Dauphin 1061/ Reese 1994 |
| M | Calymperaceae | Syrrhopodon | rigidus Hook & Grev. | b | Di | Neo | Dauphin 1100/ Reese 1994 |
| M | Dicranaceae | Campylopus | savannarum (C. M.) Mitt. | h | Di | Neo | Dauphin 1223/ Allen 1994. |
| M | Dicranaceae | Dicranella | harrisii (C.M.) Broth. | s | Di | C,Ca | Dauphin 991/ Allen 1994. |
| M | Dicranaceae | Dicanella | hilariana (Mont.) Mitt. | s | Di | Neo | Dauphin 988, 1120/ Allen 1994. |
| M | Fissidentaceae | Fissidens | angustifolius Sull. | r | Au | Neo | Dauphin A-4/ Pursell 1994. |
| M | Fissidentaceae | Fissidens | elegans Brid. | r | Au | Neo | Dauphin A-4/ Pursell 1994 |
| M | Fissidentaceae | Fissidens | intramarginatus (Hampe) Jaeg. | s | Au | Neo | Dauphin 1071/Pursell 1994 |
| M | Fissidentaceae | Fissidens | leptophyllus Mont. | r | X | Neo | Dauphin 1110/ Pursell 1994 |
| M | Fissidentaceae | Fissidens | microcladus Twait & Mitt. | X | X | X | Weber 199320 |
| M | Fissidentaceae | Fissidens | neglectus Crum | r | X | Neo | Dauphin 1009/ Pursell 1994 |
| M | Fissidentaceae | Fissidens | pellucidus Hornsch. | s | Au | Pan | Dauphin 1065/ Pursell 1994 |
| M | Fissidentaceae | Fissidens | sp. | r | X | X | Dauphin 1154. |
| M | Hypnaceae | Ctenidium | sp. | h | X | X | Dauphin 1043. |
| M | Hypnaceae | Isopterygium | tenerum (Sw.) Mitt. | h, rb | Au | Neo | Dauphin 1047, 1051/ Ireland 1992. |
| M | Hypnaceae | Vesicularia | vesicularis (Schwaegr.) Broth. | r | Au | Neo | Dauphin 1126/ Cru 1994g. |
| M | Leucobryaceae | Leucobryum | martianum (Hornsch.) Hampe ex C.M. | s,r | Au | Neo | Dauphin s.n., 1236/ Allen 1994. |
| M | Leucobryaceae | Octoblepharum | albidum Hedw. | rb | Au | Pan | Dauphin 1048/ Salazar Allen 1994. |
| M | Leucobryaceae | Octoblepharum | cocuiense Mitt | b | Au | Neo | Dauphin 962/ Salazar Allen 1994. |
| M | Leucobryaceae | Octoblepharum | pulvinatum (Doz. & Molk.) Mitt. | b | Au | Neo | Dauphin 933, 1062/ Salazar Allen 1994. |
| M | Leucobryaceae | Octoblepharum | stramineum Mitt | lb | Au | Neo | Dauphin 1081/ Salazar Allen 1994. |
| M | Leucomiaceae | Leucomium | strumosum (Hornsch.) Mitt. | x | Syn, Au | Pan | Williams 192421/ Crum 1994. |
| M | Meteoriaceae | Squamidium | isocladum (Ren. & Card.) Broth. | lb | Di | Neo | Dauphin 935/ Alen & Crosby 1986. |
| M | Meteoriaceae | Zelometeorium | patulum (Hedw.) Manuel | lb, h | Di? | Neo | Dauphin 934, 938, 22/ Crum 1994. |
| M. | Neckeraceae | Neckeropsis | undulata (Hedw.) Reichardt. | lb | Syn | Neo | Dauphin 917/ Smith 1994. |
| M | Orthotrichaceae | Groutiella | mucronifolia (Hook. & Grev.) Crum & Steere | b | Au | Neo | Dauphin 946, 1194/ Vitt 199423 |
| M | Pottiaceae | Hyophila | sp. | r | X | X | Dauphin 1017, 1192. |
| M | Rhizogoniaceae | Pyrrhobryum | spiniforme (Hedw.) Mitt. | rb | Di | Pan | Dauphin 941 / Crum 1994b. |
| M | Sematophyllaceae | Acroporium | pungens (Sw.) Broth | lb,b | Au | Neo | Dauphin 970, 975/ Magill 1994. |
