Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.46 n.3 San José Sep. 1998
Abstract
Two cowpea (Vigna unguiculata L. Walp) varieties, IT84E-124 and Vita 7 of the International Institute of Tropical Agriculture, Ibadan, were exposed to varying doses of chemical and physical mutagens. Optimum doses of 10mM EMS for 6hr and 0.1mM and 1.0mM NaN3 for 2hr, determined from seeding growth tests and 100R and 200R gamma radiations were applied to 2000 seed samples of each genotype. Screening of the M2generation revealed that the mutagenic treatments induced morphological, physiological and biochemical changes in the genotypes. A spectrum of mutations which included variants with respect to anthocyanin pigmentation, leaf morphology, maturity date, male sterility and insect pest resistance qualities were observed. Lines with significant increases in yield parameters such as number of seeds per pod, peduncles per plant, 100 seed weight and seed storage proteins were selected.
Key words
Vigna unguiculata, cowpea, induced mutations, mutagens, varieties.
Cowpea is one of the most important pulse crops in tropical Africa. The seeds are a major source of dietary protein in most developing countries (Duke 1990). Induced mutation breeding which has been recognized as a valuable supplement to conventional breeding in crop improvement has been least applied in grain legumes. For example, only eight out of over 1000 improved mutant varieties of different crops released up to 1989 in over 48 countries were cowpeas (Micke et al. 1990).
In Kenya, Pathak (1991) obtained two aphid resistant mutants of cowpea from an M2 population of susceptible seeds irradiated with 20kr of gamma rays. Other attributes of the improved lines include increased pod length, semi-erect plant habit and higher grain yield. In Nigeria, crop breeding through induced mutation is limited (Odeigah 1991). The present investigation was undertaken to induce viable mutations in quantitative and qualitative traits which could be utilized directly or introduced into our cowpea improvement program. We report here some of our results on the use of radiation and chemically induced mutations for crop improvement in cowpea.
Materials and Methods
Plant Materials: Mature dry seed samples of two IITA cowpea varieties; IT84E-124 and Vita 7 were obtained from the Grain Legume Improvement Program (GLIP), International Institute of Tropical Agriculture (I.I.T.A.)Ibadan, Nigeria. The two cultivars have good grain yield potential but are susceptible to most cowpea insect pests. IT84E-124 is erect, determinate with ovate leaves and have big seeds with rough seed coats. Vita 7 is spreading, indeterminate with hastate leaves and have small seeds with smooth seed coats.
Mutagenic Treatment And Seedling Growth Tests: Three mutagens, 60Co gamma rays, ethyl methane sulfonate (EMS) and sodium azide (NaN3), were used. Seeds were treated with 100R and 200R of gamma rays from a 60Co source at the Seibersdorf Laboratory of the International Atomic Energy Agency (I.A.E.A..) Austria. For EMS and sodium azide treatments, seeds were presoaked in distilled water for 1 to 2 hr. at room temperature and then transferred to aqueous solutions of 5,10,25, and 40mM EMS for 3,6,9, and 12 hr respectively or to 0.01,0.1, 1.0 and 10mM NaN3for 2 and 4 hr respectively. The seeds were then washed in running tap water for 1 hr and transferred to petri dishes containing two layers of moist filter paper for germination. Five petri dishes of five seeds per treatment were planted and percentage germination and seedling variations for each treatment were subsequently determined.
Generation Of The M1 And M2Populations: 2000 seeds of each variety were treated with 100R and 200R gamma rays, 10mM EMS for 6hr, 0.1mM and 1.0mM NaN3 for 2hr. These doses were chosen because previous germination assays indicated that these doses may be suitable for mutation breeding. After postwashing, the seeds were planted (25 by 50cm) on I.I.T.A. fields. Plots were cultivated as necessary and protected with Sherpa Plus insecticide. At least three pods from each M1 plant were harvested and bulked to form the M2 generation.
2000 seeds per treatment per cultivar were planted with insecticide protection and observations on seedling emergence, survival and morphological changes were noted. Data on yield parameters like number of peduncles per plant, pods per plant, seeds per pod, seed weight and plant height were recorded and compared statistically with the controls using the Students t test. Plants of interest were harvested individually, and the rest bulked.
