Introduction
Propolis is a viscous, resinous, aromatic substance that has different colors ranging between brownish-green and coffee, depending on its origin and age. It is hard and brittle on contact with cold and when ihot, it becomes soft and sticky and is also known as bee glue (1). It is obtained from the branches of resinous trees and from the leaves of certain plants (2). The main source of propolis is Salicaceae (poplar and willow trees), whose chemical composition is very complex; about 19 components have been classified, with some of these substances belonging to the flavonoid group, isovanieline, resins, etc. All of these are characterized by their biological activity (3,4,5). The antibacterial activity of propolis is mainly conferred by flavonoids (flavones, flavonols, flavanones, flavanonol, chalcones, dihydrochalcones, isoflavones, isodihydroflavones, flavanos, isoflavans, and neoflavonoids) and terpenoids (acyclic, monocyclic, dicyclic, and derived monoterpenes), and by phenolic compounds (cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid, and derived acids (6). There are various types of propolis, depending on the plant species from which they are obtained but, in particular, 50-60% of propolis is composed of resins and balms (including phenolic compounds), 30-40% of waxes and fatty acids, 5-10% of essential oils, 5% of pollen, and about 5% of other substances including amino acids, micronutrients, and vitamins (thiamin, riboflavin, pyridoxine, vitamin C, and vitamin E) (7,8). Propolis extracts have an application in treating diseases due its anti-inflammatory (9), antioxidant (10), antibacterial (11), antifungal (12), antiulcer (13), anticancer (14), and immunomodulatory properties (15). The therapeutic application of propolis that has been most studied is its antibacterial action, which is made up of flavonoids, the main component of propolis, by enhancing the antibacterial, antiviral, and anti-inflammatory effect (16).
Applications of propolis ins dentistry
In dentistry, propolis is known as a promising compound mainly in the treatment of tooth decay and periodontitis; it is used in various presentations such as toothpastes and mouthwashes (17). The oral cavity is an environment in which there are around 700 different species of microorganisms that live in symbiosis, these known as the normal microbiota of the mouth. Because there are changes in the oral environment or under host conditions, some bacteria tend to colonize and give rise to various oral pathologies mostly related with inflammation and infection (18). The use of propolis in dentistry has been studied for a long time; there are several studies mentioning that propolis extract can inhibit the growth of Gram-positive and Gram-negative bacteria, comparing its efficacy with some antibacterial drugs such as Ampicillin and Tetracycline, or even with antiseptics such as 0.12% Chlorhexidine (19,20). Regarding the prevention of oral diseases, propolis has been employed for its anticariogenic potential and the reduction of the accumulation of dentobacterial plaque. Several studies affirm that the presence of propolis significantly reduces cavities in rats by inhibiting the synthesis of glucans and blocking the action of glucosyltransferase (21). Momen- Beitollahi et al. mentioned that propolis decreases the growth of the most prevalent oral pathogens that include Streptococcus mutans, Candida albicans, and Actinobacillus commitans (22). In addition, propolis has been successfully used in the treatment of dental pulp regeneration as a direct coating in accidental exposure (23). Meakawa et al. reported that the propolis extract was effective against the microorganisms C. albicans, Enterococcus faecalis, and Escherichia coli (24). However, Koo et al. note that the true mechanism of antimicrobial action of propolis appears to be complex and is not yet fully understood (25).
Porpolis in peridontal diseases
In the treatment of periodontal diseases, propolis has demonstrated antibacterial, anti-inflammatory, anesthetic, and healing activity in certain lesions such as chronic ulcers or periodontitis (26). Some studies claim the action of propolis against supragingival plaque by its stimulating tissue recovery and the local immune response (18). As an anti-inflammatory, it inhibits the synthesis of prostaglandins and helps the immune system by promoting phagocytosis and stimulating cellular immunity (27). Periodontal propolis has been utilized in various presentations, such as patches that continuously release propolis into the affected gingiva, promoting tissue recovery (28). As an irrigator prior to periodontal treatment, good results were observed, and these results were even noted in the treatment of the herpes simplex virus in a solution presentation, slowing the progression of skin changes at the beginning of the disease (26).