| M | Sematophyllaceae | Glossadelphus | laevifolius (Mitt.) Bartr. | r | Au | Neo | Dauphin 101424/ Crum 1994. |
| M | Sematophyllaceae | Sematophyllum | galipense (C. M.) Mitt | x | Au | Neo | Gómez 4568 (CR, MO)/ Magill 1994. |
| M | Sematophyllaceae | Sematophyllum | subpinnatum (Brid.) Britt. | x | Au | Neo | Weber 1993. |
| M | Sematophyllaceae | Taxithelium | planum (Brid.) Mitt. | b, lb,r | Au | Neo | Dauphin 922, 1041, 932/ Crum 1994. |
| M | Sematophyllaceae | Trichosteleum | cf. fluviale (Mitt.) Jaeg. | rb | Au | Ca,Nsa | Dauphin 937pp./ Crum 1994 |
| M | Thuidiacea | Cyrto-hypnum | involvens (Hedw.) Buck | x | Au | Neo | Weber 199325. |
1 Class: A=Anthocerotae, H=Hepatics, M=Mosses.
2 Substrate types: b=bark, e=leaves (epiphylls), h=humus, Lb=living bark, r=rocks, rb=rotten bark, s=soil, x=unknown.
3 Sex distribution: Au=autoicous, Di=dioicous, Mo=monoicous, x=unknow.
4 Distribution types: Aa=amphiatlantic, C=caribbean, Ca= Central aerican, Co=cosmopoitan, E=endemic, Es=subnemic, Neo=neotroical, Nesa=NE outh America, Na=Northern South America, Pa=Pantropical, x=unknown.
5 Clark(1953) included also Frullania cucuata Lindnb. & Gott. and F. gymnotis Nes & Mont.
6 Clark 1953, Fosberg & Klawe 1966 as F. cocosenis Steph.
7 As. A. juliformis (Nees) Gradst.
8 After the author it could be a ynonim of C. maritima (Spruce) Steph., known only form the original description. The type is not available at the GIS Herbarium.
9 The genus Hygrolejeunea is considered a synonim of Lejeunea (e. g. Yano & Gradstein 1997). Because of the ocelli, this taxon does not fit in the present concept of the genus Lejeunea (Gradstein 1997). Mizutani 1971 placed it as Pycnolejeunea cocosensis Mizut. After He (pers. Comm.) it oes not belong in that genus. Only recorded from Cocos Island,
10 As Rectolejeuna phyllobola (Nees & Mont.) A. Evans.
11 Clark 1953 as L. cocosensis sp. nov.
12 Clark 1953 as L. muelleriana (Gott.) Schiffn.
13 New to Costa Rica.
14 Clark 1953 reported other species now put under synonymy by Gradstein 1994.
15 Also as P. andersonii Aognst.
16 Fosberg & Klaw 1966 as P. gracillima Aongstr; Weber 1993.
17 Williams 1924 (as Pilotichum obtusatum sp. nov.), Fosberg &v Klawe 1966, Crosby 1969 (as P. corrugatum Ren. & Card.).
18 Fosberg & Klawe 1966 as C. oerstedianum C.M.
19 Bartram 1949 as C. donnellii Aust.
20 As F. garberi S. & L.
21 Fosberg & Klawe 1966 as L. cuspidatifolium (C.M.) Mitt.
22 Fosberg & Klawe 1966 as Meteoriopsis patula (Hedw.) Broth.; Weber 1993.
23 = Groutiella apiculata (Hook.) Crum & Steere.
24 Williams 1924 as Hookeriopsis cocosensis sp. nov; Bartram 1949 as G. cocosensis (Williams)Bartra), Fosberg & Klawe 1966 also as G. longisetus Bartr., Weber 1993 as Taxiphyllum laevifolium (Mitt.) W. R. Buck
25 Fosberg & Klawe 1966 as Thuidium involvens Hedw.












 uBio
uBio