Introduction tl the microbiota, a flourishing research topic
Oral and general health influence each other. Recent scientific work shows that oral diseases can impact general health, and vice-versa. The idea that oral health can impact general health conditions is not new. For instance, Hypocrates recommended tooth extraction to cure arthritis, and Miller in 1890 suggested to remove tooth decay to treat general diseases (1). However, the biological mechanisms hypothesized that could explain these links remain misunderstood. Some evidence suggests that the oral microbiome and microbiota could be an understudied pathway explaining how oral health can impact and modify systemic diseases. Exploring the impact of microbiomes on general health could contribute to better conceptualize the potential associations between the overall microbiome composition and systemic conditions.
The aim of this review is to discuss current evidence explaining the potential mechanisms playing a role in the associations between the oral and general systemic health. The hypothesis is that the oral microbiome could be a missing link that can partially explain the influences of oral diseases on general health. The final objective is to present recent scientific evidence to update the general knowledge in this topic of professionals in dentistry. We will introduce the conceptual bases, describe the main methods used in microbiology to characterize the oral organisms, summarize the main evidence of a biological plausibility linking oral microbiome and systemic diseases, and conclude with some future research recommendations.
Concepts and importance to the human host
Both “Microbiota” and “microbiome” are expressions frequently used on scientific papers. A quick search on PubMed, arguably one of the most consulted search engines for bibliographic information, shows an exponential growth in biomedical literature using these appellations. From less than 100 papers in 2000, the number of hits rocketed to near 11.000 articles in 2018 (Figure 1).
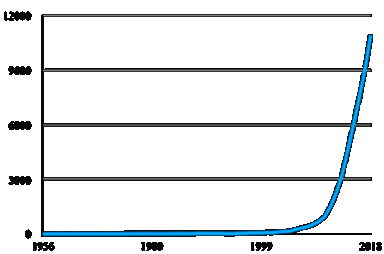
Figure 1 Number of papers per year (from 1956 to 2018) retrieved on Pubmed using either “microbiota” or “microbiome” as search terms.
Usually used as synonyms, both terms represent different but related concepts. On the one hand, “microbiota” refers to the group of beneficial and/or detrimental microorganisms that live on one host, so it is related to its composition and taxonomy. On the other hand, “microbiome” is all the microbiota genomic material, a so-called “metagenome”, a functional term including the microbiota’s physiology and metabolism (2,3,4). A microbiota is composed of diverse prokaryotic species (Bacteria and Archaea) as well as fungi, protozoa and virus. By far, the most studied component of microbiota is the bacterial one, termed “bacteriota/bacteriome” but most times, in an oversimplification, used interchangeably with “microbiota/microbiome” (5,6).
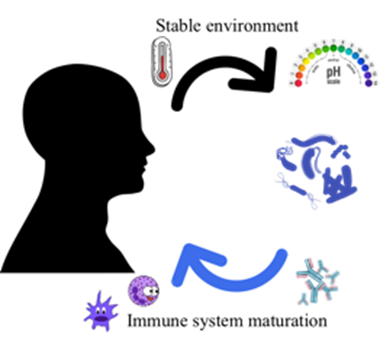
Humans have co-evolved with bacteria in a symbiotic relationship. Previously believed to be outnumbered 1 to 10, more recent estimations of the number of human cells (3·10¹³) and bacterial cells within us (4·10¹³) propose that the human “superorganism” or “holobiont” is composed of roughly the same amounts of eukaryotic and prokaryotic cells (4,7,8,9,10). In this close partnership, the host provides a more stable environment (e.g. temperature, pH and nutritional sources) while the microbiota play important roles in vitamin production and mucosal immune maturation (Figure 2), as well as other functions such as acting as a barrier to exogenous colonization or modulate the immune response e.g. downregulating unwanted proinflammatory responses (4,8,9).
Study methods and the human microbiome project
Methods to study the microbiota are classified as culture independent and culture-based. Historically, isolation-dependent methodologies (e.g. microscopy, biochemical tests or growth conditions) were used (11,12). With the advent of molecular biology, new technologies emerged such as DNA microarrays and PCR-based methods (13). In the early 1990s, the use of the evolutionary conserved 16S rDNA sequences to study prokaryotic diversity widened our knowledge about the richness of microbiota (14). However, the revolutionary methodology that exponentially expanded our understanding in this topic was the rise in sequencing capacities called massively parallel sequencing or next-generation sequencing (NGS) and the use, as mentioned, of the 16S rRNA gene (11,13,14). Finally, a new technique, sequencing the entire DNA present in a sample, whole-genome shotgun metagenome sequencing, could be performed to study the microbiome (11). Conventional microbiology (culture-based), although deemed less advanced, complement culture independent methodologies (sequencing) by providing information on the microbes physiological and metabolic properties, deepening our understanding of microbiota and microbiomes (15,16).
