Introduction
Dental decay is a multifactorial disease that involves bacterial growth and a capacity to destroy a hard dental surface. The principal consequences are pain and demineralization at the enamel/ dentine interface. It is caused by the presence of acid products and the consequent carbohydrate metabolism in dental plaque (1,2,3,4,5).
Enamel is a hard tissue composed principally of hydroxyapatite crystals (Ca5(PO4)3(OH)) assembling in rod and prism shapes at micrometer size. A hydrophilic characteristic is present due to a 10% water composition in porosities. On the other hand, when demineralization affects enamel, it induces a phenomenon of diffusion and substance exchanges, favoring decay, pain, and pulp damage (6). To prevent demineralization, some clinical strategies have been developed in enamel; principally the topical application of fluor, the sealing of prisms by resins, and modification of the hydrophobic surface (7,8,9).
A modification of the hydrophobic surface can involved different methods, of which silanization is an example. The goal is to chemically change the hydrophilic to the hydrophobic condition on the enamel’s surface. To obtain this condition, a pretreatment protocol with EDTA and NaOCl is necessary for exposed collagens fibers and hydroxyapatite film (HA) for the silanization protocol (10). This strategy is employed with excellent results in dentistry, and in the textile, electronics, telephonic, construction, and in the food industries (11,12,13).
Currently, silanization is employed in endodontic and prosthetic processes; however, the goal is its possible use in preventive dentistry. For example and reciently Icon® (DMG America) is a dental material that act as infiltrators in spaces created in the demineralization process, it is a 15% hydrochloric acid gel that fills cavities, preserving healthy dental structures. It is not similar to silanization, but it is a recent clinical tactic in preventive dentistry that employs resin infiltration to prevent decay development (14).
In the literature, there are reports on the chemical modification of intra-radicular dentine by silanization with octadecyltrichlorosilane (OTS) and octadecyltrietoxisilane (TEOS). In these studies, it was demonstrated that the dentin surface acquired a better root seal on proceeding from a hydrophilic to a hydrophobic state after silanization with OTS (15,16,18).
Patiño et al. developed a protector effect on hydroxyapatite (HA) film against acid erosion with silanization by OTS. These authors demonstrated the protector effect of a hydrophobic surface against acid erosion employing OTS on hydroxyapatite (HA) films and on dental samples in which no significant erosion was observed. These results validate that a hydrophobic surface can be an effective dental protector against acid erosion (10).
On the other hand, Reis et al. (17) evaluated dental composites and their capacity of enamel remineralizing containing silica-hydroxiapatite (Si-HAp) nanoporus particles charged with sodium fluoride (NaF). These authors mentioned that composites presented a remineralizing activity in human enamel and that this ability underwent a subtle reduction after particle silanization (17).
Therefore, the objective of this study was to evaluate the silanization of dental enamel using octadeyltrichlorosilane (OTS) and octadecyltriethoxysilane (TEOS) for the prevention and demineralization of dental enamel through by means of an ex-vivo assay.
Materials and methods
Biethical considerations
This protocol was authorized by COFEPRIS with authorization number CI-UAE-15-2020.
Sample collection
Thirty premolars extracted for orthodontic reasons were collected at the Multidisciplinary Clinic of the Master of Biomedical Sciences Program of the Autonomous University of Zacatecas (CLIMACB) and were stored for 2 weeks in distilled water until the procedure with the different materials. To obtain a flat enamel surface, the teeth were fragmented and mounted in epoxy resin. After a sixty-four samples (square, 5 x 5mm) were sanded until the concave enamel was removed and specimens 3mm in thickness were obtained. Later, these were recovered from the epoxy resin and cutted with a high speed motor (RAY-FOSTER, USA), burn 701 (DENTSPLY, SIRONA, USA), and carbide disc (DENTSPLY, SIRONA, USA). Afterward, in an optic microscope (MICROVISION, USA), the simples were evaluated to rule out the presence of epoxy- resin residues.
Pretreatment
All samples were immersed in 17% EDTA (3min), 2.5% NaOCl (5min), 17% EDTA (3min), and finally, were rinsed in deionized water. The aim of this protocol was to expose the collagen fibers and the HA indispensable for developing Si-O bonds in this silanization protocol (Figure 1) (16,10).
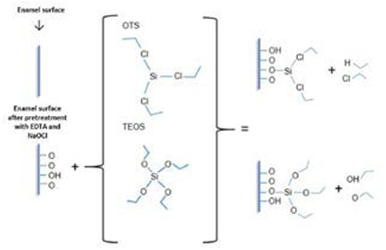
Figure 1 Schematic representation of silanization in dental enamel. In the schema, a pretreatment protocol and silanization were observed, On the left, a raw enamel surface raw is pretreated with EDTA and NaOCl for exposed collagen fibers and HA in enamel. After a silanization protocol, OTS and TEOS were developed. Finally, covalent bonds between Si-O are developed in enamel and indicated a hydrophobic surface.