Extraction And Determination Of Seed Protein Content Of M3 Selected Plants: Two seeds from the same plant of each M3selection listed in Table 3 were separately dehulled and ground in a mortar and the extracts were defatted by washing with three changes of cold acetone for 4 to 6hr. The acetone was removed by filtration and the extracts were air-dried at room temperature. The proteins from the defatted meal were precipitated with 10% trichloro-acetic acid and recovered by centrifugation at 5000rpm for 30 min at 40C. The protein content was then determined colorimetrically according to the method of Lowry et al (1951) using bovine serum albumin (BSA) as standard.
Insect Pest Resistance Screening: The M3 generation was grown without insecticide protection. 347 plants selected across the different populations as having the least aphid populations were screened for aphid resistance qualities. They were screened in the greenhouse according to the method of Jackai and Singh (1988). Briefly, the test materials along with a susceptible check were planted in single rows in wooden trays filled with soil. About 10 days after planting, each plant was infested with five fourth instar nymphs using a camel-hair brush. Infested trays were transferred into cages with fine saran mesh in the greenhouse. About two weeks after infestation, plants were assessed for vigour. Seeds of some lines were also screened for bruchid resistance following the procedure of Jackai and Singh (1988). Briefly, 20 seeds of each test material were placed in small plastic boxes with two pairs of day-old adult bruchids to oviposit for 24 hours. Five days after infestation, eggs laid per seed lot were counted and starting from 25 days after infestation, emerging adults were counted and removed until no further emergence from the susceptible control. Suitability index which is
= log percentage adult emergence
mean development time
was used to estimate the resistance status of test materials.
M4 Generation: 24 different mutant plants from among the M3 generation selections were advanced with their parents in a randomized block design with four replications. Each line was grown in 2 rows of 2.5m.
Results
With respect to the germination seedling growth test, 60Co gamma radiation produced a number of physiological effects including reduced and late germination in comparison to the controls in both cultivars. 200R produced more effects that 100R. The response of the seeds to EMS and NaN3 treatments at different doses and durations is shown in Fig 1a and b respectively. For EMS treatment, a low dose (5mM) gave high germinability but little or no variability while a high dose (40mM) gave good morphological variations but low seed survival or germinability. For all doses, long duration of exposure led to significant decrease in germinability. Long exposure to a high dose had a cytotoxic effect leading to lack of germination of the seeds. The overall data revealed that a dose of 10mM for a duration of 6hr gave good germination and morphological variations and is thus appropriate for mutation breeding (fig. 1a). The overall response of the varieties to NaN3 treatment was similar to that of EMS. Doses as low as 0.1mM for 2hr duration resulted in more than 50% germination (fig. 1b) and variations in the plant morphology. Although the responses of the two varieties to EMS and NaN3 treatments were similar, more pronounced effects were observed in IT84E-124 (see fig. 1a and b).
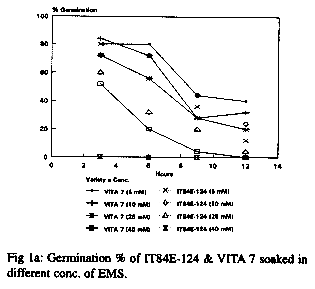
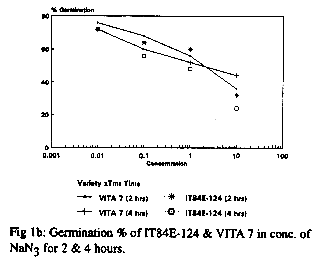
The three mutagens (60Co gamma radiation, EMS and NaN3) at the doses used induced a broad spectrum of mutations in the M2 plants of the two varieties. The changes observed were morphological, physiological and biochemical. Both desirable and non-desirable effects were observed. Plants with branched peduncles, anthocyanin pigmentation in the pods and stem, changes in leaf and flower shape and colour, male sterility, early maturity and aphid and bruchid resistance were recognized across the different treatments. The bruchid resistant selection had percent adult emergence and suitability indices of 30.56 and 1.193 respectively compared to 45.46 and 1.715 of the control. Non-selectable mutant (plants with least/poor agronomic values) observed include plants with stunted growth, twining stem and spreading growth habit.
Tables 1 and 2 show the yields and the corresponding increases and decreases of M2 generation yield components induced by the various treatments in var. IT84E-124 and var. Vita 7 with respect to the controls. Varietal differences were noticed in the two varieties. EMS treatment and 100R gamma irradiation did not effect any improvement in the yield parameters of IT84E-124 (Table 1). For Vita 7, 100R gamma irradiation made positive changes in peduncles and pods per plant and the flowering date, while EMS affected the 100 seed weight and seeds per pod positively (Table 2). 200R gamma irradiation and the two NaN3 (0.1 and 1.0mM) treatments induced significant improvements in the plant height, peduncles per plant and pods per plant in both IT84E-124 and Vita 7 (see tables 1 and 2).
