Introduction
Oral submucous fibrosis (OSMF) is a chronic debilitating oral disease characterized by progressive inflammation leading to fibrosis of the deeper connective tissues and lamina propria in the submucosal tissues (1). OSMF is frequently reported in the Indian subcontinent and Southeast Asian countries (2). In India, the prevalence of OSMF has been estimated to be 0.2-2.3% in men and 1.2-4.6% in women, in the age range of 11-60 years (3). Over the past decade, the prevalence of OSMF has increased to 6.42% in India (4). The etiopathogenesis of OSMF is multifactorial; however, the areca nut component of betel quid is critical for the pathogenesis of OSMF (5).
OSMF results from an imbalance between collagen synthesis and degradation in the extracellular matrix (6). The clinical presentation of OSMF includes a burning sensation in the oral mucosa, pain with or without ulceration preceded by vesicle formation, restricted gnathic movements, depapillation of the dorsum of the tongue, blanching of the oral mucosa, and leathery texture with loss of pigmentation, leading to progressive restriction of mouth opening (7).
Binne and Cawson (8) were the first to report muscle involvement in OSMF in an ultrastructural study using light microscopy. In 1985, Labban and Canniff (9) studied the degeneration of muscle fibers in OSMF using an electron microscope. Connective tissue alterations were characterized by dense collagen fiber deposition, fragmentation, hyalinization, and a wavy worm-like appearance of fibers, representing the elastotic degeneration of collagen. Chawla et al. (10) described muscle alterations in OSMF, where they observed four main structural changes, namely, highly eosinophilic muscle fibers lacking muscle striations, fragmentation, multiple nuclei, and internalization of nuclei. Chakravarthy et al. (11) established a causal relationship between increased masseter muscle thickness and increased duration and frequency of the habit. The proposed explanation for this was the overdemand of the masseter muscles during excessive chewing. Masseter muscle hypertrophy is a measurable component of OSMF.
Electromyography (EMG) is a technique for measuring muscle physiology by recording myoelectric signals and analyzing their development and quantification (12). It assesses the active characteristics of a particular muscle (13). Myofibrils (14) and sarcolemma, (15) which is the plasma membrane in muscle fibers, are responsible for muscle contraction. When muscle fibers shorten, the calcium ions remain in the sarcoplasm to bind with troponin as long as the availability of ATP is adequate (16). The muscle relaxes once the ATP is depleted, and this silent period contributes to ''resting EMG''(17). Carlo Matteucci (18) was a pioneer in developing EMG equipment. The parameters measured by EMG are the timing of muscle activation and force of muscle contraction, thereby allowing researchers to measure muscle performance (19).
The muscle action potential generated during a response triggers an action sequence that results in the contraction and relaxation of muscle fibers (20). The independent discharge of a single motor unit produces a muscle action potential. Morphological or functional disturbances in the motor unit during nerve diseases or muscle changes lead to altered electrical signal discharges, which are visualized as waveform alterations. The surface EMG signal is the totality of action potentials generated by the motor units present in the range of volume detected by the electrodes over them (21). The onset of muscle activity and force of contraction were measured quantitatively and expressed in microvolts and milliamperes, respectively (22).
During the management of OSMF, the existing assessment parameters for measuring treatment prognosis are not related to direct measurement of the affected muscle. Therefore, this systematic review aimed to assess whether there were any changes in masseter muscle activity levels in patients with OSMF and to determine whether the alteration in masseter muscle activity has any influence on the outcomes of different treatment modalities. The structured question was ''Can EMG of the masseter muscle be a reliable assessment during OSMF treatment?''The PICO was: Population (P)- OSMF patients; Intervention (I)- EMG; Comparison (C)-Masseter; and Outcome (O)- Muscle activity.
Methods
Protocol and registration
This systematic review was registered in the PROSPERO database (23) under the registration number CRD42022310589. Institutional review board and ethics committee approval was not required for this project. This report followed PRISMA's transparent reporting of systematic reviews (24).
Eligibilitu¿y criteria
Studies that used EMG to measure masseter muscle activity in the management of OSMF, irrespective of the treatment modality, met our inclusion criteria and were included in this review. We included controlled clinical trials (CCTs), randomized clinical trials (RCTs), and non-RCTs (NRCTs), with no restrictions on age, sex, demographics, and study setting. Studies published in languages other than English, literature reviews, letters to the editor, and case reports were excluded.
Information sources and search strategy
The databases used for our literature search were PubMed, Google Scholar, Science- Direct, Cochrane Library, and Latin American and Caribbean Health Sciences Literature (LILACS).
