One of the main characteristics of the Jatropha curcas plant is the high production of non-edible oil, widely used for the making of biodiesel. However, several residues are generated during the production process. One of these residues is the seed husk, which represents the third part of the seed mass (Heller, 1996; Sotolongo, Díaz & Montes de Oca, 2007; OCTAGON, 2007).
The seed husk chemical composition was published by Makkar, Becker & Schmook(1998) where several analysed varieties presented a high content of lignin, over 45% on a dry basis. Lignin is a natural heterogeneous polymer, based on three monomers: guiaicyl, syringyl
and p-hidroxyphenylpropane. (Tejado, Peña, Labidi, Echeverria & Mondragón, 2007). Based on the phenolic structure, lignin presents some chemical characteristics that allow it to substitute phenol on several resins and adhesives, such as phenol-formaldehyde resins, decreasing the use of phenol (Pérez, Rodríguez, Alonso, Oliet & Echeverría, 2007).
Phenolic resins are thermo-stable polymeric structures, which are produced by reacting phenol (or a substitute phenol) with formaldehyde. Based on the components ratio, they are called resols or novolacs. They are stable at high temperature, have electric isolation properties and present chemical stability. These properties have opened their use to many applications (Kopf, 2000).
It has been reported the use of lignin in several phenolic resin formulations, substituting phenol partially or completely (León & Sanabria, 1988; Alonso, Oliet, Pérez, Rodríguez & Echeverría, 2004; Derek, 2008; Effendi, Kalaycioglu & Hiziroglu, 2008; Gabilondo, López, Ramos, Echeverría & Mondragon, 2007; Nada, Abou-Youssef & ElGohary, 2003; Pérez et al., 2007).
In most cases they obtained a resin with good properties only if they substituted phenol partially. Using a high percentage of lignin affects the mechanical and physical properties, because there is insufficient polymerization (Nada et al., 2003). This study is focused on the extraction of lignin from Jatropha curcas seed husk and its incorporation into phenolic resins, because no study has reported the use of this lignin to synthesize a modified- phenolic resin.
Materials and methods
Materials:Jatropha curcas seeds were obtained from the Fabio Baudrit Experimental Station, University of Costa Rica. This station is located in La Garita, Alajuela, Costa Rica (Coordinates: 10.004099 N, -84.267873 E). The seed husk was manually separated, and grinded in a millstone, obtaining particles of less than 1mm length.
Analysis of seed husk: Several analyses were carried out to the seed husk: moisture content, extractable substances, ashes, acid insoluble lignin and heat of combustion. In the first four analyses, specific TAPPI standard methods for each analysis were used. For heat of combustion it was ASTM D5865-07a standard method.
Lignin extraction: A pulping method was applied, it consisted of 15,0g of seed husk with 250 mL of NaOH 2 mol/L, and reflux during 8 hours with stirring. Afterwards, it was filtered while still hot to remove the seed husk particles, and the liquid was acidified with H2SO4 3 mol/L until pH~2 was achieved. The precipitated lignin was filtered through filter paper and a Büchner funnel, and washed with hot water until neutrality. The lignin cake had a ~70% moisture.
Preparation of lignin-modified resin: The modified method of Khan, Ashraf & Malhotra (2004) was used. The lignin cake was mixed in a phenol:lignin ratios of 0%, 25%, 50%, 75% and 100%, based on the dry lignin weight in the cake. This resin was pre-polymerized with 37% aqueous formaldehyde as a binder, NaOH as a catalyst and methanol and water as solvents. The curing process was done at 65°C for 6 hours in the oven, without any other added substance.
Characterization of lignin-modified resin: The lignin and phenolic resins were characterized using FT-IR (Perkin Elmer, Spectrum FT 1000), in KBr pellets. The 0%, 50% and 100% lignin-modified resins and the pure lignin were analyzed with thermogravimetric analysis (TGA) (TA Instruments Q500). The chemical resistance was tested with water, 95% ethanol, acetone, 0,1mol/L aq. HCl and 0,1mol/L aq. NaOH on 0% and 50% modified resins, submerging a small fragment of resin in the solutions and monitoring weight changes through time.
