Introduction
Quality control in orofacial surgical procedu- res is critical due to its impact on both the tissue extension and occupation (1); trigeminal injuries (TI) can have an irreversible impact on orofacial perception. Preclinical research has shown these have sensory, affective, and cognitive effects (2,3). At clinical level, it has been observed that TI have an additional psychosocial impact (4). Since this work shows that oral sensations are disturbed, this evidence indicates a decrease in the patients quality of life who undergo TI (5). Despite all the existing evidence, there is no agreement regar- ding the outcome of TI, and their variability and neurobiological basis are not well understood. This review aims to present evidence of TI caused by surgical procedures and their effects on somesthe- tic perception in patients and discuss their possi- ble neural basis with a translational perspective.
Somesthetic impairment caused by maxillo- facial surgical procedures. Clinical evidence has shown that orofacial surgical procedures can induce alterations in orofacial somesthetic perception (Table 1). These occur most frequently in mental and lingual region (6). For example, a bilateral divided sagittal osteotomy had as a side effect a neurosensory injury. 127 patients were studied and grouped by age (<24,24-25 and> 35 years old), who received a short mandibular advancement (<or=7mm), large advancement (>7mm), with and without genioplasty. Results showed a smaller sensory loss in patients over 35 years old who received a large mandibular advan- cement and genioplasty. This study concluded that age and a larger advancement and genio- plasty increase the risk of a TI (7). In a different study, hypoesthesia caused by bilateral divided sagittal osteotomy was assessed. That was made by measuring the evoked somatosensory poten- tials of the trigeminal nerve of 10 patients with prognathism during surgery (4 times) and in 5 postoperative sessions. Hypoesthesia was evalua- ted through P1 and N2 waves; results showed that the latency during medial periosteum dissection increased after fixation, with fast recovery after 2 to 4 weeks. Hypoesthesia is related to a direct injury to the inferior alveolar nerve (8). In another study, the authors proposed to evaluate the long- term effects on somatosensory perception after bilateral divided sagittal osteotomy. The study included 38 patients with an asymmetry greater than 4mm, preoperative and postoperative (6 months and 3 years). An improvement was obser- ved in the midline of 56% of the patients. 58% of the patients showed a discrepancy of more than 3mm, 3 years after the surgery, and an improvement in the dental midline in 80%. 44% of the patients reported normal sensitivity on the lower lip and mental region, but a difference was obser ved in the incidence of neurosensory impairment between both sides of the osteotomy (9). Sensory alterations after a divided sagittal osteotomy alone (n=84) or with genioplasty (n=37) occurred in 37% of the manipulated sides. Incidences were 36/101 for mandibular advancement and 12/30 for a mandibular setback. This study concluded that there are no significant differences between mandibular advancement or setback combined or not with genioplasty to show somesthetic impairment (10). These sensory signs have been associated to inferior alveolar nerve injury, produced by a divided sagittal osteotomy and distrac- tion osteogenesis. 