The seed protein contents (mg/ml) of some of the selected M3 mutants are shown in Table 3. As can be seen, some mutants have higher values while some have lower values compared to the original or parent lines. In IT84E-124 the highest increase of 13.3% was found in line AR-3 which is an aphid resistant line obtained by 1.0mM NaN3 treatment while the highest increase in Vita 7 was 13.64% found in line 7P which showed no visible morphological or physiological change. Tables 4 and 5 show the mean yield of different parameters in the two varieties and their M4 mutants. The tables show variations in all parameters studied. For example the aphid resistant lines of IT84E-124 (AR-1, AR-2, AR-3) produced seeds per pod or higher 100-seed weight than the control. Also the Vita 7 mutant, lanceolate leaf, LL, produced more peduncles and pods/plants than the parent.
Discussion
Development of improved plant cultivars is restricted by limited genetic resources. Mutation induction could create additional genetic variability to supplement conventional crop breeding. NaN3, EMS and 60Co gamma radiation induced genetic variability in the two varieties, IT84E-124 and Vita 7. Lines were produced with agronomically useful traits as well as lines with traits that can be used only as genetic markers. Responses of the two cultivars to the different treatments differ. Fehr (1987) reported that differences exist among species and among genotypes within a species for sensitivity to mutagen treatment. It has been shown in the pulse crop, lentil (Lens culinaris) that the mutation spectrum depends greatly on the genotype and the type and dose of mutagens used (Shaikh and Begum 1991). Similar findings were observed in the two cowpea varieties (Vita 7 and IT84E-124) which are products of different genetic crosses and differ in a number of morphological and physiological characters.
Use of induced mutations for obtaining early maturing cultivars has been a frequent breeding objective (Micke 1979). The early maturing selections could serve as candidates for short season cowpea. Ashraf (1985) found early maturing cowpea useful in areas with short rainfall and as relay crops in rice paddies. Pulses generally have yield per hectare lower than cereals. Plant with increases in yield parameters and total protein content have a promising possibility of improving total protein yield per hectare. Similar findings have been reported in pea mutant (Jaranowski and Micke 1985). Increases in a polygenic character like yield could result from changes in simply inherited traits (Micke et al 1990) or mutations at the structural loci (Evans 1987).
We are currently studying the three aphid resistant lines and a bruchid resistant line that we selected. These and plants with anthocyanin pigmentation in pods and stems which are less attractive to insects (Thorstenison 1980) could find application in integrated pest management program. Cowpea, a basically self-fertilizing crop could benefit from genetic male sterility in the improvement of the low level of outcrossing found in the crop. Studies of the different male sterile lines and the associated floral aberrations gave an insight into the crops genome (Odeigah et al. 1996).
This study, as well as many previous ones, in which agronomically useful mutants and those that could serve only as genetic markers have been produced through mutagenesis indicate that induced mutation breeding is a valid and effective crop breeding method even in cowpea.