In December 2007, the National Institutes of Health (NIH) launched the Human Microbiome Project (HMP) with the mission to characterize the healthy human microbiota, by identifying the most prevalent taxa in five body sites: nasal passages, oral cavity, skin, gastrointestinal tract and urogenital tract (17). The composition of microbiota was found to be “personalized” or site-specific, each sampling location containing its exclusive microbiota and metagenome (15,18,19). The most colonized sites were found to be the mouth, gut and vagina, while the most diverse bacterial populations were found in the gastrointestinal tract and the mouth (18).
Variation between microbiota is important but there is less variability in the microbiota of the same anatomic sites among different individuals than in microbiota from different locations on the same person. These variations introduced two concepts, first, the “core microbiota”: shared by all or a significant number of individuals and composed of predominant species at different body sites. Secondly, the “variable microbiota” is only shared by a subgroup of hosts and is believed to be the corollary of the evolution between host and microbiota, consequence of the host’s unique lifestyle as well as phenotypic and genotypic determinants (15). Importantly, scientists are now aware that the community composition does not necessarily reflect the active members of the community (20) and new methodologies to study genomic expression (metatranscriptomics) are being used (11).
Introduction to the oral microbiota
Of the studied microbiota, the mouth is one of the most heavily colonized (18) and the oral microbiota can be described as a group of diverse microbial biofilms (13). The oral cavity has the second most complex microbiota in the human body after the gut (10). It has the highest diversity (species richness and evenness or alpha-diversity) and, when comparing microbiotas from the same sites among individuals (beta diversity), the oral sites were the less diverse which means that oral communities shared more similar bacteria than communities in other body sites (21,22).
Moreover, the oral microbiota is considered more stable over time (5), i.e. suffering short-term alterations in composition after external stresses like antibiotic usage. This stability is called “resilience” and could be divided into two concepts: “resistance” which is the capability to cope with stress factors and “recovery” from perturbations. Overall, resilience is the capacity of a community to deal with perturbations without shifting to a perturbed state (23).
In recent years, the interest in characterizing the oral microbiome has grown arguing that perhaps dentists, microbiologists and other health professionals were missing an important element that can partially explain oral diseases and even the correlations found between oral and general health as the microbiota may represent potential biomarkers for early diagnosis and prognosis (24).
In this review, divided in two parts, the current evidence explaining the potential mechanisms playing a role in the associations between the oral and general systemic health will be discussed. This first part will focus in an introduction to the subject and specifically to the oral microbiota in order to understand its characteristics in a state of health. On the next part, the focus will be on prevalent oral diseases and their crosstalk with the oral microbiota and their impact on systemic health. The general hypothesis is that the oral microbiome could be a missing link that can partially explain the influences of oral diseases on general health.
The oral microbiota: what do we (don’t) know?
A GENERAL CHARACTERIZATION OF THE ORAL MICROBIOTA
Bacteria in the mouth are uncommon elsewhere, neither in the environment nor in other habitats of the human body (25). The oral community contains in total over 700 species that contribute to health (15,26). However, on average, any single person harbors from 100 to 200 species (9). The dynamic composition of the mouth microbiota could be the result of the oral cavity’s continuum with the external environment (15). As previously mentioned, oral bacteria communities are described as organized into biofilms (13,21,27).
As for any microbiota, the oral microbiota composition can be studied at diverse taxonomic resolution. The highest, most general, being the phylum up to the most specific, the strain, passing through the family, the genus or the species levels. The type of sequence data available (16S rDNA or whole genome), the bioinformatic analysis pipeline and the databases may limit the taxonomic resolution (28).
At the phylum level, 80-99,9% of the taxa of oral bacteria belong to only six phyla (21,22,23, 29). Less than 16 genera represent up to 88% of all bacteria in the oral cavity (21,25,29); other less frequent genera are also present but found in few numbers (15) (Table 1).