130 cases of inferior alveolar injury were studied (70 bilateral divided sagittal osteotomies and 60 distraction osteogenesis). Results showed 23 cases of perception disorders, of which 14 were subjected to a bilateral divided sagittal osteotomy and 9 distraction osteogenesis. Furthermore, 107 inferior alveolar nerves did not show somesthetic impairment, of which 56 corresponded to bilateral divided sagittal osteotomy and 51 to distraction osteogenesis. It was also shown that there were no significant differences in inferior alveolar nerve injury between both surgical techniques (11). The maxillary nerve can also be injured after a Le Fort I bone fracture, with a postoperative evaluation in 12 men and 13 women. Skin, oral mucosa, and teeth sensitivity were assessed. An increase in the mechanical threshold of gums and palate was observed, and a higher thermal sensi- tivity in the infraorbital region 12 months after surgery. Sensory change was determined for the eyes, upper lip, gums, palate, and teeth (12). Other study assessed sensory recovery after orthog- nathic surgery. 47 patients (26 men, 21 women) were evaluated, 1, 3, and 6 months after surgery, using a visual analog scale. It was found that tactile sensory alterations occurred on the mental region in 55.7% of the cases and on the lips in 27.3%, similar to paresthesia, which decreased over time. This study concluded that somesthetic impairment is an inevitable complication but can be solved spontaneously (13). Another study used self-monitoring of sensory impairment in patients, and it was compared with a quantitative sensory assessment instrument. These evaluations are important to obtain better quality control in orthognathic surgery and decrease risk factors. In 2 groups of patients programmed for bi-maxillary orthognathic surgery (cases and controls study), sensory tests were applied in 6 trigeminal sites and 1 extratrigeminal site; patients sensitivity to toothbrush bristles, prick, pressure, among others, was evaluated. 8% of the patients showed postoperative intraoral sensory disorders, and 46% showed extraoral sensory disorders. One and a half years after surgery, many patients reported sensory disturbances, such as mechanical hypersensitivity, compared to control groups or preopera- tive conditions. Patients could report their sensory conditions in addition to clinical assessment using sensory measurement instruments (1). Further- more, the infraorbital nerve was assessed after zygomatic bone fractures. In a study with 25 patients, 15 displaced fractures from the zygomatic complex and 10 with minimal displacement. Of these cases, 7 were left with no treatment, 8 were treated with bone reduction but no fixation, and 10 cases with fixation, and somatosensory function was improved with electrical stimulation. This occurred 6 months after the TI produced on the group that received a bone fixation. Thus, bone fixation might allow the recovery of infraor- bital nerve injury after bone fracture (14). This is corroborated by displacement of the zygomatic- maxillary fracture, which can induce peri-orbital hematoma, edema, diplopia, and mouth opening limitation, which is solved during the postoperative period. However, in 44% of displaced fractures, somatosensory impairment persisted until the end of follow-up (after 6 months), unlike non-displaced fractures, in which incidence is much lower (15). In a study that analyzed the somatosensory function of the lower lip and mental region after divided sagittal osteotomy, 22 patients with skele- tal class III were recruited. A quantitative sensory test was applied before the surgery, and 1 week, 1, 3, and 6 months after, cold, heat, and pressure pain threshold were measured on the lower lip and mental region. Results showed a decrease in sensitivity for all tests, except for pressure pain. After 3 months, threshold values returned to the baseline; thus, somatosensory function might fully recover after 6 months (16). It has been found an increase of cold and heat threshold after divided sagittal osteotomy (17). Other studies showed that the risk of hypoesthesia after a bilateral osteotomy increases with age (5%) (18). For intraoral verti- cal osteotomy, the development of hypoesthesia in the lower lip has been observed. It has also been suggested that bone displacement can delay the recovery of lower lip hypoesthesia (19).
Table 1 Sensory changes associated with orofacial surgical procedures.