Silanization protocol
OTS silanization
Two different storage solutions were prepared for the silanization process: solution 1: OTS 1% (10 ml ethyl acetate + 100 µL OTS), and solution 2, OTS 4% (10 ml ethyl acetate + 400 µL OTS). The enamel fragments were divided into the following eight groups (n = 4 per group): (OTS 1%+ 5min; OTS 1% + 8h; OTS 1% + 5min + 60 days in citric acid; OTS 1% + 8h + 60 days in citric acid; OTS 4% + 5min; OTS 4% + 8h; OTS 4%+ 5min + 60 days in citric acid, and OTS 4% + 8h + 60 days in citric acid). These were randomly distributed and silanizated for 5min and for 8h, respectively (10,16).
TEOS SILANIZATION
In the same manner, storage solution 1, consisting of TEOS 3% (300 µL TEOS + 10ml ethanol + 200 µL acetic acid), and storage solution 2, consisting of TEOS 6% (600 µL TEOS + 10ml ethanol + 200 µL acetic acid) were prepared. Another eight groups (n=4 per group), including (TEOS 3% + 5min, TEOS 3% + 5min + 60 days in citric acid, TEOS 3% + 8h, TEOS 3% + 8h + 60 days in citric acid, TEOS 6% + 5min, TEOS 6%+ 5min + 60 days in citric acid, TEOS 6% + 8h, and TEOS 6% + 8h + 60 days in citric acid) were silanized for 5 min and for 8h (10,16).
On finalizing the silanization protocols, the groups were evaluated in terms of Contact Angle, Atomic Force Microscopy, Scanning Electronic Microscope, and Fourier Transformed Infrared Spectroscopy.
Contact angle (CA)
Ten-µL drops of water were randomly disposed on different silanized enamel zones and contact angle (CA) measurements were developed in a Goniometer (DIGIDROP, FRANCE) at room temperature 10 times.
Fourier transformed infrared spetroscopy (FTIR)
An FTIR evaluation was made in a spectrophotometer Nicolet Nexus 470 FTIR Spectrophotometer (NICOLET, MADISON, WI, USA). The bonds PO₄³ ̄, OH ̄, C-Cl, Si(CH)₃, Si-OH, and Si-O-Si were analyzed on silanized surfaces. All samples and control were analyzed at wavenumber 3,000-500 cm ̄¹.
Scanning electronic microscopy (SEM)
A microscope (JSM-6510; JEOL, JAPAN) was employed at 550X magnification. In all samples, EDX analyses were processed. The samples were dried in alcohol at different concentrations and then dried in an oven for their coating with gold and their analysis.
Atomic force microscopy (AFM)
An Atomic Force Microscope (AFM) (Bruker, Dimension Edge model, USA) was utilized to evaluate the morphology and roughness of the silanized surfaces before and after remaining in citric acid on a 20-µm x 20-µm surface; the quadratic Roughness (Rq) of each specimen was obtained.
Confocal microscopy (CM)
Some complete premolars dental organs were pretreated as described previously. The coating solution with 1% and 4% OTS, or with 3% and 6% TEOS, was mixed with the compound Rhodamine (Sigma-Aldrich, 99% pure) to obtain a final concentration of 20 µg/ml. Subsequently, the pieces were varnished in enamel with the solution containing the silanizing agent and the Rhodamine; subsequently, the dental organs were immersed in citric acid for 8h. After 8h, the pieces were sectioned (square, 5 x 5mm) with an isometric seal (CIENTEC) for their subsequent analysis in enamel by Laser Confocal Microscopy (LCM).
Results
Contact angle (CA)
Figure 2 presents the averages of the CA measurement. Under a black bar, samples presenting less than 90 degrees are considered hydrophilic surface, and above the bar, we find all silanized specimens with a CA of more than 90 degrees are a hydrophobic surface. Specimens coated with 4% OTS for 8h, exhibited the highest average CA measurement (>130°), while those silanized with 3% TEOS for 5 min had the lowest average CA (<75°).