Table 1 The six major phyla and genera in the oral microbiota with some examples of less frequent genera.
| Major phyla | Major genera | Minor genera |
|---|---|---|
| Firmicutes | Streptococcus, Veillonella, Selenomonas, Gemella, Oribacterium | Granulicatella, Lactobacillus, Peptostreptococcus, Staphylococcus, Eubacterium |
| Actinobacteria | Actinomyces, Corynebacterium, Rothia | Propionibacterium |
| Bacteroidetes | Prevotella, Capnocytophaga, Porphyromonas | Bacteroides |
| Proteobacteria | Neisseria, Campylobacter, Haemophilus, Lautropia | Eikenella |
| Fusobacteria | Fusobacterium, Leptotrichia | Sneathia |
| Spirochaetes | Treponema | -- |
It should be noted that most studies of the oral microbiota composition are similar in two aspects: i) the dominance of the Firmicutes phylum and of Streptococcus at the genus level even if several individuals do not fit this paradigm; and ii) the presence of so-called periodontopathogens in healthy individuals e.g. Porphyromonas or Treponema (25). Furthermore, different bacterial groups can fulfill certain microbiota functions, a feature known as functional redundancy (23). As an example of functional redundancy beneficial to the host, some resident oral bacteria of very distant phylogenetic groups participate in the reduction of dietary nitrate to nitrite. These groups include, for example: Actinomyces, Rothia or Propionibacterium (Actinobacteria); Staphylococcus and Veillonella (Firmicutes); Prevotella (Bacteroidetes) and Neisseria and Haemophilus (Proteobacteria). The reduction of dietary nitrate helps reducing blood pressure and promotes vascular health (9,30).
Mouth specificities and oral health
The description of the microbiota associated to the whole oral cavity lacks precision since it is not a homogeneous environment. Moreover, several factors (Table 2) may impact the oral microbiota composition and metabolism such as host lifestyle and health status (31,32,33).
Table 2 Host factors influencing the oral microbiota either modulated or not intentionally modulated by the host.
| Intrinsic factors | Extrinsic factors | |
| Modulated çby the host | Adaptive psychosocial coping mechanisms with stressors | Diet, oral hygiene, tobacco intake, medication |
| Not intentionally modulated by the host | Host immune response, hormonal fluctuation, genetics, quality and quantity of saliva | Socioeconomic status, access to dental care, cultural conditions |
Different anatomic sites, although joined by saliva, constitute distinct habitats (28). There are considerable distinctions among the oral surfaces: the teeth as a hard nonshedding surface and the mucosa whose cells fall regularly and that is dived into keratinized, nonkeratinized and specialized types of mucosa such as the tongue dorsum with papillae (25,28). To these specificities could be added the crevicular epithelium and prosthodontics or orthodontic appliances (34). Each type of surface has a particular microbiota organized as a biofilm (28). Even within the same environment of teeth surface (either supragingival or subgingival), bacterial community composition varies considerably based on tooth location and site (35). Accordingly, each microhabitat maintains a unique ecosystem with distinct pH, oxygen partial pressure, salinity, water activity, temperature, redox potential or nutritional compositions, influencing the settling of microorganisms and creating a complex microbiota with symbiotic interactions among the various microbes within that ecosystem and the host (15,34).
A modulator of these cited physicochemical parameters is the difference of saliva flow over oral biofilms. Its velocity influences the rate at which small molecules are transported into and away from the biofilm (28). These oral biofilm communities constitute a dynamic metabolic network with interconnected metabolism that can undergo important and rapid changes in composition and physiology, shifting in time and location (27). A long-term balanced state (eubiosis) is therefore the resultant of all interactions, antagonistic and synergistic, which will determine the modulation of the oral microbiota, contributing to its composition and behavior (4,33). Therefore, the key to oral health seems to be an ecologically balanced and diverse microbiota that practices commensalism within itself and mutualism with its host (15).
In this dynamic oral environment, a resident bacteria are the ones that can colonize and its population can grow by adhering to the surfaces or to partners in the multi-species biofilm as well as occupying a specific niche into the structure in which chemotaxis can also be important. On the other hand, the transient microbiota is composed of bacteria that are just “passing through” and consequently are in low numbers. But being consistently in low-abundance does not mean that they are transient as they could act as resident keystone taxa (28). A keystone bacterium can shape the community with an influence out of proportion to its abundance (36).