| Surgical procedure | Sensory affection | Reference |
|---|---|---|
| - | Oral surgery (Inferior Alveolar Nerve) | - |
| Third molar extraction | Tongue numbness. Increase in cold sensitivity and cold and heat pain. | (Yan et al., 2020) |
| Coronectomy | Lower lip numbness | - |
| Bilateral divided sagittal osteotomy | An increased tactile threshold in the lower lip, two-point discrimination. Reduced pulp threshold. | (Thygesen et al., 2008) |
| Mandibular nerve lateralization during implant placement | Anesthesia (81 sites).Hypoesthesia (9 sites). Burning sensation (9 sites). Pain (8 sites).Tickling (1 site). | (Hashemi, 2010) |
| Third molar extraction | A reduced electrical detection threshold. | (Barron et al., 2004) |
| - | Maxillofacial Surgery | - |
| Bilateral divided sagittal osteo- tomy/Le Fort I/Genioplasty | Dysesthesia. Hypoesthesia. Paresthesia. | (Y. K. Kim, Kim, & Kim, 2011) |
| Le Fort I | Increase of tactile threshold in gum and palate. Increase of thermal sensitivity in infraorbital region. Changes of eyes, upper lip, gum, palate, and teeth sensitivity. Increase of electrical stimulation pulp threshold. | (Thygesen, Bardow, Norholt, Jensen, & Svensson, 2009) |
| Bimaxillary orthognathic surgery | Increased sensitivity to brush stimulation. Decrease of two-points discrimination threshold (mental region). Hypersensitivity to brush, prick, two-point discrimination, and pain. | (Baad-Hansen, Arima, Arendt- Nielsen, Neumann-Jensen, & Svens son, 2010) |
| Sagittal split ramus osteotomy | Decrease of sensitivity of lower lip and mental region: cold. heat. cold-pain. heat-pain. detection of mechanical stimuli. mechanical-pain. | (He et al., 2021) |
| Zygomaticomaxillary fracture fixation | Hyperesthesia. Anesthesia. Paresthesia. In the infraorbital and paranasal region, for displaced fractures (88.2%). | (Mueller, Zeiß, Mtsariashvili, Thorwarth, & Schultze-Mosgau, 2012) |
| Bilateral divided sagittal osteotomy | Lower lip/mental region sensitivity: Normal 44%. Slightly reduced (34%). Markedly reduced (10.5%). Hypersensitivity (7.9%). Complete loss (3.6%). | (Hågensli, Stenvik, & Espeland, 2014) |
| Zygomatic fracture fixation | Electrical stimuli: Hypersensitivity (8,3%). Hyposensitivity (50%) Fine mechanical stimuli hypoesthesia (55%). Thermal detection threshold: Hypersensitivity (10%). Hyposensitivity (40%). | (Benoliel, Birenboim, Regev, & Eliav, 2005) |
| Vertical intraoral ramus osteotomy with and without Le Fort I | Lip hypoesthesia. | (Ueki et al., 2009) |
| Bilateral divided sagittal osteotomy | Trigeminal hypoesthesia Increase in the latency of evoked somatosensory trigeminal potentials | (Nakagawa, Ueki, Takatsuka, Takazakura, & Yamamoto, 2001) |
| Divided sagittal osteotomy | Anesthesia (3 nerves). Hypoesthesia/paresthesia. (11 nerves). | (Baas, de Lange, & Horsthuis, 2010) |
| Distraction osteogenesis | Anesthesia (2 nerves). | - |
| Bilateral divided sagittal osteotomy | Increase in sensory thresholds (over 35 years old, with genioplasty) Tactile sensory loss (advancements of 7 mm or more, patients over 35 years old). | (Van Sickels, Hatch, Dolce, Bays, & Rugh, 2002) |
| Glossectomy | No sensory alterations were observed in sensory tests after tongue reduction. | (Matsumoto, Morita, Jinno, & Omura, 2014) |
| Bilateral divided sagittal osteotomy | Hypoesthesia. | (Politis, Sun, Lambrichts, & Agbaje, 2013) |
For divided sagittal osteotomy, an increase in the tactile threshold for the lower lip and a decrease of self-perception of mouth opening have been found. These changes depend on the intrao- perative risk factors and individual preoperative sensation (20). This result could lead to prediction and prevention of the development of disturbances in somesthetic perception after osteotomies. In soft tissue surgical procedures, it has been less likely to find somatosensory changes, as reported for macroglossia surgical reduction (21). Some procedures have been proposed to reduce the risk of trigeminal injuries, such as the coronectomy (22). Practicing coronectomies for dental extrac- tions have been shown to decrease the sensory changes observed in conventional extractions, such as increased thermal and pain sensitivity. Therefore, coronectomy might imply a lower risk of TI than a conventional total extraction (23). One of the main problems in assessing the somesthe tic changes associated with TI caused by surgi- cal procedures is the absence of an agreement for it. In a prospective study of up to 20 years, postoperative sensory changes were measured following 75 mandibular surgical procedures. Most of the procedures were osteotomies and bone fractures, compared to third molar extractions. This study used the light touch test with Semmes-Weinstein filaments, accompanied by a visual analog scale, to evaluate sensitivity (24). Another efficient strategy to prevent sensory impairment caused by sagittal mandibular osteotomies is steroids in patients over 40 years old, whose incidence increases the risk of developing an orofacial somesthetic perception disturbance (25). Dipyrone and preoperative dexametha- sone treatment can prevent the development of somatosensory alterations induced by third molar surgery (26).