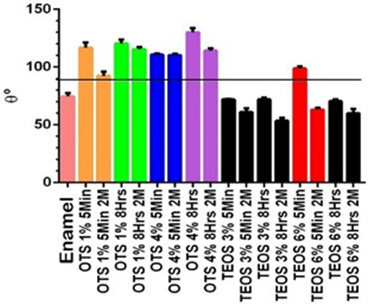
Figure 2 Contact angle measurements. Average of samples in the contact angle. Enamel was control without pretreatment and all silanized groups were measured. The test was conducted immediately and after 2 months in citric acid in all groups. The groups above the black bar (>90 degrees) are considered a hydrophobic surface.
Fourier transformed infrared spectroscopy (FTIR)
In Figure 3, a control (enamel) presents characteristic bonds (3,200 cm-1) of the elements in hydroxyapatite (P-OH, OH). These bonds are indispensable in the silanization process. In silanized groups, there are present the characteristic bands of Si-OH (3,200 cm-1) and Si-O-Si (1,500-750cm-1), in contrast with the CA frequency of Si-O-Si and Si-OH bands. The samples expressed that certain elements can be variable depending on the percentage of silica on the modified surface.
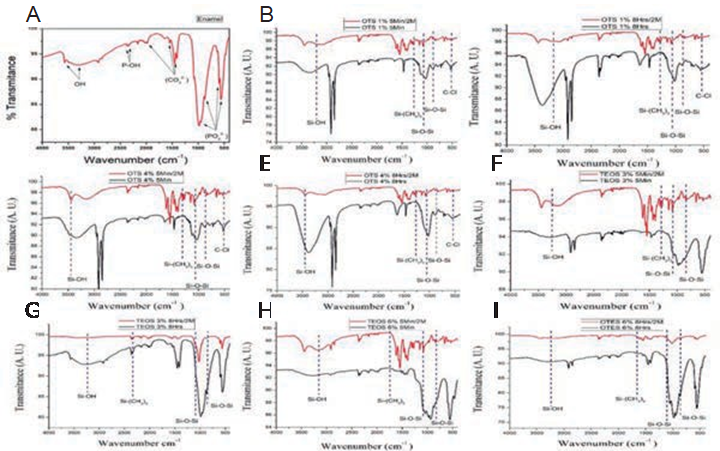
Figure 3 FTIR spectra. The FTIR enamel spectrum (A) has shown the characteristic bands of hydroxyapatite. The FTIR spectra of the specimens coated with OTS at 1% for 5 min (B), OTS at 1% for 8h, (C), OTS at 4% for 5 min (D), and OTS at 4% for 8h (E), those coated with TEOS at 3% for 5min (F), and at 3% for 8h (G), while TEOS at 6% for 5min (h), 6% for 8h (I) are shown in the histograms in black bands, compared with the spectra of the same samples after remaining in citric acid for 60 days. The comparative bands are shown in red.
Scanning electronic microscopy (SEM)
In the SEM analysis, Figure 4A represents enamel without silanization. Images 4B-4Q represent all groups silanized with OTS and TEOS. In each image, a Si percentage was determined with EDX analysis.
Atomic force microscopy (AFM)
Figure 5 shows the histograms of the groups roughness and topography. The enamel presents a surface that is uniform and smooth. Other histograms represent that were silanized and left in citric acid after 60 days. The TEOS groups possess minor modifications after their remaining in the citric acid. The average Rq obtained with silanization was 1.0849 µm and, after being in citric acid, the Rq was 2.486 µm.
Confocal microscopy laser scanning (CMLS)
In the microphotographs (Figure 6) of OTS and TEOS coating with Rhodamine, the silanizing agents are observed as red in color on the surface of the dental enamel. This indirect marking allows us to observe the homogeneity and continuity of the silanizing coating, as well as whether it possesses fractures or partial or total loss of the coating after remain in the citric acid.
