Introduction
In today's clinical dentistry, composite resin materials are popular because of their aesthetical and mechanical properties. However, the clinical success of a restorative material should not be evaluated solely by these features. The material's biological reliability and biocompatibility with dental tissues should also be considered. The biocompatibility of a restorative material is predominantly determined by the amount of substances released due to incomplete polymerization and/or resin degradation over time, and cytotoxic effects are caused by these substances (1,2,3).
No matter how big and deep cavities are, only a small amount of composite resin material is used for restorations. As a result, the material may expire before all of it is used. The expiration date of a dental material indicates the duration of time (beginning from the date of manufacturing) over which a material retains the mechanical, aesthetical, optical, and physical features required to achieve its intended purpose (4). In vitro studies have shown that, even when the expiration date is not exceeded, components (i.e., monomers, photoinitiators, and fillers) added to the structure of the composite resins to improve the properties of the materials can cause cytotoxic effects (5,6). In clinical practice, the changes in the material's properties over time may not be conspicuous, but these changes may have an effect on the longevity, success, and biocompatibility of the restoration (7,8). However, insufficient data are available on the biocompatibility of expired composite resin materials. Although usage of expired restorative materials is not strictly recommended, some clinicians may use these materials due to their expense, ignoring the unpredictable results after usage. Therefore, evaluating the biocompatibility of expired composite resin materials and comparing the data with non-expired materials are crucial. Since the biological compatibility of the material after the expiration date is unknown, such compatibility should be investigated. This information is important because it may allow for extending the recommended shelf life of these expensive restorative materials (9).
Therefore, the aim of this in vitro cell culture study was to investigate the cytotoxicity of expired (six months) and non-expired current composite resin materials on L-929 mouse fibroblast cells using direct contact cell culture method with the sulforhodamine B (SRB) test. The null hypothesis was that expired composite resin materials would be cytotoxic on cells.
Materials and methods
In this in vitro study, expired (six months) and non-expired composite resin materials were used (Tetric N-Ceram Bulk-Fill (IVA) Ivoclar Vivadent (TNB), Tetric N-Ceram (A1) Ivoclar Vivadent (TN), and Clearfil Majesty ES-2 (A1 Dentin; CM) (Table 1).
Cell cultures
In the study, L-929 mouse fibroblast cell line (NCTC clone 929 (ATCC® CCL1™), USA) was used. Cells were cultured in Eagle's minimum essential medium (EMEM; Sigma-Aldrich, Germany) containing 10% horse serum (Merck, Germany) and 1% penicillin/streptomycin (Sigma-Aldrich, Germany). In each well of the 24-well plates, 2x104 cells were seeded with 500 μl of the medium. Then, the cells were incubated at 37°C with 5% CO2 and 95% air mixture for 24 hours.
Sample preparation
Plastic rings (2 mm depth x 5 mm diameter) that had previously been sterilized twice were filled with non-polymerized composite resin materials. Later, the medium covering the cells was removed, and the rings were placed on direct contact with the fibroblast cells that were cultured one day prior the experiment. Then the rings were polymerized with an LED light curing unit according to the manufacturer's recommendations (Valo, Ultradent, USA) (wavelength range of 385-515 nm, power irradiance: 1000 mW/cm2). Polymerization was carried out directly under the cell culture conditions.
After polymerization, the removed medium was added to the cells in each well. Two groups were used as the positive control: (1) the cells that were left without medium during the preparation of the experimental groups (WOM) and (2) the cells that were exposed to the light curing unit during polymerization of the experimental groups (LCU). Cells without any treatment were used as the negative control group (C). Three samples were prepared for each group (n=3) (10). And all samples were prepared by the same operator. Cells were incubated with the tested materials for 7-days to evaluate the cytotoxic effects.
Determination of cell viability by sulforhodamine B Test
Cell viability was calculated using the SRB (Sigma-Aldrich, Germany) test (11). In an SRB assay, a bright-pink aminoxanthene dye forms an electrostatic complex with the amino acid residue of proteins in slightly acidic conditions; thus, the assay measures the total biomass of the cells. SRB has been widely used to determine the toxicity of drugs and different materials on cancerous and non-cancerous cells (12,13).
At the end of seventh day, the cells were fixed adding 500 µl/well of cold 1% trichloro- acetic acid (TCA; Sigma-Aldrich, Germany). They were then incubated for 30 minutes at 4ºC. Then, the supernatants were disregarded, and the plates were washed with water and dried at room temperature. The cells were stained with 500 µl/ well of 5% SRB in 1% acetic acid and incubated for 30 minutes at room temperature.