These effects on somatosensory perception are more significant if the surgical procedures are directly performed in nervous branches (27).
Neurobiological Aspects of trigeminal injury. Additionally to their clinical study, orofacial nervous injuries have been evaluated experimentally, mainly in murine models. The scientific assessment of these injuries has allowed to study them in a controlled way and, despite these models do not completely reflect the clinical conditions of trige- minal injuries, they have been helpful to assess these phenomena in a greater depth, from a molecular to a behavioral approach (Figure 1).
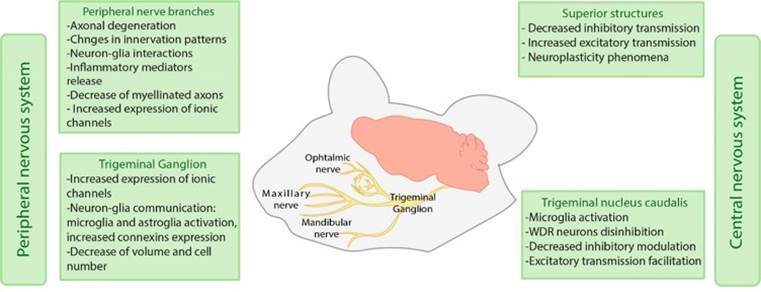
Figure 1 Mechanisms involved in orofacial chronic neuropathic pain in both peripheral and central nervous systems in preclinical models.
The studies mentioned above have been made at peripheral level, evaluating the trigeminal branches and trigeminal ganglion; and at the central level, studying the different brain structures involved in orofacial areas somatosensory processing. For peripheral structures assessment, the changes after mental nerve injury include axonal degeneration and alterations in the lower lips innervating patterns, involving sensory and autonomous fibers. Autonomous fibers show changes in their length and the innervation site (28). These changes occur during the first days after the injury and are maintained over several weeks, suggesting long-term effects. These effects are associated with hypersensitivity in rodents, observed through an increased grooming behavior.
Nerve degeneration signs have been obser- ved in infraorbital nerve compression models, showing a deficient repair process compared to what can occur in a facial nerve injury. That highlights the importance of the consequences of nerve injuries in somatosensory functions (29). It has been proposed that sensory impairment extends beyond the injured nerve region, as observed after a mental nerve constriction and transection. The rodents showed hypersensitivity signs on the whisker pad, a region innervated by the maxillary branch of the trigeminal nerve (28). Hypersensitivity signs in a region beyond the territory of an injured nerve indicates that its changes might affect a more significant proportion of the trigeminal system, not only locally.
The effects of TI on the trigeminal ganglion have also been assessed, and there have been observed both molecular and cellular alterations. After a mandibular branch injury, axonal damage is seen as a high expression of the transcription factor-dependent ATP (ATF3). However, not only the neurons that showed axonal damage participate in the sensory alterations induced by TI, since the neurons in the trigeminal ganglion that did not express ATF3 showed an increased expression of the transient receptor potential vanilloid 1 (TRPV1) channels, involved in the processing of noxious stimuli (30,31). Different evaluations suggest that these are long-term changes because around 180 days after trigeminal injury, the volume and number of neurons in the trigeminal ganglion decrease and do not recover over time (32). Regarding neural activity in the trigeminal ganglion, inferior alveolar transection can produce a state of hyperexcitabi- lity of nerve fibers. This phenomenon is associated with hypersensitivity signs (33).