Electroisolating property was tested using the Mena, Castro & Castellón (2012) method. The equipment was composed of a RadioShack multimeter, model 22-821, and two metal rod (diameter= 0,470cm, resistance= 2Ω). Pressure was applied using a 2kg weight.
Results
The results of the lignin physicochemical analyses are presented in Table 1, with a comparison with several published data by Makkar, Becker & Schmook (1998).
Table 1: Determination of composition of J. curcas seed husk, with data published by Makkar et al. (1998)
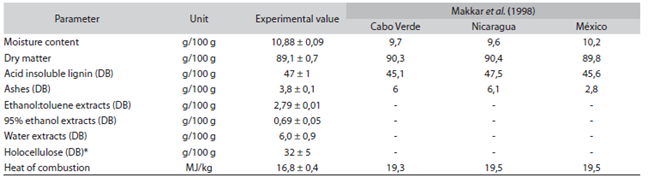
*Calculated on the difference between 100% and the sum of all measure components; DB: dry basis.
Next, the lignin was extracted using the selected method. About 50% of the lignin in the seed husk was extracted, which represented the 23% of the husk weight. To achieve these percentages, several conditions had to be optimized, such as heating time, liquid: solid ratio and lignin precipitation techniques.
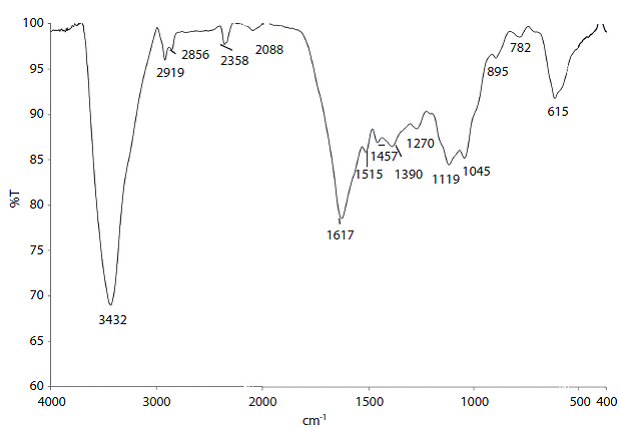
After the extraction method, a lignin cake was obtained with approximately 70% moisture. This cake was kept refrigerated until resin preparation. The cake was characterised using FT-IR analysis (Figure 1). The spectra shows specific bands for guaiacyl units (near 1275, 1153 and 1037 cm-1), while syringyl unit bands are missing (near 1330, 1220 and 1120 cm-1), pointing out a high quantity of guaiacyl units in the extracted lignin (Ibrahim, Agblevor & El-Zawawy, 2010).
The lignin TGA (Figure 2.A) shows quick thermal degradation between 125-320 ºC, while it has been reported that this thermal degradation for lignin occurs between 200-400ºC (Ibrahim, Zakaria, Sipaut, Sulaiman & Hashim, 2011).
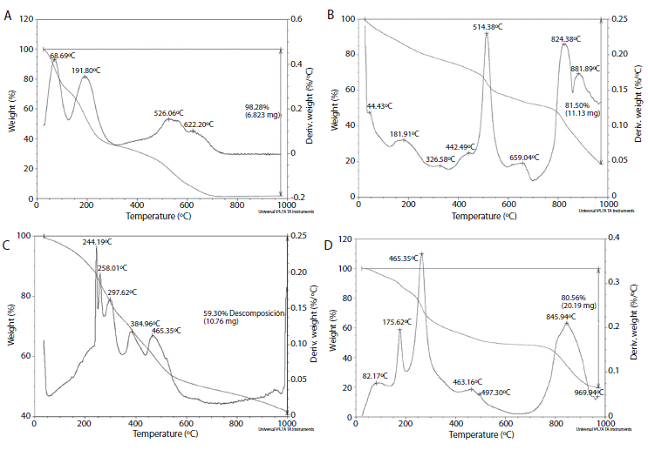
Figure 2: TGA thermograms of A) lignin, B) control resin (PF), C) 50% lignin-modified resin and D) 100% lignin-modified resin.