Chronic periodontitis (CP) is the most common periodontal disease. It is defined as an infectious inflammation that compromises the supporting tissues of the tooth, causing loss of insertion and alveolar bone. Its asymptomatic behavior prevents the disease from being diagnosed in early stages; thus, the clinical manifestations are more severe (29). It is initiated by bacteria present in the biofilm; however, the specific immune response and inflammation play an important role in the development of the disease (30). The assessment of periodontal status should be performed by a clinical evaluation of the inflammation of periodontal tissues (31). To assess these clinical parameters, an index system with several scores was designed, including the gingival index (GI) (32), the dental plaque index (PI) (33), bleeding after probing (BP) (34), and the periodontal index (PI) (35). The treatment for CP is undoubtedly scaling and root planing (SRP), since it allows for the elimination of microorganisms that give rise to inflammation and tissue damage. However, it has been observed that adjuvant treatment with antiseptics permits better results in the clinical and microbiological parameters of the patients with this condition without neglecting bacterial resistance, which is a worldwide problem and in which the majority of CP pathogens present genes for resistance to the most widely employed antibiotics (36,37). The most widely used adjuvant treatment in patients with periodontitis is Chlorhexidine but, in recent years, propolis has been investigated to a greater extent in presentations such as gel and toothpaste as natural products, which offer even better results than Chlorhexidine (38,39,40). Therefore, the aim of this study was to determine the effect of propolis on non-surgical periodontal therapy in patients with CP as it appears in the recent literature as an adjuvant for the improvement of its clinical parameters.
Materials and methods
The PRISMA Statement checklist for preferred reporting items for systematic reviews and meta-analyzes (41) was used to conduct this systematic review. This review was registered in the International Prospective Register of Systemic Reviews in Health and Social Care (PROSPERO) under protocol number CRD42020191473.
Search strategy and study selection
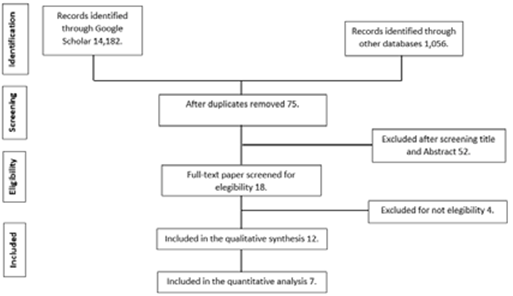
An extensive search of the literature was conducted using the electronic databases in PubMed, Google Scholar, Web of Science, and Science Direct. The keyword strings employed were the following: “Propolis AND periodontal disease”; “Propolis AND red complex”; “Propolis AND periodontal surgery", and "Propolis AND scaling and root planing", finding a total of 15,238 articles relating to the subject. All of the articles included in this review were based on the following criteria: (1) time interval ranging from 2013-2021; (2) randomized clinical trials (ASE), with cohort or cases and controls were considered; (3) patients considered had chronic periodontitis diagnosed with an intraoral examination, with nonadjacent teeth with periodontal bag; (4) propolis management in any presentation; (5) comparison with any other antimicrobial agent, and (6) response, including improvement in clinical and microbiological parameters. Then, the full texts of the possible eligible articles were evaluated considering the following exclusion criteria: (1) patients with gingivitis; (2) in-vitro studies; (3) in-vivo studies (mouse, rat, rabbit, dog, etc.); (4) patients requiring endoperiodontal treatment; and (5) patients with peri-implantation disease. Duplicate revisions were deleted in the different databases; thus, a total of 75 articles were obtained, which were analyzed by title and abstract, their being useful in only 18 bibliographies. Of these, four were discarded due to their failing the eligibility criteria (one on gingivitis, another with a focus on immunodetection, and two on plaque control). In the final analysis, 12 articles were evaluated for qualitative data collection and seven for quantitative data collection (Figure 1).