The immune response role can also affect the development of sensory impairment after a nerve injury, and a cross-communication might explain it between nervous and immune system cells. Thus, nerve damage causes the activation of immune cells and the release of inflammation mediators, stimulating nerve cells. This stimulation can origin the peripheral sensitization phenomenon, facilitating chronic neuropathic pain development (34). Immune system participation does not only occur in the nerve endings, where an injury is made, but also in different sites, such as the trigeminal ganglion, where an increase of inflammatory mediators has been observed, for example, interleukin 1β (IL-1β) after mental nerve transec- tion (28).
Although glial cells do not directly parti- cipate in nervous impulses transmission, they could be essential in establishing and maintaining chronic neuropathic pain; the activation of micro- glial cells and the increase of inflammatory mediators in cells such as astrocytes since the early stages of the injury (35) and a possible neuronglia communication in the trigeminal ganglion. It has been suggested that this increase in the cross-communication might be due to an increase in connexins expression in glial cells, which correlates with the presence of spontaneous pain (36), in addition to the satellite glial cells, found near neuronal somas in the trigeminal ganglion. This process can increase paracrine signaling and cell sensitization mediated by the activation of puriner- gic receptors. Such activation might increase the intracellular calcium levels, facilitating an increase in neural activity (37).
One of the most studied structures in the central nervous system is the trigeminal nucleus caudalis since it is the site where the first synapse occurs in the trigeminal sensory pathway. This nucleus has been shown to suffer changes in both humans and rodents in the context of trigeminal injury and chronic neuropathic orofacial pain. It has been found that wide dynamic range neurons (WDR), which process somatosensory information, have increased evoked and spontaneous responses after lesioning the infraorbital nerve. One possible explanation for this event can be attribu ted, at least in part, to the decrease in inhibitory transmission mediated by gamma-aminobutyric acid (GABA) (38). Moreover, it has been observed microglia and astroglía activation since the first days after a trigeminal injury (39); an increase in connexin 36 (CX36) expression, favoring neuronglia communication (40); as well as the increase in functional connectivity between rostroventral medulla (RVM) and the trigeminal nucleus caudalis (41). These data suggest a possible impairment in modulatory systems that reduce pain perception. It has been recently reported that orofacial pain induced by mental nerve injury can facilitate nerve transmission in the trigeminal nucleus caudalis. Such effect is, in part, mediated by TRPV1 receptors (42). Additionally, different supraspinal structures have been associated with the development of sensory, affective, and cognitive alterations due to nerve injuries. Nonetheless, such alterations are beyond the purpose of this review, and they would be worthwhile to be addressed in an additional review.
As has been already mentioned, ectopic innervation of peripheral fibers, crossed excitation of injured and uninjured nerves, expression of cell-membrane and signaling molecules, and neuronglia interactions can produce changes in the electrical properties of nervous fibers, altering the balance between excitatory and inhibitory transmission. The augmented excitatory transmission and the diminished inhibitory transmission can be manifested in the characteristic symptoms of chronic pain and the sensory alterations that can co-occur (43). These alterations might be hypersensitivity phenomena, such as allodynia and hyperalgesia (44), spontaneous pain episodes (45), or other sensory alterations reported due to trigeminal injuries, such as paresthesia, dysesthe- sia, anesthesia, among others (46).
Conclusion
Surgical procedures performed in adults have the highest risk of generating perception impairment, combined with the extension and tissue invasion. The clinical and experimental basis shows that the main somatosensory alterations after trigeminal injury are the development of chronic pain, hypersensitivity, and hypoesthesia. There are plenty of pathophysiologic mechanisms underlying these alterations, and therefore, a prevention strategy is necessary for every surgical intervention.