After incubation, the unbonded dye was washed with 1% acetic acid five times, and the plates were then air-dried. The bonded dye was solubilized with 200 µl/well of Tris buffer (pH=10) for 10 minutes, and the absorbance was measured at 515 nm using a microplate reader (Varioscan, Thermo Scientific, USA). Percentage of viability was calculated according to absorbance values, and the viability versus concentration graph was plotted (11). The cytotoxicity of each sample was compared to the reference value represented by the cells of the control group (100%).
Statistical analyses
The cell viability was calculated as a percentage (%). The data were statistically analyzed with one-way ANOVA via post-hoc Tukey tests. A p-value <0.05 was considered significant.
Results
Comparison between the expired (E) and non-expired (NE) groups of the same composite resin materials did not result in statistically significant differences (p>0.05), except for the comparison between TN NE and TN E (p<0.05). TN E (46.33%) was significantly more cytotoxic tan TN NE (67.29%). The toxicity of TNB E (53.87%) was less than that of TNB NE (44.36%), although in a statistically insignificant manner (p>0.05) (Figure 1).
When the NE composite resin (TNB NE, TN NE and CM NE) groups were compared to each other, a statistically significant difference was found between the TNB NE and TN NE groups (p<0.05). No significant differences were observed between TNB NE and CM NE or TN NE and CM NE groups (p>0.05). Among all tested groups, TN NE showed the least cytotoxic profile (67.29%). When the E (TNB E, TN E and CM E) composite resin groups were compared to each other, no statistically significant differences were determined (p>0.05) (Figure 1).
All experimental groups showed a statistically significant cytotoxicity (p<0.05) when compared with the negative control group (C). A statistically significant difference was found between the TNB NE group and WOM, LCU, and C groups (p<0.05). When the positive control groups (WOM and LCU) and negative control group (C) were compared to each other, a significant difference was shown between the LCU and C groups (p<0.05). No significant difference was observed between the WOM and LCU groups or WOM and C groups (p>0.05) (Figure 1).
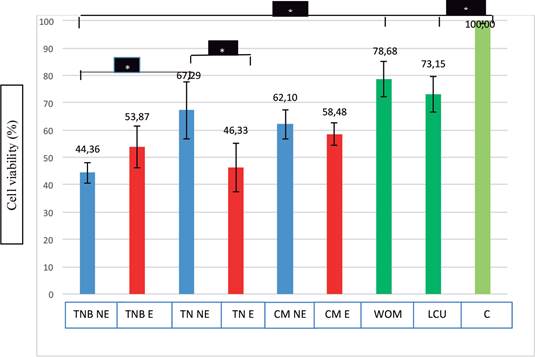
TNB NE: Tetric N-Ceram Bulk-Fill Non-Expired, TNB E: Tetric N-Ceram Bulk-Fill Expired, TN NE: Tetric N-Ceram Non-Expired, TN E: Tetric N-Ceram Expired, CM NE: Clearfil Majesty ES-2 Non- Expired, CM E: Clearfil Majesty ES-2 Expired, WOM: Without Medium, LCU: Light Curing Unit, C: Control
Table 1 The tested composite resin materials and the composition of each material.
| Product name Shade Abbreviations | Material type | Composition of materials | Manufacturer |
|---|---|---|---|
| Tetric N-Ceram Bulk-Fill (IVA) (TNB) | Bulk-fill (hybrid) composite resin | Bis-GMA, Bis-EMA, UDMA, Camphorquinone (468 nm) and Ivocerin (408 nm) Barium aluminium silicate glass filler, Ytterbium trifluoride, spherical mixed oxides, prepolymers | Ivoclar Vivadent AG, Schaan, Liechtenstein |
| Tetric N-Ceram (A1) (TN) | Nanohybrid composite resin | Bis-GMA, Bis-EMA, UDMA, Camphorquinone (468 nm) Barium glass, ytterbium trifluoride, oxides, prepolymers | Ivoclar Vivadent AG, Schaan, Liechtenstein |
| Clearfil Majesty ES-2 (A1 Dentin) (CM) | Nanohybrid composite resin | Bis-GMA, hydrophobic aromatic dimethacrylate, Camphorquinone (468 nm) Silanated barium glass filler, prepolymerized organic filler | Kuraray, Tokyo, Japan |
Bis-GMA: Bisphenol A glycidylmethacrylate.
Bis-EMA: Ethoxylated bisphenol A dimethacrylate. UDMA: Urethane dimetacrylate.
Discussion
In the present in vitro research, the cytotoxicity of expired (E) and non-expired (NE) composite resin materials on L-929 mouse fibroblast cells was evaluated using the SRB test. According to the results; the cell viability of the expired composite resin materials was as follows: TNB E: 53,87%, TN E: 46,33%, and CM E: 58,48%. This means that all of the expired composite resin materials were found cytotoxic on cells. Because according to ISO 10993-5, when a material has cell viability lower than 70%, it is accepted that this material has a cytotoxic potential (14). Therefore; the null hypothesis was accepted.