Bacterial species called “generalists” require few host-associated benefits and occupy a wide range of habitats, while specialist species are confined to a narrow range of habitats; in addition, some generalists have site-specific subtypes (35). For example, the genus Streptococcus, a known generalist has species specialized for the tongue such as S. salivarius and S. parasanguinis, and S. sanguinis and S. gordonii for dental plaque. Some other species are “true” generalists, e.g. Porphyromonas pasteri is abundant in all tested oral niches. However, this seem to be a unique example rather than a generality and the major conclusion is that most oral microbes show strong preferences for individual habitats within the mouth. As a result of their specialization, when bacteria are out of their preferred niche, their growth is reduced and their gene expression altered (28). Understanding the ecology and physiology of the microbiota will help the interpretation of metagenomic and metatranscriptomic data (37).
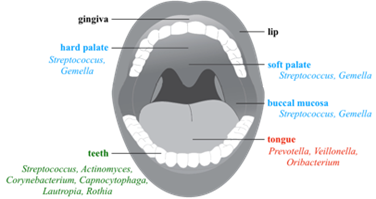
Taken together, the characteristics of the oral cavity and their resident microbiota could be used to better understand the hallmarks of each community associated to the mouth compartments. For instance, saliva has no true microbiota because swallowing occurs shortly after its secretion and there is not enough time for bacterial growth, especially at the low levels of nutrients present in saliva. On the papillate surfaces of the tongue, the microbiota is skewed towards anaerobic genera whereas the ventral surface of the tongue and other mucosal sites (buccal mucosa, keratinized gingiva and hard palate) bears a community rich in Firmicutes (25, 28, 38). Finally, the tooth surfaces, which represent a stable location for long-term biofilm development, are the more diverse (37) (Figure 3).
Oral biofilms formation
Colonization of oral surfaces is a non- random process in which bacterial species are somehow selected by adherence properties and result in successful colonization when bacteria can grow in the specific niche. Bacteria in the mouth can reach all the surfaces (teeth and the different types of mucosa) thanks to the saliva flow, but the characteristics of the different tissues determine which species are able to adhere and colonize them. These adherence features may be driven by coevolution of microbe-microbe interactions e.g. syntrophy (the metabolic products of one species are used by another as nutritional source), coaggregation (attachment of different bacterial species to one another using specific molecules), antagonism (some bacteria might inhibit adherence or growth of another species) and communication (i.e. quorum sensing); as well as adaptation to the host (23,35).
Dental biofilms develop by waves of colonization, and its diversity increases over time. Early colonizers attach via their fimbriae to a pellicle formed by host salivary glycoproteins on the tooth surface (39). Then, they modify the environment, enabling other species to adhere and to establish afterwards (9). At first, coaggregation occurs between Firmicutes and Actinobacteria genera such as Streptococcus, Veillonella and Actinomyces. Afterwards, Fusobacterium interact with those initial colonizers (25). These colonizers play positive roles in maintaining oral health, establishing a complex interdependency among its members, and contribute to preserve the biofilm resilience (9).
If oral hygiene is neglected, later bacteria recognize polysaccharide or protein receptors on the early colonizers and attach to them. As a result, a mature subgingival biofilm forms also called subgingival plaque, consisting of approximately 5-25% bacterial cells and 75-95% matrix (39). At first, homeostasis is maintained with Firmicutes and Actinobacteria being dominant. With time, the accumulation of cytotoxic metabolites such as endotoxin and lytic enzymes pass into the gingivae, inducing irritation and inflammation. This host immune response deepens the periodontal crevices and elevates the secretion and flow of gingival crevicular fluid (13,40). This serum ultrafiltrate is rich in protein and promotes the growth of proteolytic bacteria such as Fusobacterium and Prevotella (40). Prevotella is preponderant in the shift from the early supragingival plaque (aerobic and facultative) to the anaerobic physiology of mature and subgingival plaque. Additionally, Fusobacterium physically connects initial colonizers with periodontopathogens (25). This is only the first step in the establishment of a complex and highly diverse microbiota in the subgingival pocket (41).
Thus, when the microbial equilibrium is broken a process called dysbiosis occurs and pathogens thrive and disease occurs (34). This deleterious change in the natural balance of the microbiota is a consequence of the overgrowth of indigenous pathogens in the resident microbiota rather than as a result of exogenous infection (18).
On the next and final part of this review, the genesis of the two most prevalent oral diseases as well as their impact on general health will be discussed.