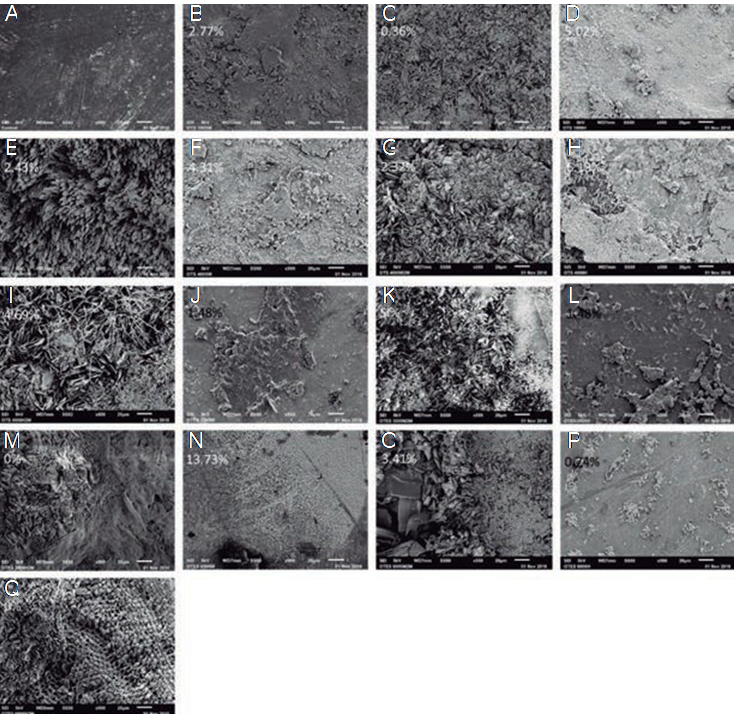
Figure 4 Scanning Electronic Microscopy analysis. SEM images showing analyzed the surface morphology and percentage of Si, (A), enamel, (B), 1% OTS + 5min, (D), 1% OTS + 8h, (F), 4% OTS + 5min, and (H), 4% OTS + 8h, and the silanized surfaces: (J), with 3% TEOS + 5min, (L), 3% TEOS + 8h, (N), 6% TEOS + 5min, and (P). 6% TEOS + 8h. After immersion in citric acid for 60 days: (C), 1% OTS + 5min, (E). 1% OTS + 8h, (G). 4% OTS + 5min, (I), 4% OTS + 8h, (K), 3% TEOS + 5min, (M), 3% TEOS + 8, (O), 4% TEOS + 5min, and (Q), 4% TEOS + 8h.
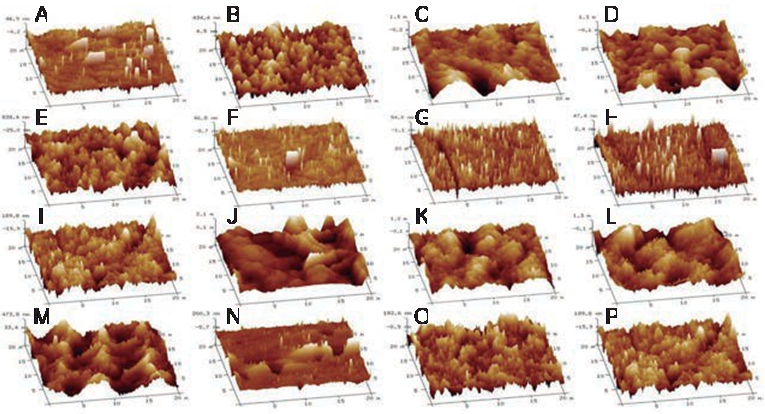
Figure 5 Atomic Force Microscopy (AFM) evaluation. AFM images showing the morphology of the surfaces of the specimens analyzed, depicting the morphology enamel (A), to the surface of the specimens silanized with 1% OTS + 5min (B), 1% OTS + 8h (D), 4% OTS + 5min, (F), 4% OTS + 8h, and (H) with 3% TEOS + 5min (J), 3% TEOS + 8h (L), 6% TEOS + 5min (N),6% TEOS + 8h (P). The topography of the silanized specimens is also shown after they have been immersed in citric acid for 60 days, with 1 % OTS + 5min (C), 1% OTS + 8h (E), 4% OTS + 5min (G), 4% OTS + 8h (I), 3% TEOS + 5min (K), 3% TEOS + 8h, and (M), 6% TEOS + 5min.
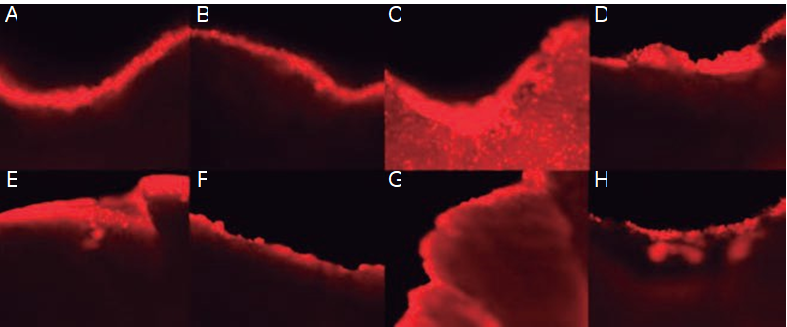
Figure 6 Confocal Microscopy analysis. Confocal microscope images of the surface of the dental organs varnished with 1% and 4% OTS stained with Rhodamine (A and C) and with 3% and 6% TEOS stained with Rhodamine (E and G). Images of the same specimens coated with 1% and 4% OTS stained with Rhodamine (B and D) and with 3% and 6% TEOS stained with Rhodamine (F and H) after being in an acidic medium for 8 h.