Composite types and application thecknesses
Resin-based composites are widely used in restorative dentistry and nano-composites are the latest type of resin-based composites. Nano-hybrid composite resins are used as universal restorative materials in a wide variety of cases. For these composite resin materials, an incremental layering technique is considered the golden standard for restoring a cavity preparation exceeding 2 mm. This layering technique helps reduce polymerization shrinkage and shrinkage stress, achieving adequate depth of cure as well as reducing elution of (co) monomers and additives (15). Additionally, this technique minimizes gap formation, achieving sufficient bonding of composite material to tooth structures and ensuring complete polymerization of composite resin material (16,17).
However, due to developments in polymer chemistry, the layering technique is no longer the only option for restorations. A new composite type-bulk-fill composites-has been introduced to clinicians; it can be applied up to a thickness of 4 mm in a single step. This type of material shows less polymerization shrinkage and shrinkage stress than conventional composite resins and also lacks negative effects on conversion degree (18). Polymerization of the bulk-fill composite material happens due to the special monomers and photoinitiators in the structure as well as the high light transmission properties of the material (19). Therefore, in this in vitro study, a novel bulk-fill and two recent nano-hybrid composite resin materials were evaluated.
Although manufacturers recommend using bulk-fill materials at a cavity preparation Depth ≥4 mm, clinicians can apply this material in different thicknesses (1, 2, or 3 mm), since a 4-mm thickness is not an obligation or a requirement. On the other hand, applying a conventional composite resin material with a thickness ≤2 mm is both an obligation and a requirement. Therefore, as an ideal-case situation, the same layer thickness -2 mm- was used for both bulk-fill and composite resin materials in this study. Thus, standard test conditions could be established, and the biocompatibility of the materials could be compared under equal conditions.
Biocompatibility
Biocompatibility is of primary importance when choosing a restorative material, since the material may cause local and/or systemic side effects during contact or interaction with oral tissues (20). When composite resin materials (non- expired) were evaluated, a correlation was found between the materials' released substances and cytotoxicity (21, 22). In addition, non-viable cells have been previously detected around non-expired composite resin sample discs (23,24,25). Therefore, in this in vitro study, the impact of cell exposure to expired composite resins was investigated, since cell death occurs even with exposure to non- expired composite resins.
This in vitro study was conducted for two reasons. First, some clinicians believe that expired (up to six months) composite resin materials can be used in clinical practice, even though their use is not recommended. They believe these materials still maintain their mechanical, physical, optical, aesthetical, and biological properties when the expiration date does not exceed more than six months. Second, the expense of the composite resin materials encourages clinicians to take the risk of using these materials. Therefore, this in vitro study was designed to exhibit the cytotoxicity of expired composite resin materials and to compare the results with those of non-expired ones.
Cytotoxicity and cell viability
A wide variety of methods have been used to evaluate the cytotoxicity of restorative materials. One such method, cell culture systems, provides convenient, controllable, and repeatable data for the initial evaluation of cytotoxic responses to materials (26). This study utilized an in vitro cell culture test method, in which an experimental system artificially reproduces the environmental conditions necessary to guarantee the viability of cells or tissues harvested from a living organism (27). Cytotoxicity is tested using a direct method that simulates clinical conditions and ensures that the material’s specimens are in direct contact with the cells in a biological solution (i.e., culture medium) (28).
Cell viability is a cytotoxicity detection method that provides information about the biocompatibility of a material. The SRB assay is a common and efficient method for detecting cell viability. Additionally, this assay is one of the modern colorimetric cell-based assays. The amount of SRB dye extracted from the stained cells is directly proportional to the total protein mass and, therefore, correlates with living cell numbers (29,30,31).
Fibroblast cell line
Continuous cell lines, such as 3T3 and L-929 mouse fibroblast, are commonly utilized for in vitro tests (28,32,33). In this in vitro study, L-929 mouse fibroblast cell line was preferred because this cell type is the most common in dental pulp, which is the target of chemical components released from resin-based composites (34). Furthermore, fibroblasts are ISO-approved cells, and they have been used for many years in routine biocompatibility studies (14).
Discussion of statistical significances
Different types of composite resin materials are widely used in clinical practice (35). In most cases, these restorative materials may come in direct contact with the soft and/or hard dental tissues for a prolonged period of exposure time and might affect the surrounding tissues. Hence, the biocompatibility of these materials due to various monomers, additives, and photoinitiators in their composition is crucial. In this study, when the expired (E) and non-expired (NE) groups of the same composite resin materials were compared to each other, a statistically significant difference was only observed between TN NE and TN E groups (p<0.05). TN E group was significantly more cytotoxic than the TN NE group.