| | | | | | | |
| | ||||||
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
*Better than control, p<0.05
| | | | | | | |
| | ||||||
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
*Better than control, p<0.05
| Line | | |
| P124 | Erect, ovate leaves, brown rough seed, white flowers, (parent) IT84E-124 | 110.0±.0339 |
| SS | Smooth seed | 115.0±.0707 |
| 4P-1 | Like the parent | 100.0±.0530 |
| SM | Very small seed | 120.0±.0884 |
| SL | Smooth and light brown seed | 87.5±.0494 |
| AR-1 | Aphid resistant, small, slightly speckled seed | 117.5±.0707 |
| AR-2 | Aphid resistant, robust and big seed | 115.0±.0707 |
| AR-3 | Aphid resistant | 125.0±.0884 |
| CS-1 | Cream seeds, flowers with anthocyanin | 115.0±.0707 |
| RS | Very rough seed | 110.0±.0707 |
| SSA | Smooth seeds, anthocyanin in stem and peduncles | 111.25±0.0707 |
| BS | Big seeds | 120.0±0.0849 |
| PV7 | Semi erect, hastate leaves, smooth brown seeds, anthocyanin in flowers, (parent Vita 7) | 112.5±0.0686 |
| GA | Giant, anthocyanin in pods, dark brown seeds | 112.5±0.0636 |
| EF-2 | Early flowering | 77.5±0.1767 |
| MS | Maroon seeds | 92.5±0.0636 |
| 7P | Like the parent | 127.5±0.0707 |
| LBS | Light brown seeds | 115.75±0.0884 |
| GLF-1 | Giant, anthocyanin, late flowering and reduced fertility | 126.75±0.0955 |
| LL | Lanceolate leaves, late flowering and reduced fertility | 117.5±0.0778 |
| Lines | Peduncle/Plant | Pods/Plant | Plant height (cm) | Seeds/Pod | 100 Seed Weight (gm) | | 50% Flowering |
| P124 (Parent) | 12.00±2.40 | 8.20±3.81 | 17.12±0.25 | 10.14±0.13 | 13.13±0.42 | 87.5 | 41 |
| AR-1 | 13.50±1.07 | 19.00±5.95 | 16.67±0.15 | 10.08±0.20 | 13.80±0.14** | 82.5 | 41 |
| AR-2 | 10.50±3.05 | 17.90±3.98 | 18.65±0.28** | 12.03±0.15** | 12.70±0.21 | 90 | 39 |
| AR-3 | 13.70±2.31 | 18.12±4.13 | 17.60±0.90* | 10.68±0.10** | 13.00±0.29 | 87.5 | 38 |
| MS-1 | 10.70±1.76 | 16.10±6.58 | 16.83±0.21 | 9.02±0.17 | 12.60±0.21 | 92.5 | 38 |
| MS-2 | 13.10±2.17* | 21.10±3.36* | 16.24±0.61 | 9.12±0.18 | 12.90±0.48 | 80 | 35 |
| 4P-1 | 12.90±2.70 | 16.30±2.23 | 18.10±0.13** | 10.16±0.13* | 13.40±0.41 | 85 | 40 |
| 4P-2 | 11.60±1.69 | 17.80±1.37 | 17.95±0.40** | 11.62±0.19** | 13.10±0.43* | 90 | 38 |
| 4P-3 | 11.80±2.05 | 19.30±4.01 | 17.80±0.60** | 10.10±0.13 | 12.90±0.27 | 85 | 38 |
| 4P-4 | 11.80±1.60 | 17.00±3.43 | 18.61±0.24** | 10.60±0.17** | 13.00±0.33 | 90 | 38 |
*Better than control, P<0.05
**Better than Control, P<0.01
| Lines | Peduncles/Plant | Pods/Plants | Plant height (cm) | Seed/Pod . | 100 Seeds Weight (gm) | Germination | |
| Pv7(Parent) | 19.75±2.06 | 20.75±1.71 | 25.80±2.37 | 12.36±0.56 | 11.60±1.45 | 82.50 | 44 |
| 7P-1 | 18.50±2.51 | 21.75±1.89 | 25.60±3.24 | 12.75±1.17 | 10.65±1.35 | 78.75 | 44 |
| 7P-2 | 17.00±0.825 | 19.25±1.26 | 32.70±1.94* | 12.60±0.64 | 11.37±1.50 | 82.50 | 43 |
| 7P-3 | 18.75±3.30 | 26.25±1.56* | 27.73±1.37 | 13.26±0.65 | 11.12±0.84 | 85.00 | 46 |
| EF-1 | 21.50±2.14 | 20.502.38 | 28.13±2.99 | 12.70±0.48 | 12.01±2.18 | 90.00 | 41 |
| LF | 14.25±2.22 | 18.50±2.38 | 29.45±2.26 | 15.45±1.01* | 10.80±1.60 | 75.00 | 51 |
| AP-1 | 20.75±2.75 | 21.75±1.71 | 22.18±1.87 | 12.57±0.68 | 11.58±1.48 | 91.25 | 43 |
| AP-2 | 16.75±2.63 | 23.25±2.50 | 29.08±2.99 | 12.76±0.67 | 10.46±2.38 | 78.75 | 43 |
| CS-2 | 20.75±3.86 | 20.50±1.29 | 28.78±1.69 | 12.45±0.76 | 11.73±1.93 | 80.00 | 45 |