The Medical Subject Heading (MeSH) search terms used were ''Oral Submucous fibrosis,''''OSMF,''''Electromyography,''''EMG,''and ''Masseter muscle activity.''The final search string was (((oral submucous fibrosis) OR (OSMF)) AND ((electromyography) OR (EMG))) AND (((masseter) OR (masseter muscle)) OR (masseter muscle activity)).
Study selection and data collection
Two trained independent reviewers screened the studies for review selection and data extraction was performed. Data extraction content included primary information about the study, characteristics, interventions, follow-up, key elements of the risk-of-bias assessment, and outcome data. The exclusion criteria were EMG studies other than OSMF and OSMF treatment studies that did not assess masseter muscle activity. The inclusión criterion was OSMF studies that assessed the masseter muscle activity during treatment.
Quality assessment
The Physiotherapy Evidence Database (PEDro) rating scale (25) was used to assess the studies included in this review. This scale is being increasingly used in systematic reviews to rate clinical trials, as it has the advantage of measu- ring external validity (criterion 1), internal validity (criteria 2-9), and statistical reporting (criteria 10 and 11) in a single tool. Our included articles (Table 1) ranged from fair to good quality, based on the scoring interpretation (poor: <4; fair: 4-5; good: 6-8; excellent: 9-10). However, with any other quality assessment tool, it is impossible to differentiate the methodological quality of clinical trials from the reporting quality.
Table 1 PEDro scale methodological quality assessment of included studies.
| Criteria | Kant et al. 2014, India | Sinha et al. 2018, India | Shandilya et al. 2021, India |
|---|---|---|---|
| 1. Eligibility criteria and source | Yes | Yes | Yes |
| 2. Random allocation | No | No | No |
| 3. Concealed allocation | Yes | Yes | No |
| 4. Baseline comparability | Yes | Yes | Yes |
| 5. Blinding of participants | No | No | Yes |
| 6. Blinding of therapists | No | No | No |
| 7. Blinding of assessors | Yes | Yes | No |
| 8. Adequate follow-up (> 85%) | No | No | Yes |
| 9. Intention-to-treat analysis | No | No | No |
| 10. Between-group statistical comparisons | Yes | Yes | Yes |
| 11. Reporting of point measures and measures of variability | Yes | Yes | No |
| PEDro scale score | 6 | 6 | 5 |
| Study quality | Good | Good | Fair |
Data analysis
The studies selected for this review had high clinical heterogeneity because of differences in the study setting, participants, intervention, and outcome measures. Therefore, a meta-analysis could not be performed as it would be inappropriate to obtain the overall effect from these sets of clinical studies (26,27).
Results
Study selection
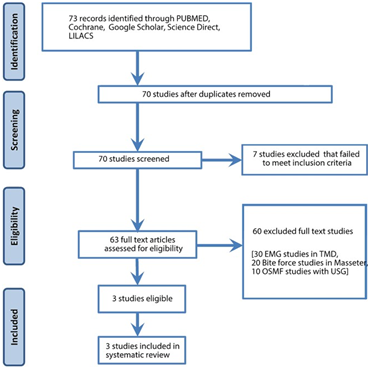
A sum of 73 studies was identified from the digital databases and screened by two independent reviewers from August 2010 to August 2022. Two articles were from PubMed, and one was from Google Scholar. Figure 1 illustrates the PRISMA flowchart of the selection process and the flow of logic in the article screening process.
Study characteristics
A total of 80 patients from three studies had undergone OSMF treatment. In two studies, intralesional corticosteroid therapy was used, and in one study, Botox-A injection followed by laser fibrotomy was employed. The muscle activity was measured using EMG before and after intervention. The characteristics of the included studies are summarized in Table 2.