Under normal conditions, lower cell viability was expected for the E composite resin groups than NE groups. While TN E (46.33%) and CM E (58.48%) groups confirmed this expectation (differences in CM groups were statistically insignificant), an unexpected result was obtained for TNB E group. The cell viability of the TNB E group (53.87%) was more than that of TNB NE group (44.36%), although in a statistically insignificant manner. It can be assumed that toxic (co)monomers and/ or additives released from TNB were separated from the structure before the expiration date, and their effects were lost over time, or their release rates decreased over time. Therefore, TNB group showed an increase in the number of viable cells after 6-months.
As mentioned above, bulk-fill composite resins are a new type of restorative material that allow up to 4-mm thickness to be cured in a single step, thereby skipping the time-consuming layering technique. Bulk-fill composite resins are capable of a higher depth of cure due to more potent initiator system used in their structure and/ or the higher translucency of the material (36,37). In this study, when NE composite resin groups were compared to each other, a statistically significant difference was found between TNB and TN groups (p<0.05). TN NE group exhibited higher cell viability (67.29%) than TNB NE group (44.36%). The fact is that both materials are structurally similar to each other in terms of monomers and fillers. The only difference between these two materials is Ivocerin, a new photoinitiator that is incorporated into the structure of TNB. Ivocerin is a dibenzoyl- germanium compound that allows for the afore- mentioned larger increments of application and curing (38). Despite this positive feature, organic germanium compounds are often characterized as slightly toxic (very low) (38). Therefore, the lower cell viability of the TNB NE group may have been related to Ivocerin. The insignificant differences between TNB NE and CM NE as well as those between TN NE and CM NE could have been due to the common organic monomer (Bis-GMA) and the organic filler (barium glass).
The thickness of TNB material used in the present study was also important. Although TNB NE was prepared at the half recommended thickness (2 mm), statistically significant and least cell viability was observed in this material when compared with WOM, LCU, and C groups (p<0.05). Even at the half recommended thickness, the bulk- fill material showed cytotoxicity on the cells.
According to ISO 10993-5, cell viability lower than 70% indicates that the material has cytotoxic potential (14). In the present study, all E and NE groups showed a statistically significant cytotoxicity (p<0.05) when compared with the negative control group (C). Therefore, all tested groups exhibited less than 70% cell viability. However, among all groups, TN NE showed the least cytotoxic profile (67.29%), while CM NE showed the second-least cytotoxic profile (62.10%). When E composite resin groups were compared to each other, they showed similar cell viability (toxicity), which were statistically insignificant (p>0.05). Based on these results, the tested materials could be considered as producing similar toxic responses to cells after the expiration date.
Led light curing unit
A conventional quartz-tungsten-halogen (QTH) lamp with a power irradiance of 400-800 mW/cm2 was the most commonly used light source for polymerization of resin composites. However, heat generation was a major disadvantage for QTH light devices (39). Recently, LED light curing units with higher power irradiances (energy output) than previous generations have been introduced to clinicians. Although LED lights are expected to produce minimal heat during polymerization of a composite resin (40,41), they usually produce a high intensity of irradiation that can endanger pulpal health. An increase in irradiance seems to correlate with increased temperature (42). Additionally, patients’ complaints about light- curing procedures have been reported, including experiences of “burning” sensations in teeth and in oral tissue (43).
In the present study, a LED light curing unit with a power irradiance of 1000 mW/cm2 (Valo, Ultradent, USA) was used for the experimental and control groups. The cells exposed to the light curing unit during polymerization of the experimental groups (LCU) showed a significant decrease in viability (73.15%) when compared with the C group (100%). Therefore, the LED light curing unit used in the present study may have caused cell death due to the increased temperature resulting in high power irradiance.
This study was an in vitro preliminary research. Cause preliminary researches contain information that needs to be verified. This kind of researches are usually used to get an idea about a particular topic and to discover the amount of information that is available on the topic. Therefore, what the authors would like to discover was the cytotoxic effects of the expired composite resin materials. As a result, the data obtained from this study is to verify that these materials should not be used even for temporary purposes in the clinic and if they are going to be used they will exhibit serious cytotoxic effects. So further studies are still needed to evaluate the biocompatibility of such kind of materials.
Conclusion
In the oral environment, dentin tissue behaves like a barrier between the substances released from the resin-based material and the pulp tissue. Additionally, the saliva buffering system and other protective systems are capable of resisting the cytotoxic effects of resin (co)monomers, photoinitiators, and/or additives. Therefore, within the limitations of the present research, the following conclusions may be drawn:
In clinical practice, expired composite resin materials should never be used.
Although a correlation was determined between the expiration date of nano-hybrid composite resin materials and cell viability, opposite data were obtained for the bulk-fill composite resin material. Therefore; studies are still required to evaluate the biocompatibility of bulk-fill composite resin materials at various thicknesses with current light curing units.