Results
According to the bibliographic review, seven articles related to the use of propolis as an adjuvant of periodontal treatment were compared in study subjects with chronic periodontitis with an approximate age ranging from 25-63 years; the variables are described in Table 1. Some authors describe their methodology by dividing their study population into three groups: a control group; a comparative group, and a group without adjuvant treatment. Gebara et al. considered 20 patients for their study, with three non-adjacent teeth with periodontal pocket. Each of these sites was assigned to a group as follows; group A irrigated with hydroalcoholic extract of propolis twice weekly for 2 weeks; group B irrigated with placebo in the same manner as the first group, and group C, which did not include additional treatment. The analysis of clinical and microbiological parameters was analyzed after 6 months, yielding a promising and more effective result in the group irrigated with propolis (42). Coutinho et al. included the same number of patients with the same characteristics and divided them into the same three groups; analysis of clinical and microbiological parameters was performed after 2 months with positive results in the propolis group (43). Shalaby et al. evaluated 45 subjects with stage II or stage III periodontitis with grade B, who were included in the study, according to the 2017 World Workshop Classification of Periodontal Disease (44). Each subject was randomly assigned to a study group as follows: group 1 with 15 subjects who only received SRP; group 2 with 15 subjects who received SRP accompanied by daily dietary supplementation with omega 3 for 6 months, and group 3 with 15 patients who received SRP with daily propolis supplementation for 6 months. Statistical analysis revealed that both propolis and omega 3 are significant and that they decrease periodontal disease and improve the level of clinical insertion at 6 months (45). Therefore, Shalaby et al. suggest dietary supplementation with propolis or omega 3 as an adjuvant of periodontal therapy (46). Sanghani et al. analyzed 20 patients with a minimum of two deep periodontal pockets. Sites were randomly assigned to the control group who received only SRP, and to the test group who received SRP and propolis. Clinical parameters were evaluated, and subgingival plaque samples were taken on 15 days per month. These results revealed better clinical and microbiological results at sites treated with propolis (47). Kumar et al. also analyzed 40 patients in two groups with CP, separated into group A and group B; the first group used toothpaste with aloe vera, and the second used toothpaste with propolis, with clinical and microbiological parameters by polymerase chain reaction (PCR). At the beginning of the study and at the end of 3 months, the results were statistically significant for propolis both on plaque and in clinical and microbiological parameters (39). El-Sharkawy et al. studied a population with chronic periodontitis and type 2 diabetes mellitus (DM2), for 6 months. The analysis was divided into the placebo group and the propolis group; these patients were administered SRP and a 400-mg tablet according to the group to which they belonged. The results included changes in hemoglobin and in fasting plasma glucose and, as secondary parameters, a decrease in periodontal clinical parameters. The results render it noteworthy that a treatment for 6 months with a daily dose of 400 mg of propolis improves hemoglobin and glucose levels in plasma, as well as improving clinical parameters in chronic periodontitis in this type of patient (48). De Andrade et al. used two groups for the analysis of a 20% propolis hydroalcoholic extract as an adjuvant to periodontal treatment, the two groups denominated the control group and the test group. There were 14 patients in the test group, in whom 65 teeth received SRP and irrigation with propolis extract. In the and finally in the control group, there were 52 teeth that received SRP and a saline irrigation. Clinical parameters were analyzed on days 0, 45, 75, and 90. The results indicated that the use of propolis as an adjuvant of periodontal treatment is more effective than the use of water-saline irrigation, placebo, and omega 3 (49). The clinical parameters discussed in the previously mentioned articles are summarized in Table 2.
Table 1 Study design.
| Author (Reference) | Extract type | Groups | Sample size | Control group | Age (range) | Dose presentation | Time of the study | P value |
|---|---|---|---|---|---|---|---|---|
| Gebara (34) | Hydroalcoholic | 3 | 20 | Placebo | 25-57 | Solution | 6 months | <0.05 |
| Coutinho (24) | Hydroalcoholic 20% | 3 | 20 | Ethanol 14% | 25-57 | Solution | 2 months | - |
| Sanghani (31) | LDD | 2 | 20 | NS | 25-50 | Local agent | 1 month | <0.01 |
| Kumar (25) | - | 2 | 40 | Aloe Vera | 35-55 | Toothpaste | 3 months | - |
| El-Sharkawy (23) | - | 2 | 50 | Placebo | 38-63 | Tablet | 6 months | - |
| De Andrade (26) | Hydroalcoholic 20% | 2 | 18 | Saline Solution | ≥ 30 | Solution | 6 months | - |
| Shalaby (30) | - | 3 | 45 | Omega 3 | 35-55 | Capsule | 6 months | <0.01 |
NS=not shown.