Khan, Ashraf & Malhotra (2004) modified method was used to obtained the modified-phenolic resins. The control resin (without lignin, named PF) was very hard to grind and presented a dark purple shining colour. The lignin-modified phenolic resins (LPF) were hard to grind (not as much as the control resin) and presented an opaque dark brown colour. The resins with the highest percentage of lignin (75% and 100%) showed fractures at the edges.
As the lignin:phenol ratio was higher, several bands were affected in the FT-IR spectra (Table 2). The 2826 and 2715 cm-1 bands indicates a bonding between Jatropha lignin and resin due to condensation of the lignin aldehyde (-CHO) group with a phenolic resin methylol group, confirming the incorporation. Also, the increasing intensity of the 765 cm-1 band, associated to the aromatic-aliphatic C-C bond, shows reaction between the lignin and phenol (Sarkar & Adhiraki, 2001).
Table 2: Peak assignment and variations observed in IR spectra for control resin (PF) and modified resins (LPF)
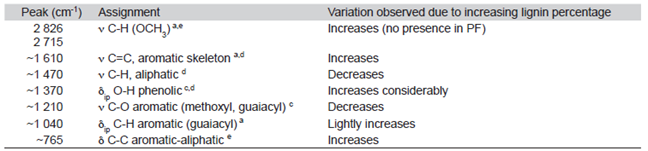
ν: Stretching; δ: deformation; ip: in plane; op: out of plane.
Sources: a) Ibrahim et al., (2011); b) Khan et al. (2004); c) Hergert (1959); d) Poljanšek & Krajnc (2005); e) Sarkar & Adhiraki (2001).
The TGA show the difference in thermal behaviour between the control resin (no lignin) and the
lignin-modified resin. In Figure 2, the thermal degradation of lignin-modified resins occurs below the temperature where the control resin degrades. Usually the phenolic resins hold until 514°C without losing more than 40% of their original weight (Kopf, 2000). But the modified resin degrades between 200-400°C, mainly because of lignin degradation, besides there is not a perfect cross-linking in the polymeric matrix.
The resins solubility analysis (Figure 3) shows the percentage change of weight in the resins samples when exposed to different solutions. In general, there is a small decrease of weight (no more than 7% in 50 hours of contact) when using water, acetone, ethanol and 0,1mol/L aq. HCl, on the control resin and the 50% lignin-modified resin. However, when using 0,1mol/L aq. NaOH there is a high weight increase in the lignin-modified resin, because it is soluble in alkaline solutions and quickly absorbs the solution.
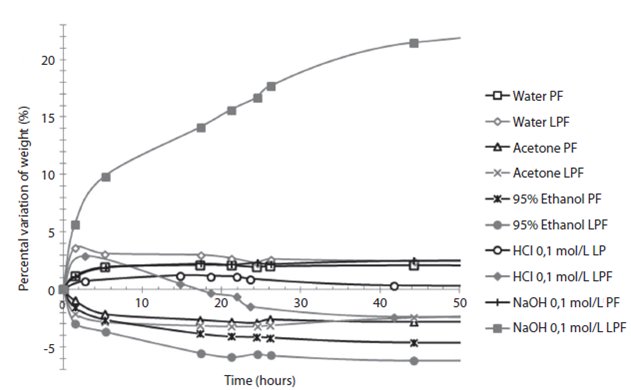
Figure 3: Variation on initial weight for control resin (PF) and 50% lignin modified resin (LPF) under contact of several common use solvents.