Discussion
The results obtained in the measurement of the contact angle showed a hydrophilic enamel (<90 degrees), this being a surface control prior to the silanization process. In the investigation carried out by Collart and collaborators in 2013 on the salinization of dentine in root canals, the authors obtained results in which the contact angle is longer than 70 degrees. The same results were obtained after the surface of the tooth enamel was silanized with Octadecyltrichlorosilane and Octadecyltriethoxysilane. In all of the surfaces coated with OTS, the contract angle was measured immediately afterward and the latter were at an angle greater than 90 degrees, indicating that the surface had undergone a modification from hydrophilic to hydrophobic. These surfaces maintained contact angles higher than 90 degrees after having been subjected to citric acid for 2 months (10,11,18).
The TEOS-coated surfaces coated with concentration of 6% and silanized for 5 min, had a contact angle measurement higher than 90 degrees. Nevertheless, when measuring of the contact angle was performed after these samples had been in citric acid for 2 months, the measurements effected rendered angles smaller than 90 degrees. In this regard, our results coincide with those of Patiño-Herrera, in which these authors synthesize hydroxyapatite and fluorapatite in gold sensors. In this research, these compounds were coated with OTS and then, several analyzes were conducted. These results were very important for this project (15,16).
The results discussed previously were not sufficient to guarantee that the tooth enamel surface was coated with OTS or TEOS. Thus, it was necessary to perform an infrared spectroscopy (FTIR) analysis, in order to verify the presence of the bonds and functional groups-of-interest that guarantee the bonding of the silanizing agent with the surface of the dental enamel. The peaks and curves that are observed at different wave numbers in the different spectra correspond to those obtained by Patiño et al., in their research in 2015 and by Collart in 2013. According to the results obtained, the silicon in the silanizing agents is bound by a covalent bond to oxygen on the surface of tooth enamel. It is noteworthy that the chemical bond between the previously mentioned elements is the strongest that exists in nature, in addition to being the most abundant element in the earth’s crust (10,15,16).
Scanning electron microscopy (SEM) was another essential analysis that was performed to ensure the existence of a coating on the tooth enamel. In this analysis, we also observed the topography of dental enamel itself, of the dental specimens recently coated with OTS and TEOS, and of the dental specimens coated with OTS and TEOS after having remained in citric acid for 2 months. In the images of the newly silanized specimens obtained in the present investigation and in that carried out in 2015 by Patiño and collaborators, we find great similarity with the images obtained from the specimens that had been subjected to citric acid. In the investigations, this image presents a less irregular surface than those we obtained, probably because these authors subjected only the silanized specimens to the demineralization process for 168h and, in the present investigation, these remained for 1,440h, that is, of 2 months. In the EDS or EDX (energy- dispersive X-ray spectroscopy) analysis was conducted to determine the percentage elements constituted the surface of the analyzed specimen, as carried out in later investigations (10,16,18).
One of the analyses employed to observe the surface topography of the dental enamel was Atomic Force Microscopy (AFM). In the investigation carried out by Patiño and collaborators in 2015, the aithors applied only this analysis to the hydroxyapatite and fluorapatite, and they synthesized and deposited the dental enamel on gold sensors; We thought it necessary to perform this analysis on fresh specimens coated with OTS and TEOS and after exposing them to citric acid for 2 months, in order to verify that the structure of the silanizing agent remained after exposure to acid. In order to have an objective result, it was necessary to obtain the quadratic roughness and the histograms of the number of frequency of the measurements procured on conducting this analysis. This indicated that, as there is less variation in the results of the histograms and that in the quadratic roughness, there is a similar or equal presence of the silanizing agent before and after the specimens were exposed to citric acid (10).
Marzuki et al., on using confocal laser microscopy (CLSM), obtained images of the dentin structure utilizing Rhodamine as a stain, in which they observed structural and mineral changes in the carious dentin (19). In our results, the specimens coated with OTS are those in which a more homogeneous coating of the tooth enamel is observed.
Conclusions
We analyzed specimens of the surface of tooth enamel coated with OTS and TEOS; there were fresh specimens that were exposed to citric acid for 2 months. Silanization with 4% and 1% OTS with a silanization time of 5 min and 8h creates a hydrophobic protective layer that remains nearly unchanged in terms of the Si percentage even after exposure to citric acid.
Therefore, we conclude that, by forming a hydrophobic layer on the surface of tooth enamel with OTS, the latter will provide a protective layer that will prevent demineralization in an acidic environment