Table 2 Clinical parameters in propolis treatment.
| Author (Reference) | PI (Baseline) | PI (Outcome) | GI (Baseline) | GI (Outcome) | BP (Baseline) | BP (Outcome) | PD (Baseline) | PD (Outcome) | CAL (Baseline) | CAL (Outcome) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gebara (34) | - | - | - | - | 57% | 43% | 93% | 64% | - | - |
| Coutinho (24) | - | - | - | - | 0% | 70% | 0% | 80% | - | - |
| Sanghani (31) | - | - | 2.04 ± 0.26 | 0.96 ± 0.09 | 2.99 ± 0.32 | 1.08 ± 0.23 | 5.35 ± 0.67 | 3.60 ± 0.68 | 3.35 ± 0.67 | 1.60 ± 0.68 |
| Kumar (25) | 1.87 ± 0.48 | 1.29 ± 0.27 | 3.08 ± 0.63 | 1.95 ± 0.23 | 2.83 ± 0.35 | 1.26 ± 0.25 | 5.57 ± 0.82 | 3.63 ± 0.67 | 4,57 ± 0,94 | 3.00 ± 0.95 |
| El-Sharkawy (23) | - | - | - | - | - | - | 6.60% | 1.90% | 4.50% | 7.80% |
| De Andrade (26) | 1.50 ± 0.94 | 0.95 ± 0.80 | 0.94 ± 0.84 | 1.10 ± 0.93 | - | - | 5,75 ± 1,17 | 5.63 ± 0.84 | - | - |
| Shalaby (30) | 2.00 ± 0.46 | 0.63 ± 0.25 | - | - | 1.98 ± 0.55 | 0.53 ± 0.26 | 5,67 ± 0,68 | 3,01 ± 1,01 | 20 ± 0.93 | 2.86 ± 0.68 |
PI=Periodontal Index; GI=Gingival Index; BP=Bleeding on Probing; PD=Periodontal Depth; CAL=Clinical Attachment Level.
Discussion
Current scientific evidence shows us that propolis is a good adjuvant in the non-pharmacological therapy of chronic periodontitis (CP). In the present review, seven clinical trials were analyzed; in all of these, the patients presented a diagnosis of chronic periodontitis (CP), in which the main clinical characteristic was periodontal pockets with a depth of ≥5mm. In the majority of studies, the experimental process was carried out during a period of 6 months, which is the time period stipulated, according to the scientific literature, during which there are better results after clinical treatment with adjuvants in CP (50). The presentation of the experimental agent was used locally as an irrigator (38,40,51), such as toothpaste (39), and the former was also tested as an oral systemic agent (tablets/capsules), exhibiting promising results in both patients with DM2 and in those with CP (37) and in patients without systemic alterations (45). Several authors in this review reported that the propolis extract utilized in their experiments was the hydroalcoholic extract at 20% (40,42,43), since it has been observed that this is the best method for extracting the highest amount of polyphenols, in that these compounds are those that confer antimicrobial properties on the propolis (52). However, authors such as Shalaby et al. and El-Sharkawy et al., who employed propolis in tablets as a vehicle, did not report what type of extract was used for its preparation (45,48). The latter is a disadvantage because it is not known whether the effect is due to the hydroalcoholic preparation or to the phenols of propolis. Similarly, Sanghani et al. and Kumar et al. did not report the methodology they utilized in the realization of their irrigation agent and toothpaste, which does not allow for a better analysis of its results (39,47). The age range used by the authors in this research was very similar, in that they included adults aged approximately 25-60 years. Researchers who employed two groups (control group and experimental group) included Sanghani et al., who worked with a total of 20 patients with a minimum of two periodontal pockets administered the SRP treatment in a periodontal pocket, and in the other, SRP and propolis as a local adjuvant, reporting the majority of the clinical parameters except for the dental plaque index (PI). This latter treatment was more favorable than that without the adjuvant (47). De Andrade et al. worked with 16 individuals; a certain number of teeth in the treatment group were treated with SRP in addition to propolis irrigation, while another number of teeth were treated in the control group with SRP in addition to saline irrigation. The authors found better results in the experimental group treated with propolis. However, the authors did not report the majority of the clinical parameters, except for