Risk-of-bias assessment
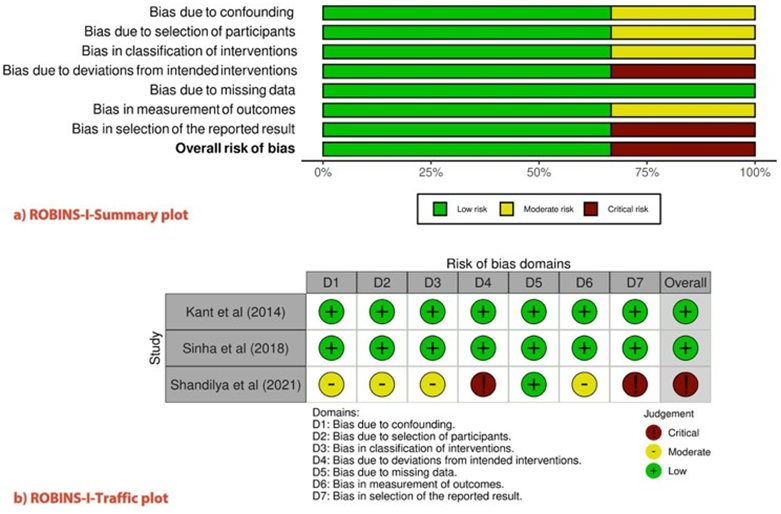
The'Risk of Bias in Non-randomized Studies of Interventions' (ROBINS-I) (28) was used to evaluate the methodological quality of the individual studies (Figure 2). This new ROBINS-I tool, previously identified as the ACROBAT-NRSI, was developed by the Cochrane Bias Methods group to estimate the effectiveness of interventions in studies that did not randomize the allocation of sample units against comparison groups. It addresses the issues before, during, and after the intervention through signaling questions. In our review, two studies had a ''low risk''of bias and one study had a ''critical risk.''
Synthesis of results
Three studies (29), (30), (31) with a sample totality of 80 patients diagnosed with OSMF who reported to dental hospitals were included in this review. The classification of OSMF was based on interincisal mouth opening, and treatment outcomes were assessed by measuring the same. The study by Kant et al. (29) proved that by measuring muscle activity using EMG, early involvement of the masseter muscle in OSMF, among other masticatory muscles, namely, the anterior temporalis and orbicularis oris, was also correlated with ultrasonography (USG), which measures the thickness of the masticatory muscles. For the masseter muscle, there was a positive correlation in Group I: >35mm mouth opening (r50.05, p50.89), Group II: 30-35mm mouth opening (r50.36, p50.29), and Group III: 20-30mm mouth opening (r50.49, p50.15), whereas there was a negative correla- tion in Group IV: <20mm mouth opening (r520.75, p50.01) and the control group (r520.03, p50.89).
Sinha et al. (30) and Shandilya et al. (31) used EMG to measure masseter muscle activity before and after OSMF treatment. Both studies described different treatment approaches in each sample group. Sinha et al. used intralesional corticosteroid therapy and Shandilya et al. used Botox-A followed by laser fibrotomy as an intervention. Compared with healthy controls, pre-treatment muscle activity increased in patients with OSMF. The post-treatment EMG activity was reduced compared with the pre-treatment levels.
In one study (30), at baseline during pre-treatment, the mean activity of the right and left masseter was 696.56±152.06 and 703.97±184.10, respectively, and that during post-treatment was 500.73±171.81 and 473.87±201.99, respectively, which was statistically significant (P<0.01). In another study (31) on patients with OSMF, masseter muscle activity reduced after treatment, which was also seen at the follow-up of 1 and 6 months. In the pre-treatment phase, the mean EMG values in the right and left masseters were 1.95 µV and 1.60 µV, 2.03 µV and 1.79 µV, and 1.14 µV and 1.65 µV during pre-injection, 1-month follow-up, and 6-month follow-up, respectively. However, the results were not statistically significant (P<0.05). This implies that there is a significant difference in muscle contraction levels before and after treatment, which can be used for prognostic quantification.
On the conglomeration of data from the included studies, the masseter muscle activity was altered in OSMF, which can be assessed using EMG. Irrespective of the intervention, the activity was altered before and after treatment; hence, this could be further pursued as an objective assessment tool for prognostic quantification.
Table 2 Data extraction and characteristics of included studies.