| MS | 19.25±2.75 | 21.75±2.36 | 24.78±2.17 | 13.70±0.88 | 10.93±0.75 | 82.50 | 47 |
| 7P-4 | 16.25±3.27 | 21.25±2.22 | 25.55±0.93 | 12.17±0.40 | 10.46±1.41 | 88.75 | 43 |
| DbS | 18.00±1.64 | 16.25±1.77 | 30.58±1.61 | 12.57±0.40 | 10.42±0.65 | 86.25 | 43 |
| GLF-1 | 18.75±2.87 | 21.50±1.29 | 54.68±4.41** | 12.40±0.52 | 13.80±0.93* | 85.00 | 47 |
| TS | 19.00±2.45 | 19.00±1.15 | 28.03±1.70 | 12.25±0.57 | 11.32±1.85 | 93.75 | 44 |
| LL | 25.50±2.40* | 29.50±2.87* | 39.75±2.03** | 12.66±0.18 | 10.50±0.88 | 82.50 | 45 |
| EF-2 | 19.25±2.75 | 18.25±1.06 | 30.40±2.09 | 11.94±0.32 | 9.60±2.48 | 75.00 | 41 |
*Better than control, P<0.05
**Better than control, P<0.01
References
Ashraf, M. 1985. Farming systems approach: Research in cowpeas and extension, p. 341-349. S.R. Singh & K O. Rachie (eds.) Cowpea research, production and utilization. Wiley, New York. [ Links ]
Duke, L. 1990. Introduction, p..1-42. In S.R. Singh (ed). Insect pests of food legumes. Wiley, New York. [ Links ]
Evans, L.T. 1987. Photosynthetic activity and partitioning, p.324-356. In L.W. Shemilt (ed). Chemistry and world food supplies: The New Frontiers-Pergamon, New York. [ Links ]
Fehr, L. 1987. Genetics of Vigna, p.311-394. In J. Janick (ed). Horticulture Review. AVI, Westport, Conneticut [ Links ]
Jackai, L.E.N. & S.R. Singh. 1988. Screening techniques for host plant resistance to insect pests of cowpea. Trop. Grain Leg. Bull. 35: 2-18 [ Links ]
Jaranowski, J. & A. Micke. 1985. Mutation breeding in peas. Mutation Breeding Rev. 2: 1-23. [ Links ]
Lowry, O., N. Rosenbrough, A. Farr & R. Randall. 1951. Protein measurement with the folin-phenol reagent. J. Biol. Chem. 193: 265-275. [ Links ]
Micke, A. 1979. Use of mutation induction to alter the ontogenic pattern of crop plants. Gamma Field Symposia no. 18, Institute of Radiation Breeding, Ohniya, Ibanraki-ken, Japan. 23p. [ Links ]
Micke, A., B. Donini & M. Maluszyonski. 1990. Induced Mutations for crop improvement. Mutation Breeding Rev. 7: 1-41. [ Links ]
Odeigah, P.G.C. 1991. In vitro mutation breeding for seed protein, a-amylase activity and herbicide resistance in bulrush millet (Pennisetum nigritarum). I.A.E.A., 1991 symposium on Plant Mutation Breeding for Crop Improvement, Vienna, Austria. (Vol. 2) 463p. [ Links ]
Odeigah, P.G.C., A.O. Osanyinpeju & G.O. Myers. 1996. Induced male sterility in cowpea (Vigna unguiculata L. Walp). J. Genet. Breeding 50: 171-176. [ Links ]
Pathak, R.S. 1991. Genetic evaluation of two aphid resistant cowpea mutants in Kenya. I.A.E.A. 1991 Symposium on Plant Mutation Breeding for Crop Improvement, Vienna, Austria. (Vol. 2) 463p. [ Links ]
Shaikh, M.A.Q. & S. Begum. 1991. Differences in radiosensitivity and mutation induction in lentil due to variation in the genotype and the type and dose of mutagen. I.A.E.A. 1991 symposium on Plant Mutation Breeding for Crop Improvement, Vienna, Austria. (Vol. 2) 463p. [ Links ]
Thorstenison, A.J. 1980. Host selection in phytophagous insect. Rev. Entomol. 5: 193-218. [ Links ]
1Department of Biological Sciences, University of Lagos, Lagos;
2Department of Biological Sciences, University of Agriculture, Abeokuta.
3Grain Legume Improvement Programme, I.I.T.A., Ibadan, Nigeria. Present address: Department of Agronomy, M.B. Sturgis Hall, Louisiana State University, Baton Rouge, LA 70803, USA.