bleeding after probing (BP) and clinical attachment level (CAL); thus, the conclusions of their study cannot be decisive in determining whether propolis could be an effective treatment, or an even better one, than that of the control group (40). The most recent study (Shalaby et al.) was one of the most complete reports in terms of clinical parameters, in which only the gingival index (GI) was not reported, however, this analysis concludes that both omega and propolis are adequately significant for the decrease of chronic periodontis (CP) (43). The clinical parameters reported in Table 2 demonstrate improvements with the use of propolis in all patients regardless of the control group used, inferring that the physicochemical characteristics of propolis have a favorable effect on CP. In the report by Gebara et al., the authors mention a 14% decrease in periodontal bleeding as a result; they also report a 29% decrease in the periodontal pocket and a decrease of Porphyromonas gingivalis and yeast colonies (42). These decreases in clinical parameters led us to suppose that adjuvant treatment with propolis induces changes in the pathophysiology of periodontal disease, in that the reductions are quite significant. In the same way, Coutinho et al. found similar results with the propolis extract as an adjuvant to periodontal treatment; this was more effective than SRP according to the evaluation of clinical and microbiological parameters (43). In another article (Sharkawy et al.), it was evidenced that there were improvements in treatment with propolis in individuals with DM2 and with chronic periodontitis, obtaining satisfactory results in both hemoglobin levels and glycemic control, as well as in the depth of the periodontal pocket, with a reduction of 4.7% and an increase in CAL of 3.3% (48). The studies reviewed in this work demonstrated that the study of propolis as an adjunct in periodontal disease is currently in progress. Propolis as an organic compound does not present cytotoxicity in bone marrow mesenchymal stromal cells (BMMSC) (53), tumor cell lines (54), and human dermal fibroblasts (HFFF2) (55), among others. However, none of the reviewed articles reported conducting tests for cytotoxicity, which would be important to perform specifically in oral cell lines. The search for an ideal vehicle for propolis continues to comprise one of the main issues in this regard since, in the oral cavity, this presentation must be suitable for the application of propolis in the oral tissues.
Conclusions
The previously mentioned articles conclude that propolis is a good adjuvant for the treatment of patients with chronic periodontitis compared to the conventional treatment (SRP), saline solution, aloe vera, and even with an effect such as that of Chlorhexidine, according to some other studies. However, all of these articles determined that further studies related to the doses of propolis are needed for antimicrobial action. Similarly, the addition is suggestive of the cytotoxicity analysis of propolis in cells of the gingival mucosa. The great difficulty in the use of propolis in general medicine and dentistry lies in the difference of the components that this antimicrobial action yields depending on geographical location, harvesting season, and method of extraction. Therefore, it is important to investigate a standardization method to determine a universal dose in the treatment of, for example, chronic periodontitis.
Author contribution statement
Conceptualization and Design: I.A.F., C.G and C.B. Literature Review: I.A.F and C.B.
Methodology and Validation: L.A, C.G. and C.B. Formal Analysis: I.A.F, C.G., L.A. and C.B.
Investigation and Data Collection: I.A.F and C.B. Resources: L.A. and C.B.
Data Analysis and Interpretation: I.A.F. and C.B. Writing-Original Draft Preparation: I.A.F.
Writing-Review & Editing: I.A.F., C.G., O.C., L.A and C.B.
Supervision: C.G., O.C., V.E., L.A and C.B. Project Administration: C.G., L.A. and C.B. Funding Acquisition: C.G., L.A. and C.B.