| Author, Year, Country | Study setting | Study design | Sample description | Purpose | Participants | Intervention group | Comparison group | Primary outcome | Secondary Outcome | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Kant et al . 2014, India | Dental hospital | CCT | Clinically diagnosed OSMF patients (Lai et al, classification) | To assess the involvement of muscles in mastication | 18-50 years | 40 OSMF patients divided into 4 groups | 20 healthy comparators | Masseter activity | Cross-sectional thickness | Early involvement of the masseter in OSMF |
| Sinha et al. 2018, India | Dental hospital | NRCT | Clinically and histopatho- logically diagnosed OSMF patients (Khanna et al classification) | To compare pre-treatment and post- treatment electromyogra- phic activity in the masseter | 20-50 years | 30 OSMF patients treated with intralesional corticoste- roids | 30 healthy comparators | Masseter activity | Improvement in mouth opening | Decreased EMG activity in the control group. Post-treatment activity decreased when compared with pre-treatment activity. |
| Shandilya et al. 2021, India | Dental hospital | NRCT | Clinically diagnosed OSMF patients (Khanna & Adradae et al classification) | To assess the effect of Botox-A in masseter followed by surgical intervention | Any age | 10 OSMF patients treated with BTX-A followed by bilateral laser fibrotom | 10 OSMF patients treated with saline followed by bilateral laser fibrotomy | Masseter activity | Improvement in mouth opening. Reduction in pain | Decreased pain in the OSMF group. One month after injection, the OSMF group showed a drop-in EMG activity |
Discussion
The use of EMG in OSMF is only at the nascent stage; hence, only a few studies were available for our systematic review. Previous studies using EMG had been conducted extensively on temporomandibular disorders and orofacial neuralgias (32), (33), (34), (35), which we had excluded in our search strategy of the systematic review. Masseter hypertrophy is a persistent enlar- gement of the muscle due to parafunctional habits and is common in younger populations because in older populations, the ability to activate muscle deteriorates. When the pathology of OSMF sets in, the masseter muscle becomes hypertrophied as a compensatory mechanism due to frequent milling of areca nut products for longer periods. The increased thickness can be assessed morpho- metrically using ultrasonography and functionally using EMG. This hypertrophied muscle causes increased myoelectric activity, which is measured as an increased amplitude on EMG. EMG is measured in a static state of rest and in a dynamic form with functions such as clenching and swallowing of the masticatory muscles.
Regarding the results, the amplitude was the only parameter measured for muscle contraction force evaluation across the time domain. This period can be customized based on study design expectations, and multiple readings can be recorded to arrive at the mean average value. Among the included three studies, OSMF pre-treatment activity was higher than in healthy controls, and two clinical trials compared post-treatment with pre-treatment values, proving that their intervention was effective in reducing masseter hypertrophy, which resulted in increased mouth opening.
A limitation of our review was the limited number of articles analyzed. The reason for this limited field of research could be that OSMF is common in developing countries where EMG measurements rely on neurological personnel that are not available in all dental settings. Additionally, loss to follow-up is higher in OSMF research because professional participants such as migrant workers and people of low socioeconomic status do not always continue the treatment as advised, although they are rigorously motivated for continuity of care. Recently, developing countries have been implementing EMG in the dental setting for muscle activity assessment under the required circumstances for a detailed understanding of the disease.
However, EMG assessment cannot be claimed to be a gold standard measurement tool because of the sensitivity of equipment usage, need for skilled technicians, and lack of standardization of baseline values. This paves the way for the definite inclusion of age- and sex-matched healthy comparators in the study design of OSMF research using EMG to maintain internal validity. We propose that further research requires studies with rigorous randomization, encompassing a larger sample size and longer follow-up periods, with the inclusion of intention-to-treat analysis.
This recommendation must be viewed with deeper knowledge, as a few limitations must be considered before direct translation to clinical practice. An important limiting factor is the use of different EMG-recording equipment, which leads to processing and recording divergence. This not only hampers data analysis but also focuses on the lacunae of standardization of EMG analysis and interpretation protocols. In addition, the difference between surface EMG and Needle EMG in masseter muscle activity must be studied, as the former is a noninvasive procedure prone to noise and impedance if skin preparation is inadequate. Additionally, all the studies were from a single country of the same ethnicity, which warrants further research as multicenter studies across demographics to provide insights into the myoelectric behavior of the masticatory muscles.
Conclusions
EMG can be advocated as a quantitative assessment tool for measuring masseter muscle activity during the management of OSMF, irrespec- tive of the chosen treatment modality. It can be considered an adjunct objective prognostic tool for assessing OSMF treatment outcomes, similar to the evaluation of improvement in mouth opening, by calculating the interincisal distance of a patient's oral cavity. An increase in EMG amplitude, i.e., increased muscular contraction force, is a consistent finding in OSMF cases, and the EMG amplitude decreases after OSMF treatment. However, the standardization of baseline values for masse- ter muscle activity to serve as reference points across geographical borders must be established. This systematic review, which adhered to the Cochrane Review Protocol, concluded that EMG can be used to assess the treatment prognosis in OSMF. Being reproducible and associated with less human error, EMG assessment can be proposed as a mandatory objective assessment tool for evaluating the prognosis of OSMF.
Author contribution statement
Conceptualization and design: P.P. and U.M.T.N.
Literature review: P.P.
Methodology and validation: P.P. and U.M.T.N.
Formal analysis: P.P. and U.M.T.N.
Investigation and data collection: P.P. and U.M.T.N.
Data analysis and interpretation: P.P. and U.M.T.N.
Writing-original draft preparation: P.P.
Writing-review & editing: P.P. and U.M.T.N.