Variation on initial weight for control resin (PF) and 50% lignin modified resin (LPF) under contact of several common use solvents.
Finally, the electric insulation analyses were done using equipment with a maximum resistance of 40MΩ. When the resins were measured, both the control and the modified resins, were over this value. When this resistance value was converted to electric resistivity, one obtained 4x106Ω×m, which is the smaller electric resistivity the resin could have.
Discussion
The chemical composition of the seed husk is similar to other husks previously analyzed by Makkar et al. (1998). Low ash content and a heat of combustion over 16MJ/ kg favours the use of J. curcas seed husk as a solid fuel in biomass combustion processes (Mendu et al., 2011), offering a possible use for this residue.
The seed husk has a notable high quantity of lignin, which could be extracted and used as feedstock for several processes. Based on this conclusion, the lignin was extracted using alkaline pulping. Several parameters had to be optimized to achieve the best extraction, specially heating time, liquid:solid ratio and the precipitation methodology. Using this optimized process, about 50% of the previously determined lignin was obtained.
The lignin FT-IR spectra showed that there is a high amount of guaiacyl units, which have a C5 free position in the ring (ortho position to the phenolic hydroxyl group), having a higher activity towards formaldehyde electrophilic substitution. This is important if one aims for a good polymerization in the phenolic resin (Tejado et al., 2007). In the syringyl units the C5 positions are occupied and the activity of this unit is minimal in phenolic resin polymerization. As a conclusion, using a lignin with high amount of guaiacyl units allow their use in modifying phenol resins.
However, the lignin TGA shows a low thermal stability, property associated to high cross-linking in the polymer and the development of highly condensated aromatic structure (Ibrahim et al., 2011). This result shows that the lignin cross-linking quality is not high enough, and can be based on the original plant nature or the applied treatment (Cheng, Winter & Stipanovic, 2012). A low thermal stability could affect the use of lignin in thermostable resins, that is why is important to determine TGA analysis on the obtained resins.
When the modified resins were made, some mechanical properties were lessened and several fractures appeared when high lignin substitution was used. The modified resin with 50% lignin presented the best mechanical properties. The FT-IR spectra showed good lignin incorporation in the resin matrix, by increasing several specific bands, shown in table 2. However, based on the fractures physical evidence, it is recommended a partial substitution, with no more than 50% of lignin.
The TGA results showed modified resins with a lower stability vs. the control resin, possibly due to lignin low thermal stability. It is not recommend the use of modified resins on high temperatures applications. However, it could be used at temperatures under 100ºC, where there would not be significant weight losses that could affect the resin properties.
The electric resistivity data confirmed the possible use of modified resins as electric insulators, because the electric resistivity obtained is similar to several electric insulating materials (Callister & Rethwisch, 2010). The specific electric resistivity value was not obtained because the resins were over the measuring equipment upper limit. But defining the lower limit of 4x106Ω×m can ensure these resins are on the typical electrical resistivity values for electric insulators.
Finally, the behaviour of the modified resins in different solutions was analyzed and shown in Figure 3. In the presence of water, 95% ethanol, acetone and 0,1mol/L aq. HCl, the 50% lignin resin did not undergo any radical changes (a maximum weight loss of 7%), which is comparable to control resin. However, when an alkaline solution was used (0,1mol/L aq. NaOH), the resin absorbed more than 20% of the original weight and the solution turned yellow. This happened because the lignin is soluble in alkaline solutions, showing its low chemical stability towards alkaline solutions.
As a conclusion, obtaining a modified phenolic resin based on J. curcas seed husk lignin is technically viable, introducing desirable characteristics of thermal, electric and chemical stability. Some previsions should be taken if the resins would be used in specific chemical and physical environments, either high temperatures or presence of alkaline solutions.
This way one could take advantage of an agro-industrial residue, improving its value and strengthening the chemical migration into technological biomaterials.












 uBio
uBio